In Situ Hydrothermal Synthesis of Ni1−xMnxWO4 Nanoheterostructure for Enhanced Photodegradation of Methyl Orange
Abstract
1. Introduction
2. Results and Discussion
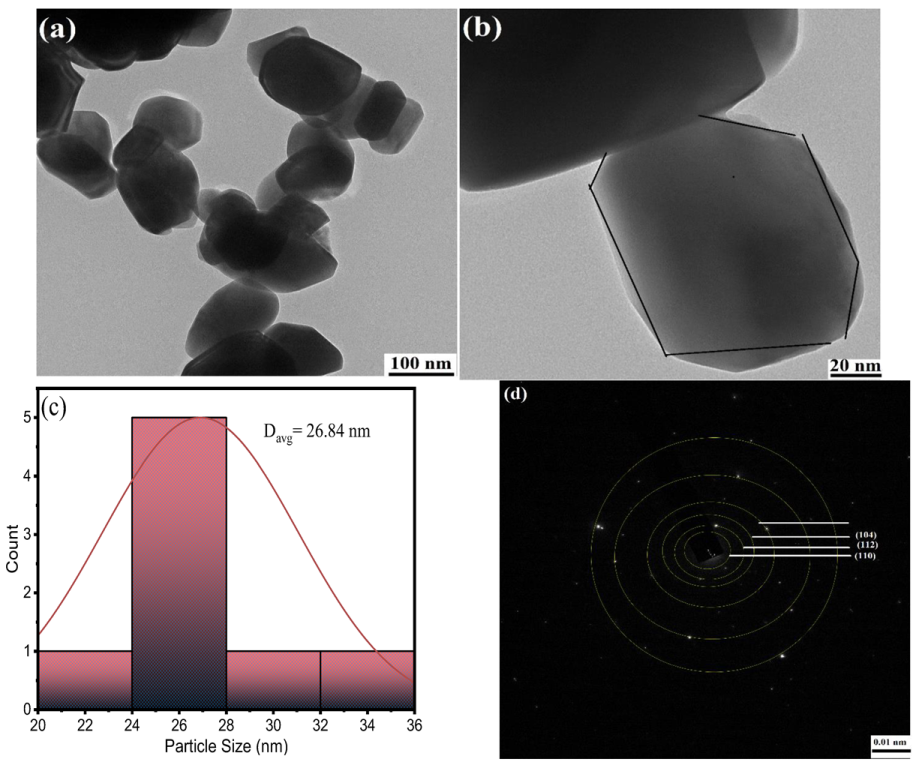
2.1. Material Characterization
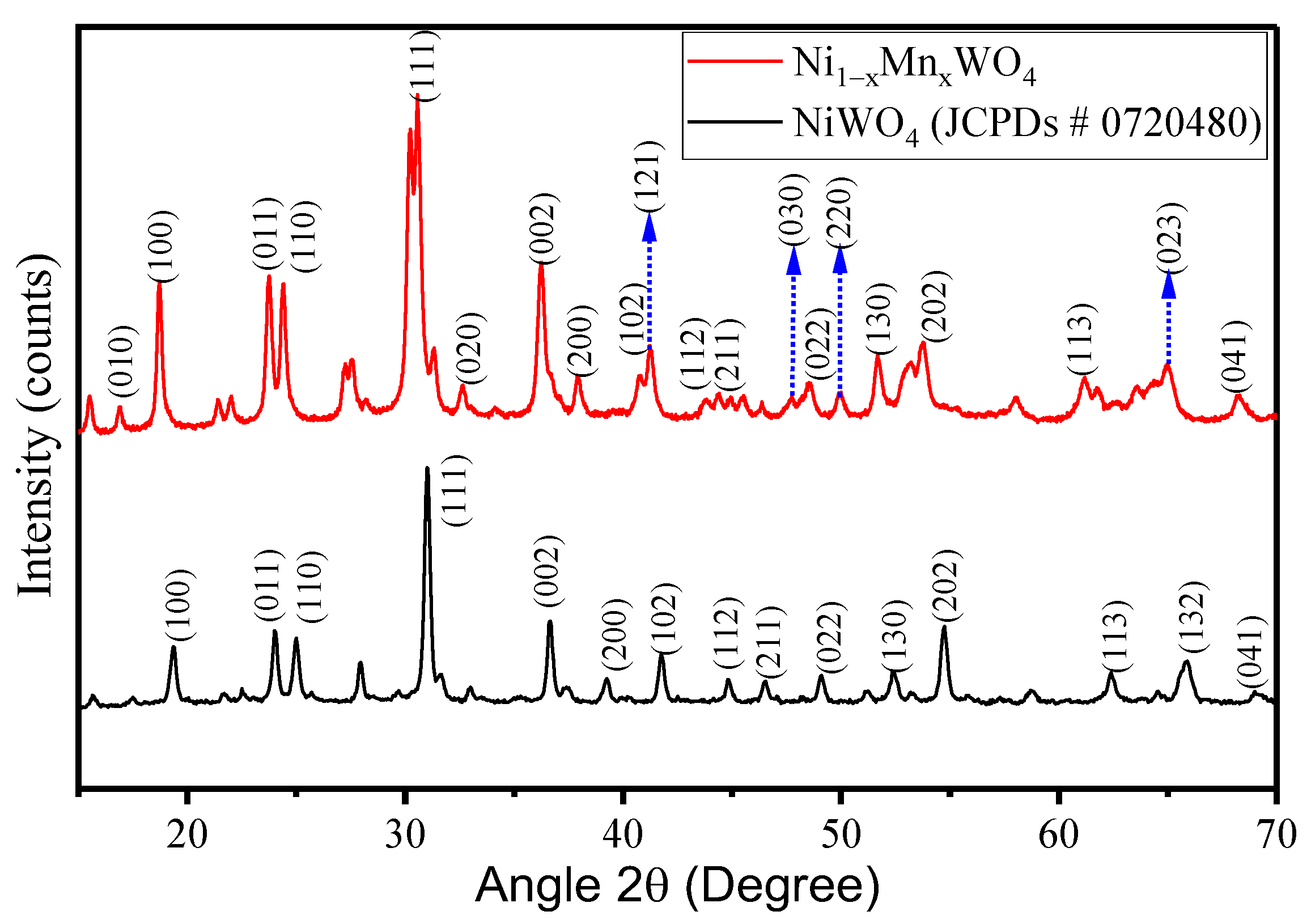
2.1.1. Crystal Structure Studies
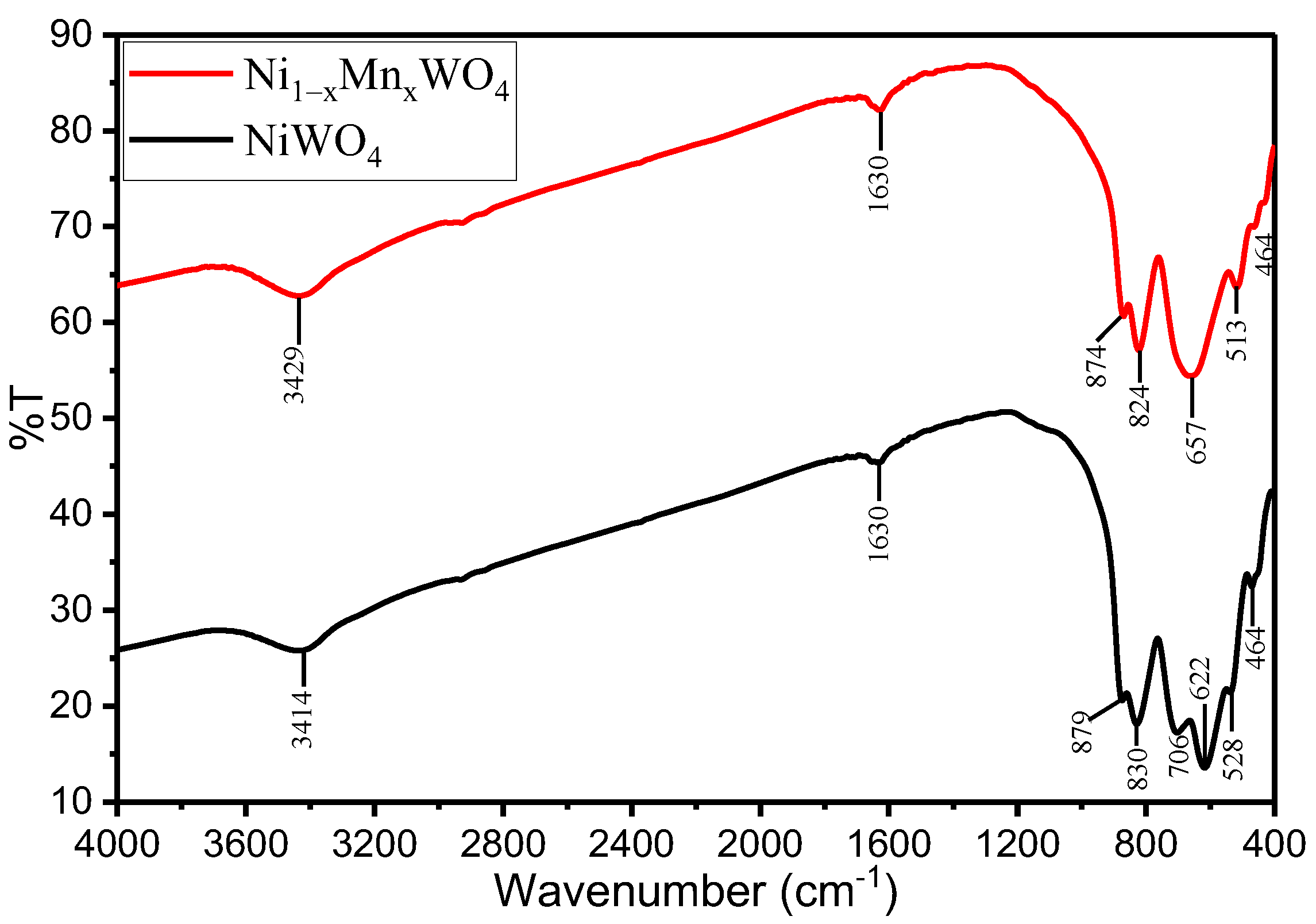
