Ferritin-Coated SPIONs as New Cancer Cell Targeted Magnetic Nanocarrier
Abstract
1. Introduction
2. Results and Discussion
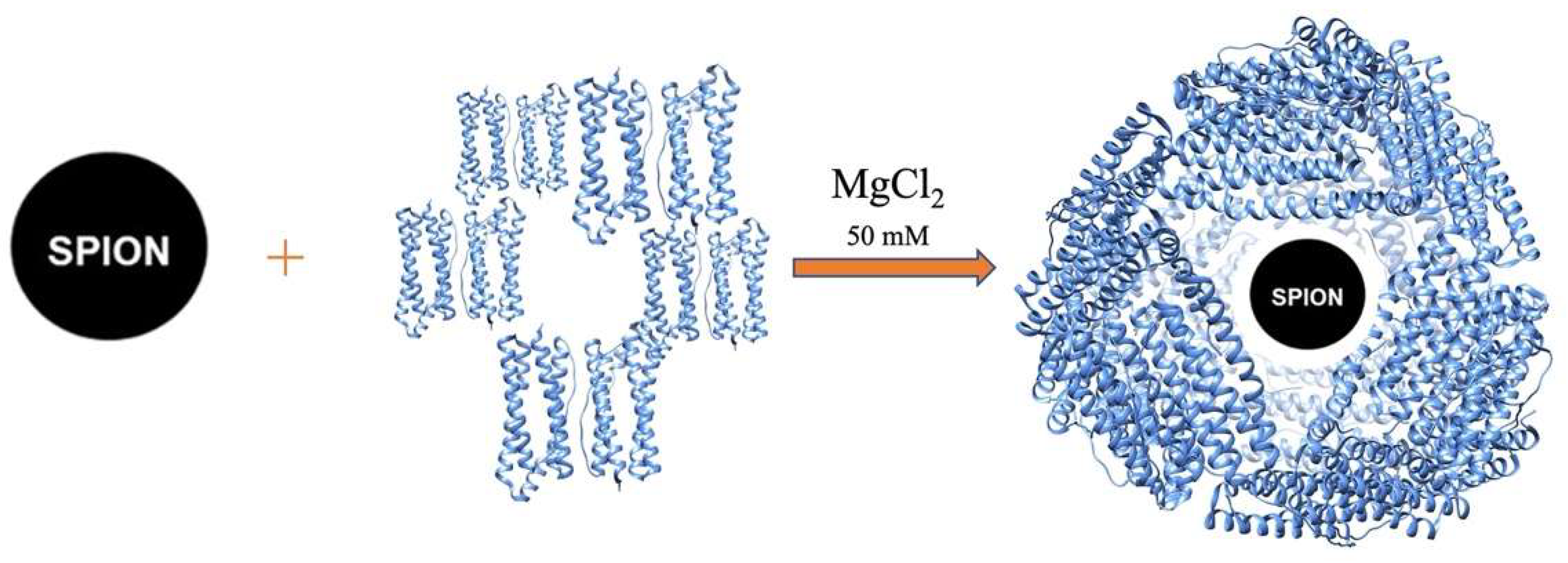
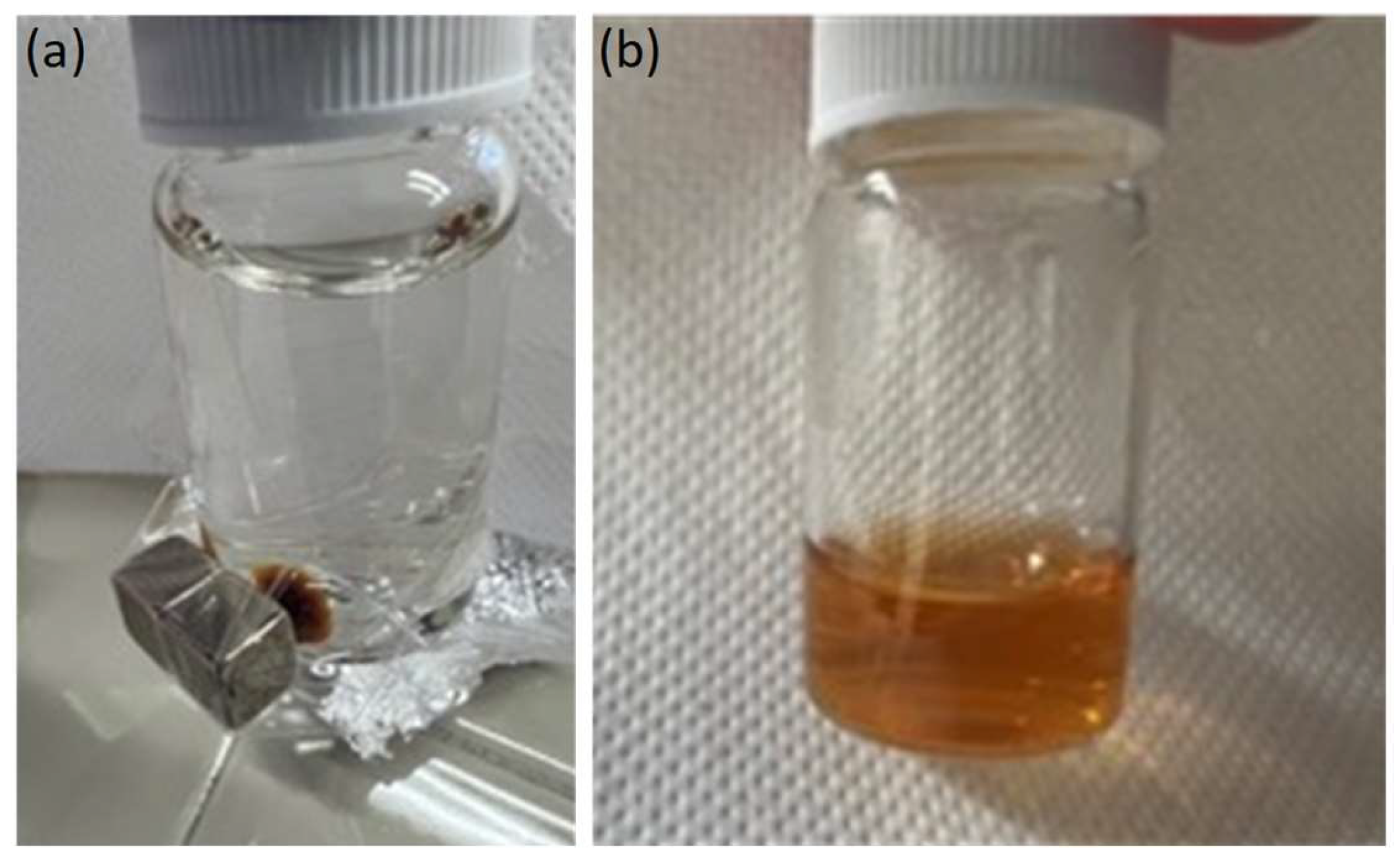
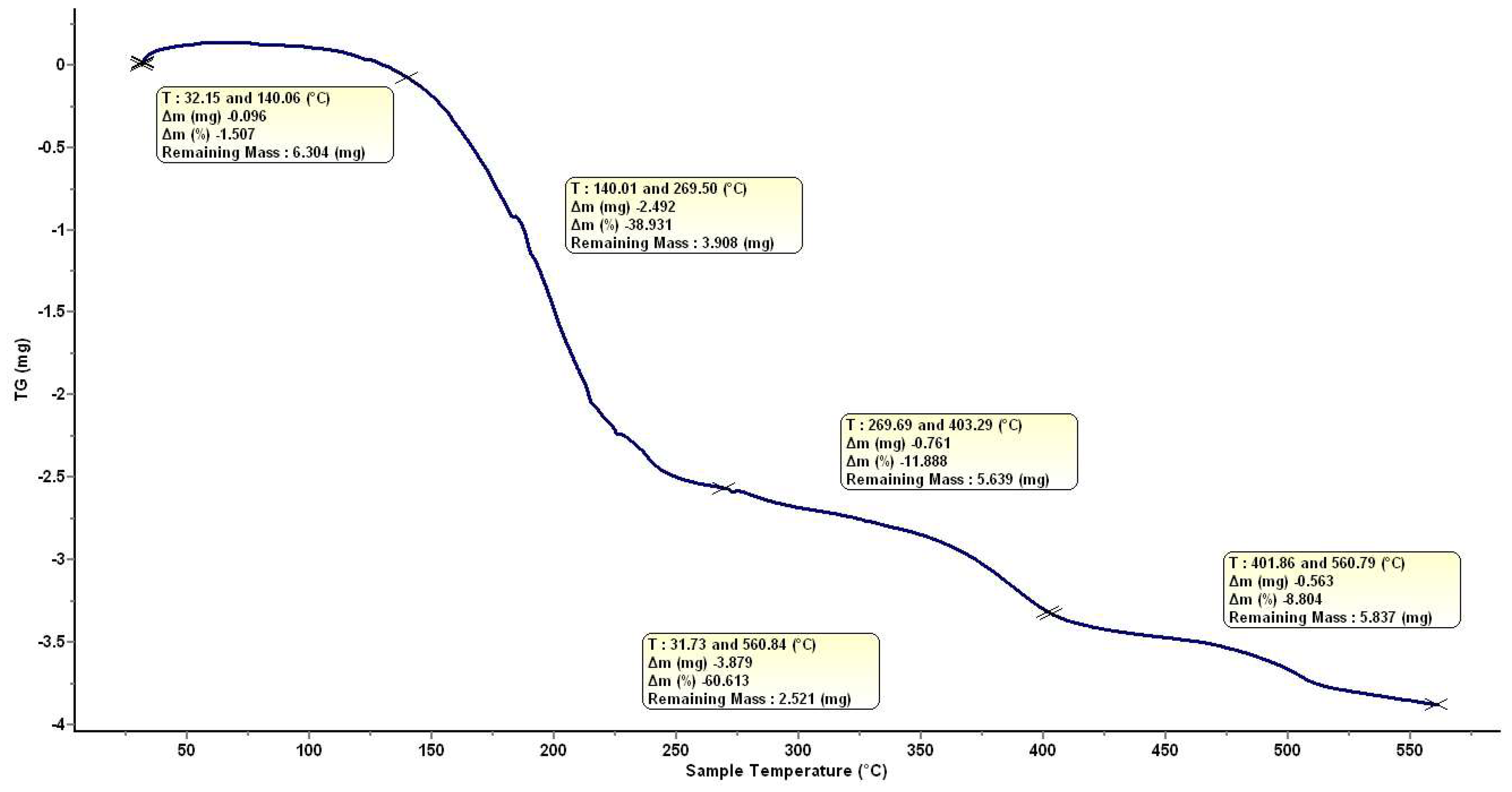
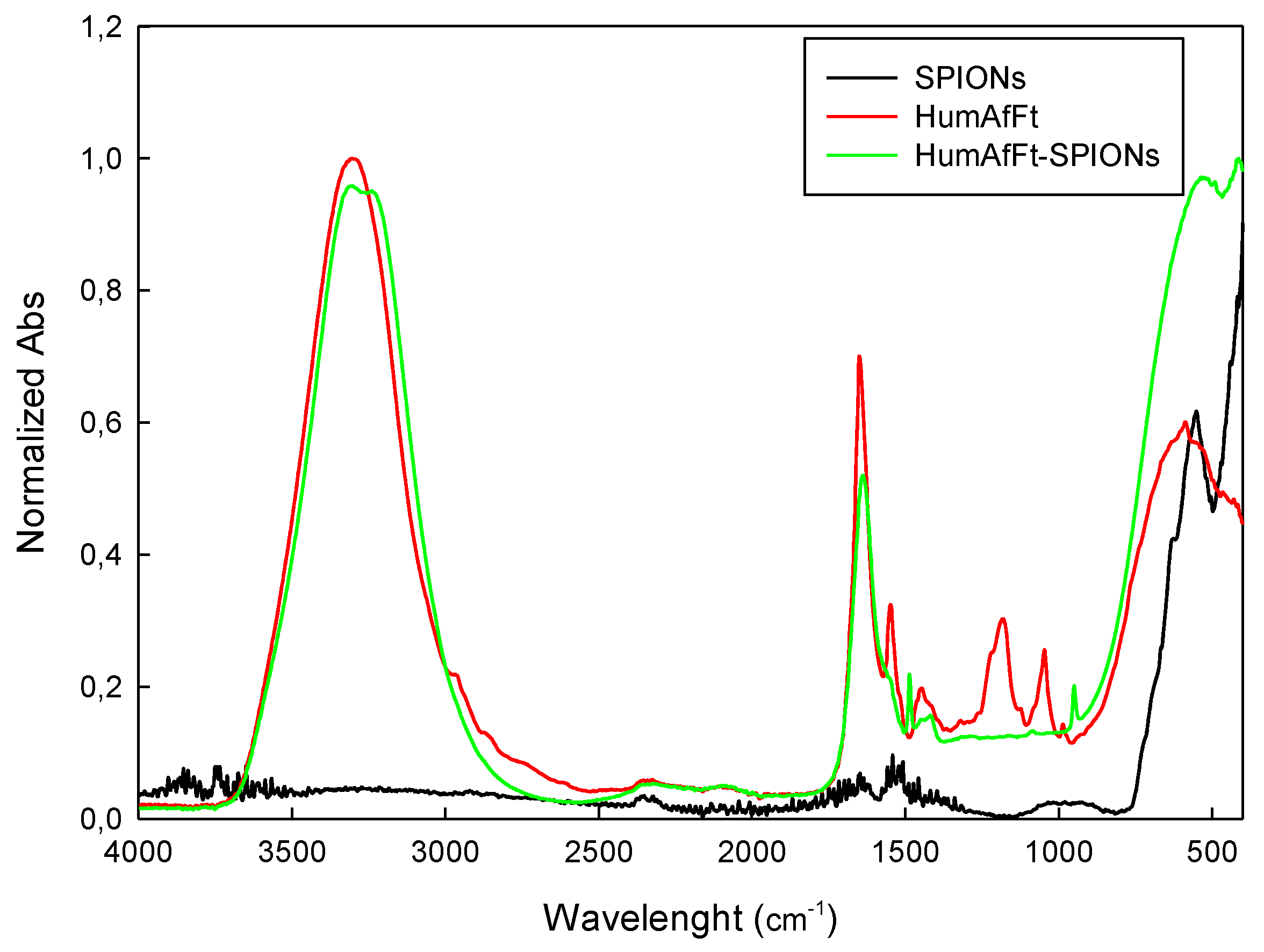
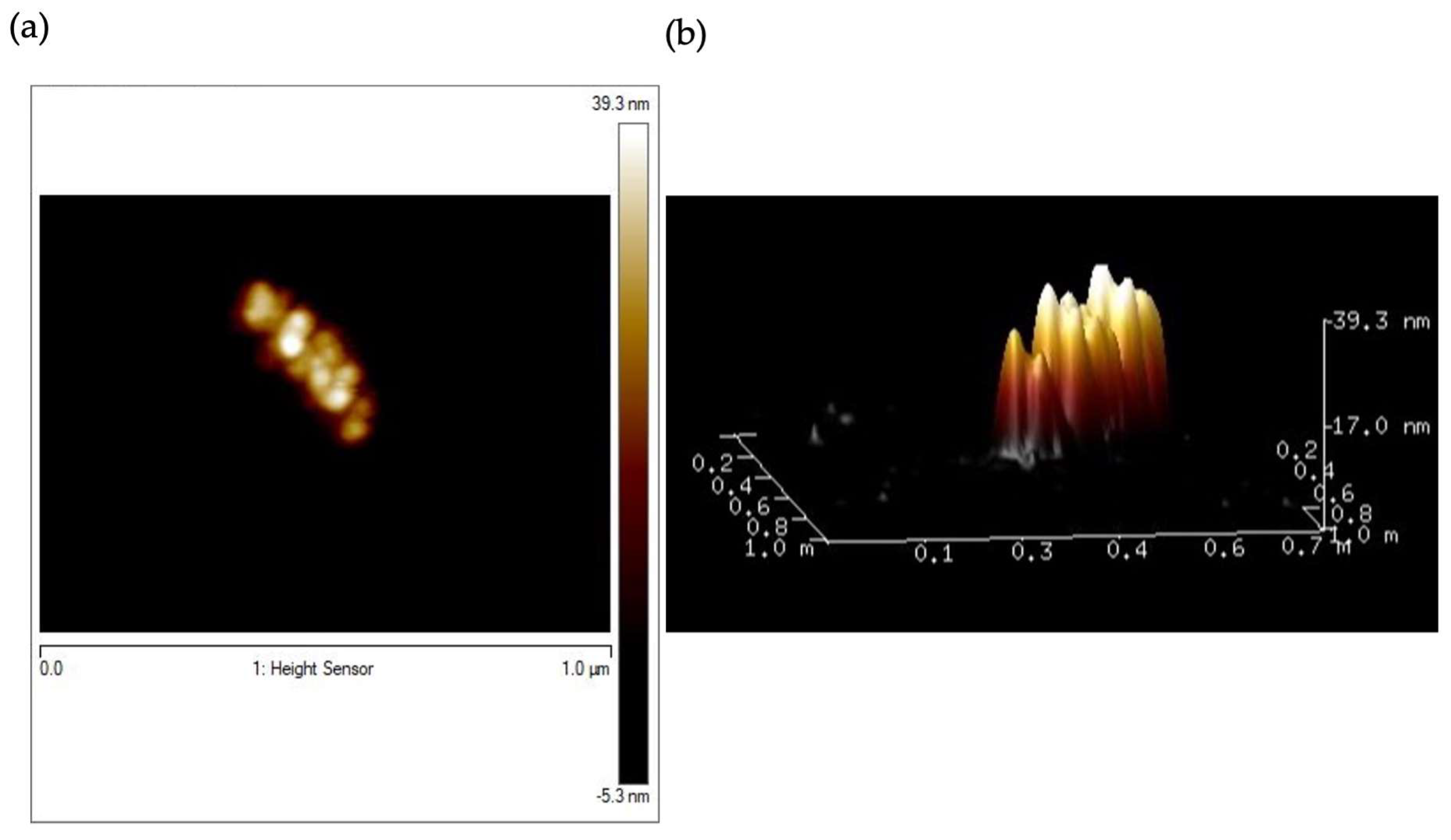
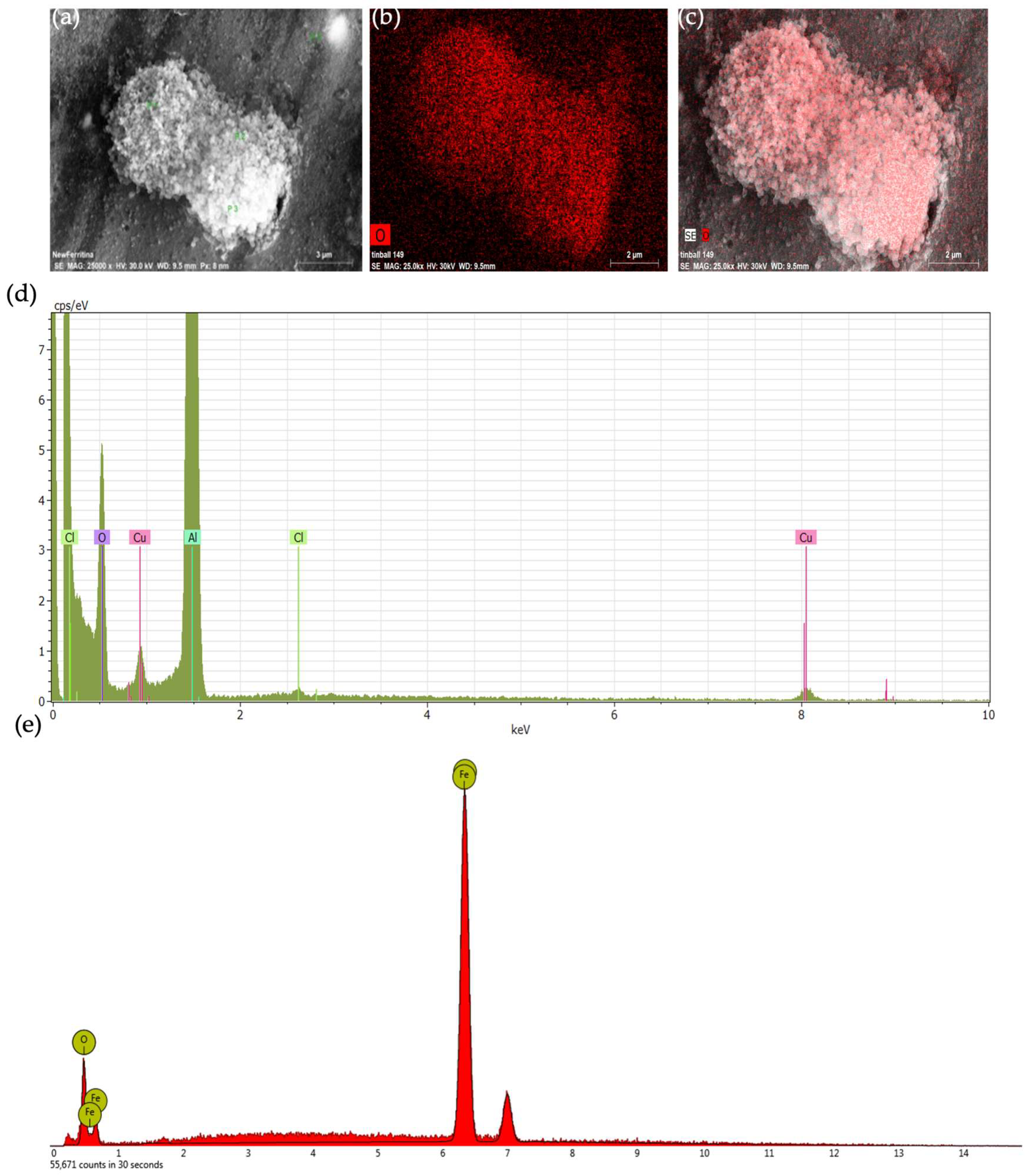
2.1. Preparation and Characterization of HumAfFt-SPIONs
Stability Studies of the HumAfFt-SPIONs
2.2. In Vitro Biological Characterization of HumAfFt-SPIONs
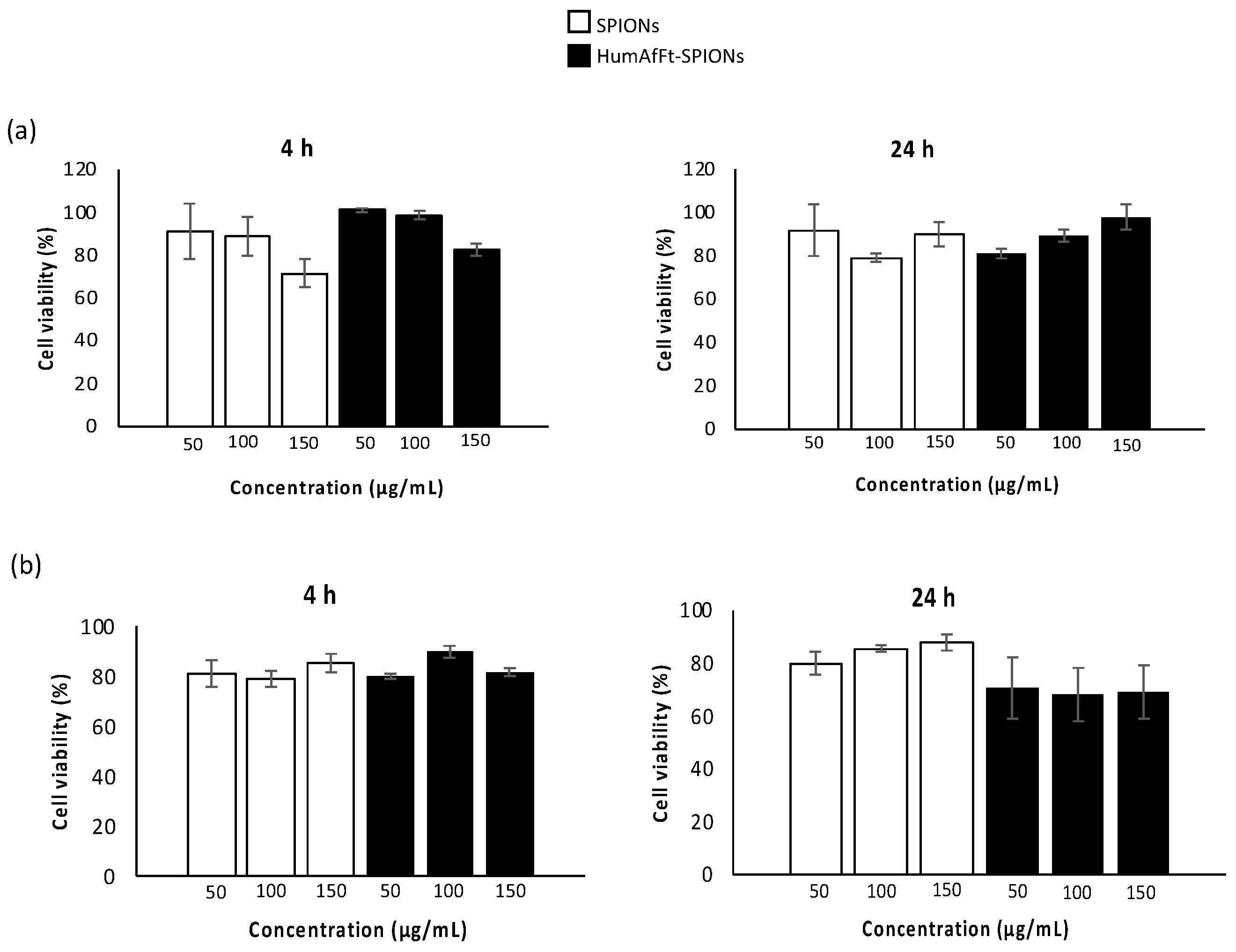
2.2.1. Cytotoxicity Assay
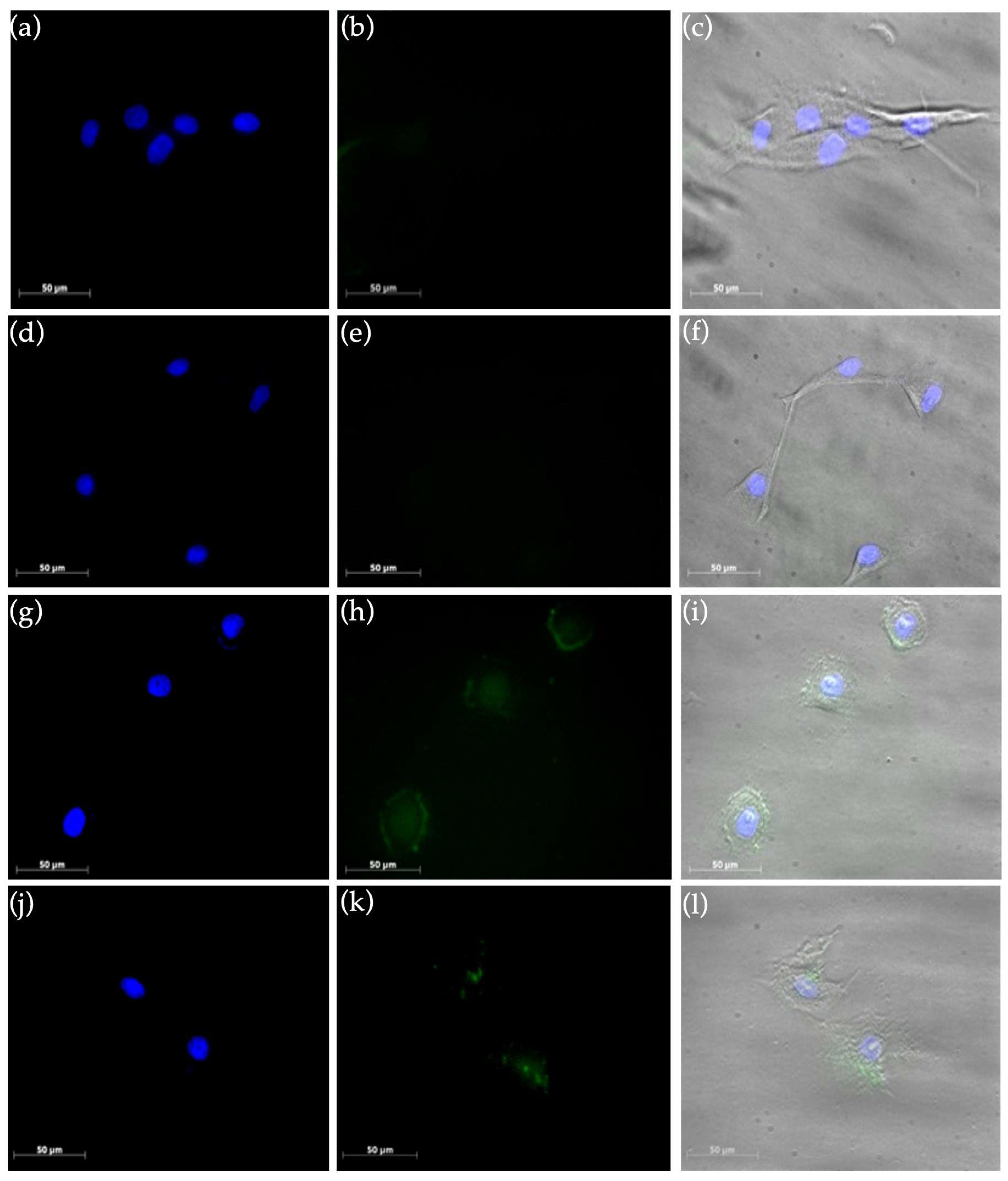
2.2.2. Uptake Studies in Cell Culture Experiments by Fluorescence Microscopy
3. Materials and Methods
3.1. Expression and Purification of HumAfFt
3.2. Preparation of HumAfFt-coated SPIONs
3.3. Characterization of the HumAfFt-SPIONs
3.4. In Vitro Biological Evaluations
3.4.1. Cytotoxicity Assay
3.4.2. Uptake Studies by Fluorescence Microscopy
3.4.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug. Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug. Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Scialabba, C.; Puleio, R.; Cassata, G.; Cicero, L.; Cavallaro, G.; Giammona, G. Smart copolymer coated SPIONs for colon cancer chemotherapy. Int. J. Pharm. 