2.1.2. Functional Group Studies
2.1.3. Optical Studies
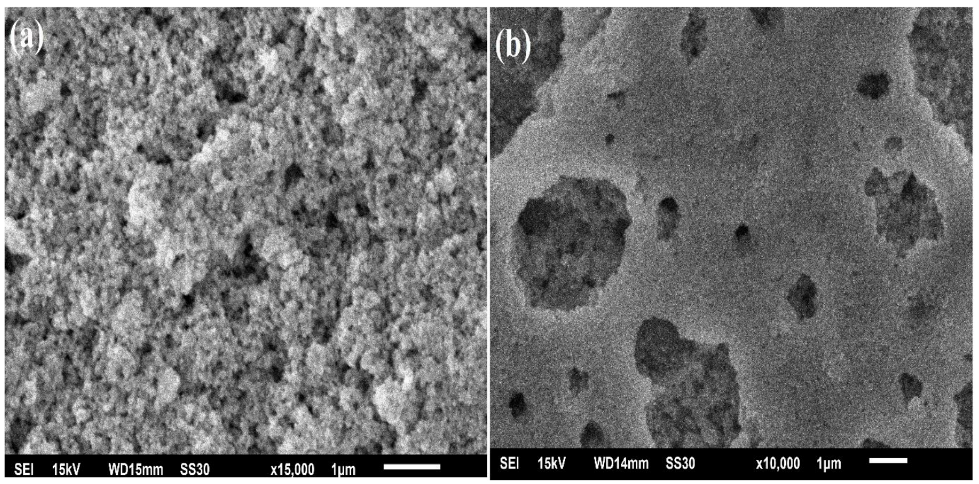
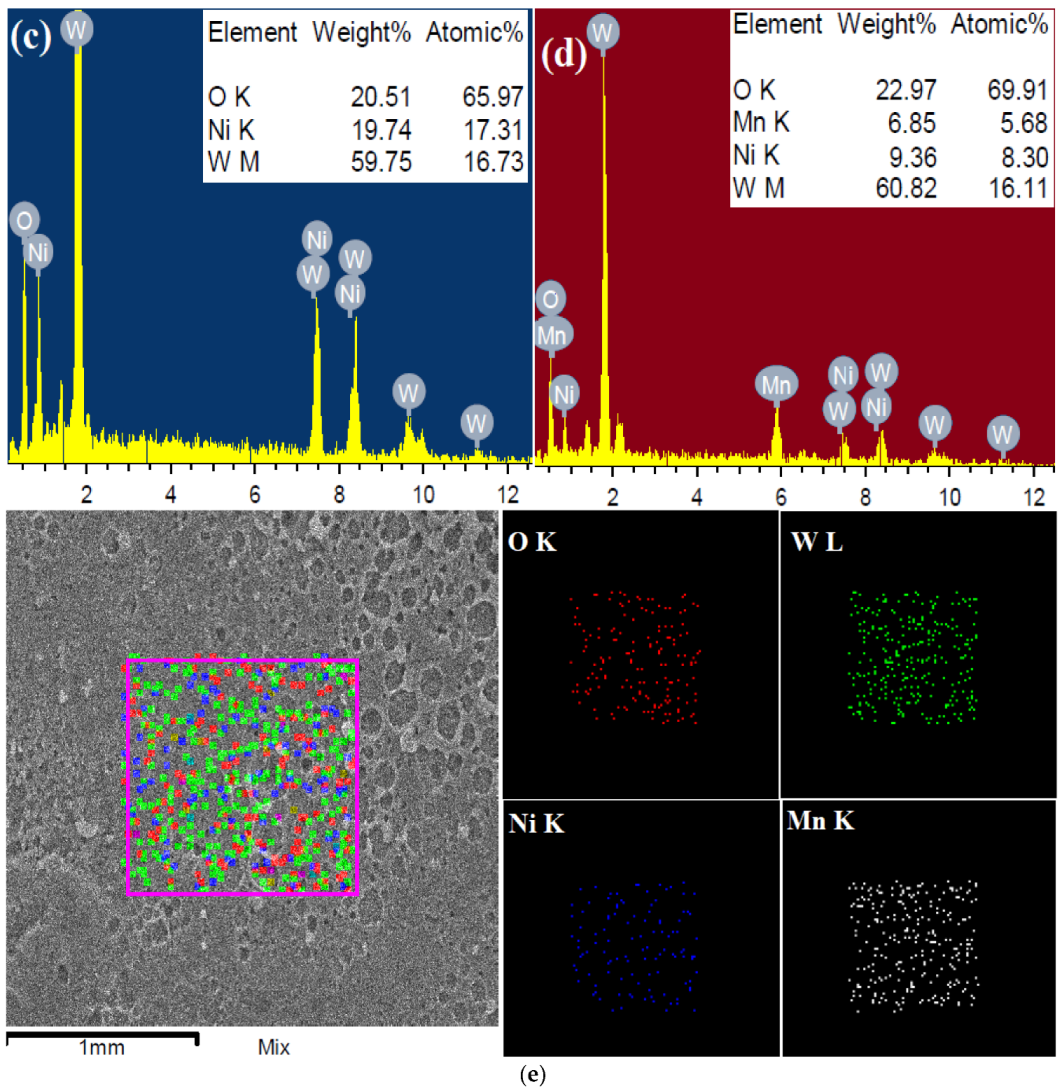
2.1.4. SEM-EDX-Mapping Analysis
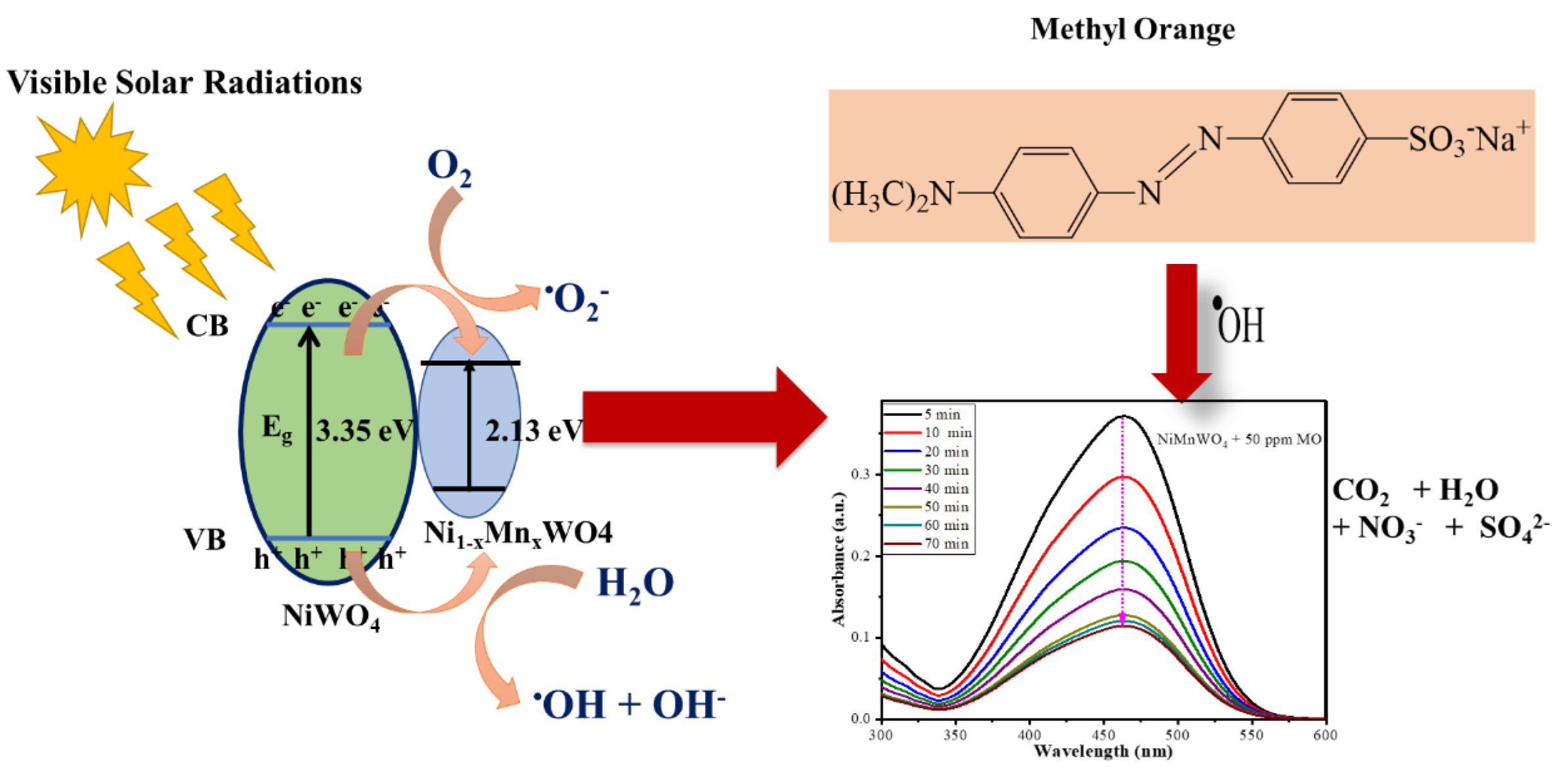
2.2. Photocatalytic Applications
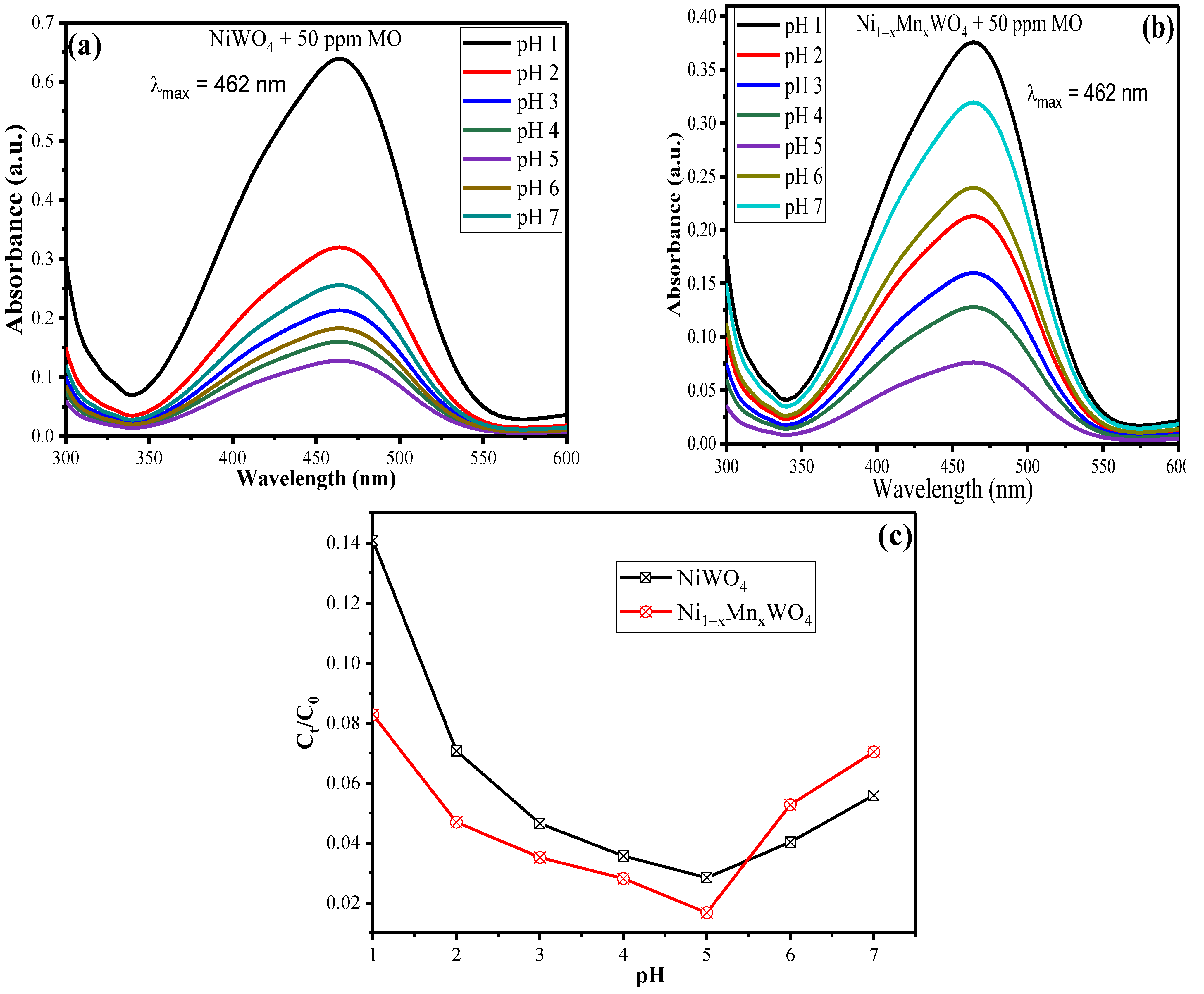
2.2.1. Effect of pH
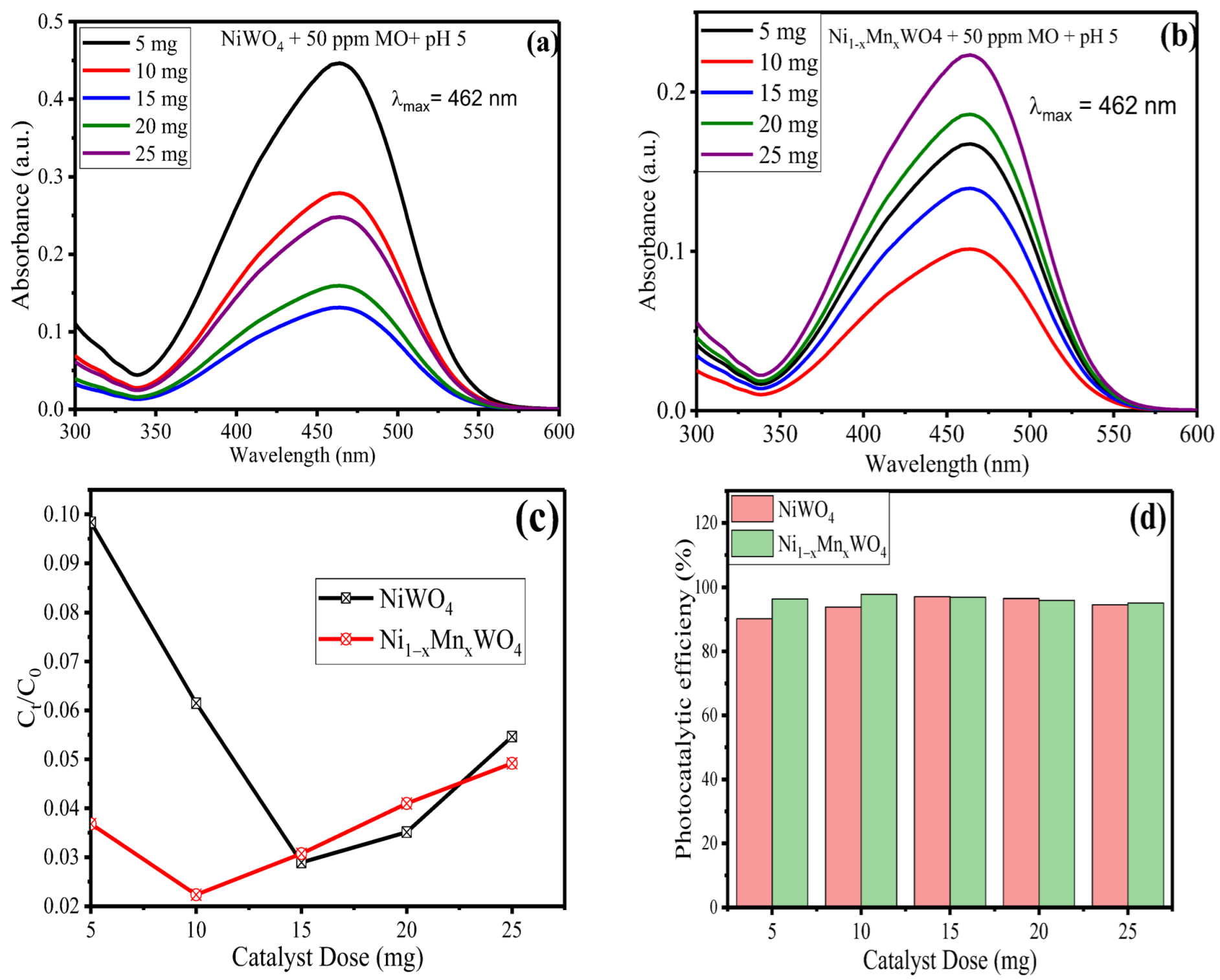
2.2.2. Effect of Catalyst Dose
2.3. Kinetics of Photodegradation and Effect of Irradiation Time
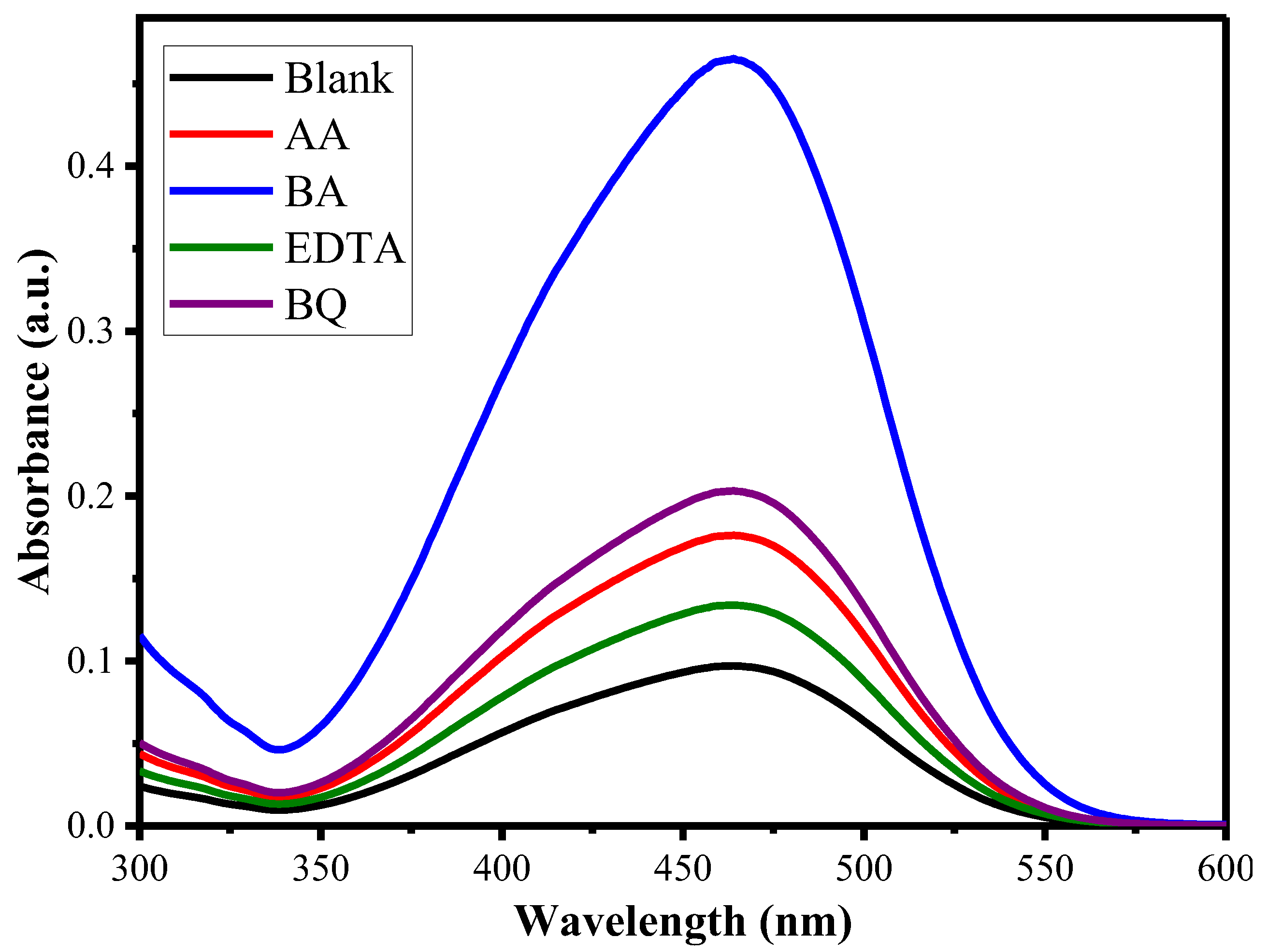
2.4. Detection of ROS
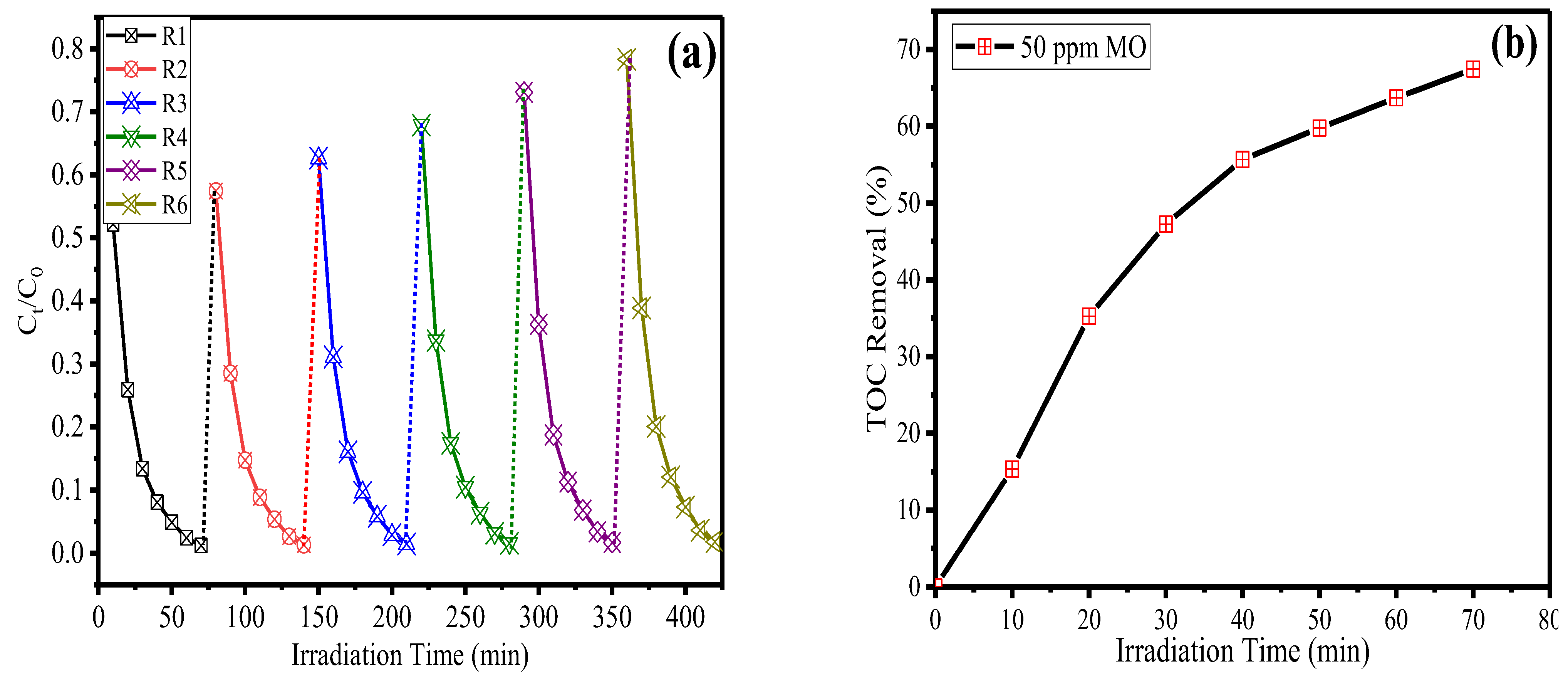
2.5. Reusability and TOC Test
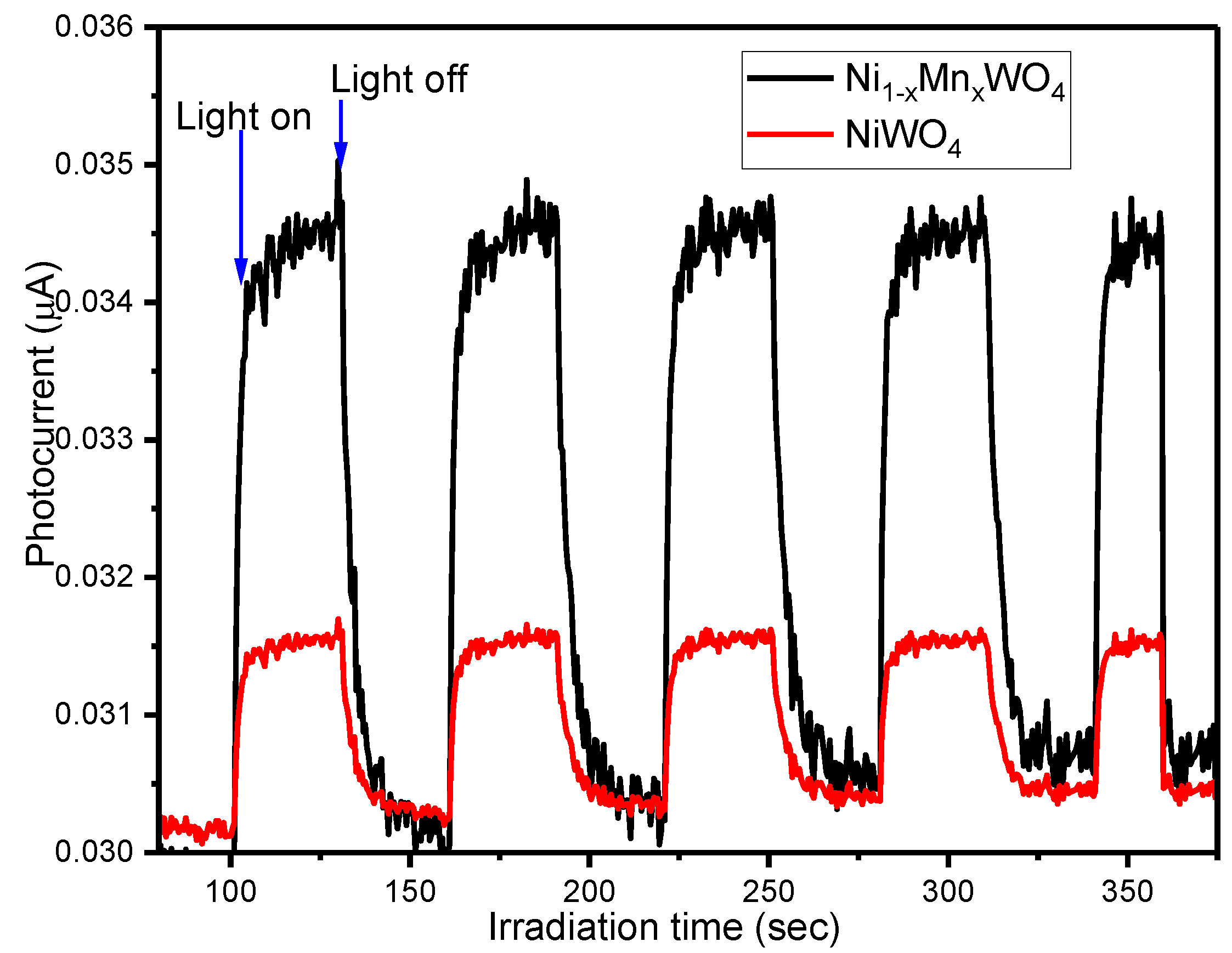
2.6. Photocurrent Measurement
2.7. Comparison with Literature