2019, 556, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Que, C.; Wang, C.; Liu, X.; Yan, H.; Liu, K. Multifunctional superparamagnetic nanocarriers with folate-mediated and pH-responsive targeting properties for anticancer drug delivery. Biomaterials 2011, 32, 185–194. [Google Scholar] [CrossRef]
- Rastogi, R.; Gulati, N.; Kotnala, R.K.; Sharma, U.; Jayasundar, R.; Koul, V. Evaluation of folate conjugated pegylated thermosensitive magnetic nanocomposites for tumor imaging and therapy. Colloids Surf. B 2011, 82, 160–167. [Google Scholar] [CrossRef]
- Scialabba, C.; Puleio, R.; Peddis, D.; Varvaro, G.; Calandra, P.; Cassata, G.; Cicero, L.; Licciardi, M.; Giammona, G. Folate targeted coated SPIONs as efficient tool for MRI. Nano. Res. 2017, 10, 3212–3227. [Google Scholar] [CrossRef]
- Wang, X.; Wei, F.; Liu, A.; Wang, L.; Wang, J.C.; Ren, L.; Liu, W.; Tu, Q.; Li, L.; Wang, J. Cancer stem cell labeling using poly(l-lysine)-modified iron oxide nanoparticles. Biomaterials 2012, 33, 3719–3732. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef]
- Zimmer, C.; Weissleder, R.; Poss, K.; Bogdanova, A.; Wright, S.C., Jr.; Enochs, W.S. MR imaging of phagocytosis in experimental gliomas. Radiology 1995, 197, 533–538. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Fu, W.; Du, K.; Sui, Y.; Chen, J.; Zeng, Y.; Li, M.; Zou, G. Preparation and magnetic properties of magnetite nanoparticles by sol-gel method. J. Magn. Magn. Mater. 2007, 309, 307–311. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the Surface Properties of Superparamagnetic Iron Oxide Nanoparticles through a Sol-Gel Approach. Nano. Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid. Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Mauro, N.; Scialabba, C.; Puleio, R.; Varvarà, P.; Licciardi, M.; Cavallaro, G.; Giam-mona, G. SPIONs embedded in polyamino acid nanogels to synergistically treat tumor microenvironment and breast cancer cells. Int. J. Pharm. 2019, 555, 207–219. [Google Scholar] [CrossRef]
- Rosen, J.E.; Chan, L.; Shieh, D.B.; Gu, F.X. Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomedicine 2012, 8, 275–290. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Cavallaro, G.; Sangregorio, C.; Fantechi, E.; Giammona, G. Cell uptake enhancement of folate targeted polymer coated magnetic nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 949–964. [Google Scholar] [CrossRef]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Hong, R.Y.; Li, J.H.; Qu, J.M.; Chen, L.L.; Li, H.Z. Preparation and characterization of magnetite/dextran nanocomposite used as a precursor of magnetic fluid. J. Chem. Eng. 2009, 150, 572–580. [Google Scholar] [CrossRef]
- Kim, D.K.; Mikhaylova, M.; Wang, F.H.; Kehr, J.; Bjelke, B.; Zhang, Y.; Tsakalakos, T.; Muhammed, M. Starch-Coated Superparamagnetic Nanoparticles as MR Contrast Agents. Chem. Mater. 2003, 15, 4343–4351. [Google Scholar] [CrossRef]
- Ma, H.L.; Qi, X.R.; Maitani, Y.; Nagai, T. Preparation and characterization of superparamagnetic iron oxide nanoparticles stabilized by alginate. Int. J. Pharm. 2007, 333, 177–186. [Google Scholar] [CrossRef]
- Ma, H.L.; Xu, Y.F.; Qi, X.R.; Maitani, Y.; Nagai, T. Superparamagnetic iron oxide nanoparticles stabilized by alginate: Pharmacokinetics, tissue distribution, and applications in detecting liver cancers. Int. J. Pharm. 2008, 354, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Wang, S.P.H.; Su, C.H.; Tsai, T.L.; Wu, P.C.; Shieh, D.B.; Chen, J.H.; Hsieh, P.C.H.; Yeh, C.S. Stabilizer-free poly(lactide-co-glycolide) nanoparticles for multimodal biomedical probes. Biomaterials 2008, 29, 2104–2112. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wells, S. Surface-Modified Superparamagnetic Nanoparticles for Drug Delivery: Preparation, Characterization, and Cytotoxicity Studies. IEEE Trans. Nanobioscience 2004, 3, 66–73. [Google Scholar] [CrossRef]
- Gupta, A.K.; Curtis, A.S.G. Surface modified superparamagnetic nanoparticles for drug delivery: Interaction studies with human fibroblasts in culture. J. Mater. Sci. Mater. Med. 2004, 15, 493–496. [Google Scholar] [CrossRef]
- Gupta, A.K.; Berry, C.; Gupta, M.; Curtis, A. Receptor-mediated targeting of magnetic nanoparticles using insulin as a surface ligand to prevent endocytosis. IEEE Trans. Nanobioscience 2003, 2, 255–261. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry. Curr. Opin. Chem. Biol. 2011, 15, 304–311. [Google Scholar] [CrossRef]
- Schoonen, L.; van Hest, J.C.M. Functionalization of protein-based nanocages for drug delivery applications. Nanoscale 2014, 6, 7124–7141. [Google Scholar] [CrossRef]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Skalickova, S.; Zitka, O.; Adam, V.; Kizek, R. Apoferritin applications in nanomedicine. Nanomedicine 2014, 9, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. PNAS 2010, 107, 3505–3510. [Google Scholar] [CrossRef]
- Kim, M.; Rho, Y.; Jin, K.S.; Ahn, B.; Jung, S.; Kim, H.; Ree, M. PH-dependent structures of ferritin and apoferritin in solution: Disassembly and reassembly. Biomacromolecules 2011, 12, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Falvo, E.; Tremante, E.; Fraioli, R.; Leonetti, C.; Zamparelli, C.; Boffi, A.; Morea, V.; Ceci, P.; Giacomini, P. Antibody-drug conjugates: Targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 2013, 5, 12278–12285. [Google Scholar] [CrossRef]
- Macone, A.; Masciarelli, S.; Palombarini, F.; Quaglio, D.; Boffi, A.; Trabuco, M.C.; Bai-occo, P.; Fazi, F.; Bonamore, A. Ferritin nanovehicle for targeted delivery of cytochrome C to cancer cells. Sci. Rep. 2019, 9, 11749–11755. [Google Scholar] [CrossRef]
- Johnson, E.; Cascio, D.; Sawaya, M.R.; Gingery, M. Schröder, I; Crystal structures of a tetrahedral open pore ferritin from the hyperthermophilic Archaeon Archaeoglobus fulgidus. Structure 2005, 13, 637–648. [Google Scholar] [CrossRef]
- Sana, B.; Johnson, E.; Lim, S. The unique self-assembly/disassembly property of Archaeoglobus fulgidus ferritin and its implications on molecular release from the protein cage. Biochim. Biophys. Acta. Gen. Subj. 2015, 1850, 2544–2551. [Google Scholar] [CrossRef]
- de Turris, V.; Trabuco, M.C.; Peruzzi, G.; Boffi, A.; Testi, C.; Vallone, B.; Montemiglio, L.C.; Des Georges, A.; Calisti, L.; Benni, I.; et al. Humanized archaeal ferritin as a tool for cell targeted delivery. Nanoscale 2017, 9, 647–655. [Google Scholar] [CrossRef]
- Calisti, L.; Benni, I.; Trabuco, M.C.; Baiocco, P.; Ruzicka, B.; Boffi, A.; Falvo, E.; Mala-testa, F.; Bonamore, A. Probing bulky ligand entry in engineered archaeal ferritins. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 450–456. [Google Scholar] [CrossRef]
- Piazza, R.D.; Viali, W.R.; Dos Santos, C.C.; Nunes, E.S.; Marques, R.F.C.; Morais, P.C.; Da Silva, S.W.; Coaquira, J.A.H.; Jafelicci, M. PEGlatyon-SPION surface functionalization with folic acid for magnetic hyperthermia applications. Mater. Res. Express. 2020, 7, 15078. [Google Scholar] [CrossRef]
- Wierzbinski, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Nowicka-Bauer, K.; Malcher, A.; Nowaczyk, M.; Krupinski, M.; et al. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 3682–3698. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Varvarà, P.; Tranchina, L.; Puleio, R.; Cicero, L.; Cassata, G.; Giammona, G. In vivo efficacy of verteporfin loaded gold nanorods for combined photothermal/photodynamic colon cancer therapy. Int J Pharm. 2022, 625, 122134. [Google Scholar] [CrossRef]
| Za (nm) | PDI | Zp (mV) | |
|---|---|---|---|
| HumAfFt | 20.42 | 0.392 | −4.1 |
| SPIONs | 27.65 | 0.295 | −31.3 |
| HumAfFt-SPION | 145.8 | 0.202 | −5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affatigato, L.; Licciardi, M.; Bonamore, A.; Martorana, A.; Incocciati, A.; Boffi, A.; Militello, V. Ferritin-Coated SPIONs as New Cancer Cell Targeted Magnetic Nanocarrier. Molecules 2023, 28, 1163. https://doi.org/10.3390/molecules28031163
Affatigato L, Licciardi M, Bonamore A, Martorana A, Incocciati A, Boffi A, Militello V. Ferritin-Coated SPIONs as New Cancer Cell Targeted Magnetic Nanocarrier. Molecules. 2023; 28(3):1163. https://doi.org/10.3390/molecules28031163
Chicago/Turabian StyleAffatigato, Luisa, Mariano Licciardi, Alessandra Bonamore, Annalisa Martorana, Alessio Incocciati, Alberto Boffi, and Valeria Militello. 2023. "Ferritin-Coated SPIONs as New Cancer Cell Targeted Magnetic Nanocarrier" Molecules 28, no. 3: 1163. https://doi.org/10.3390/molecules28031163
APA StyleAffatigato, L., Licciardi, M., Bonamore, A., Martorana, A., Incocciati, A., Boffi, A., & Militello, V. (2023). Ferritin-Coated SPIONs as New Cancer Cell Targeted Magnetic Nanocarrier. Molecules, 28(3), 1163. https://doi.org/10.3390/molecules28031163