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Synthesis of NiWO4 and Ni1−xMnxWO4 Nanocomposite
3.3. Characterization Techniques
3.4. Photocatalysis Process
3.5. Reusability and TOC Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swathi, D.; Sabumon, P.C.; Trivedi, A. Simultaneous Decolorization and Mineralization of High Concentrations of Methyl Orange in an Anoxic Up-Flow Packed Bed Reactor in Denitrifying Conditions. J. Water Process Eng. 2021, 40, 101813. [Google Scholar] [CrossRef]
- You, J.; Zhang, L.; He, L.; Zhang, B. Photocatalytic Degradation of Methyl Orange on ZnO–TiO2/SO42− Heterojunction Composites. Opt. Mater. 2022, 131, 112737. [Google Scholar] [CrossRef]
- Bian, Y.; Zheng, G.; Ding, W.; Hu, L.; Sheng, Z. Magnetic Field Effect on the Photocatalytic Degradation of Methyl Orange by Commercial TiO2 Powder. RSC Adv. 2021, 11, 6284–6291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Jung, U.; Jung, B.; Park, J.; Naushad, M. Zinc Hydroxystannate/Zinc-Tin Oxide Heterojunctions for the UVC-Assisted Photocatalytic Degradation of Methyl Orange and Tetracycline. Environ. Pollut. 2023, 316, 120353. [Google Scholar] [CrossRef] [PubMed]
- Rajapandi, S.; Pandeeswaran, M.; Kousalya, G.N. Novel Green Synthesis of N-Doped Carbon Dots from Fruits of Opuntia Ficus Indica as an Effective Catalyst for the Photocatalytic Degradation of Methyl Orange Dye and Antibacterial Studies. Inorg. Chem. Commun. 2022, 146, 110041. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Hao, W.; Sun, G.; Li, Z. Mechanism of UV-Assisted TiO2/Reduced Graphene Oxide Composites with Variable Photodegradation of Methyl Orange. RSC Adv. 2015, 5, 72916–72922. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Wu, J.; Zheng, X.; Liao, S.; Ou, B.; Tian, L. In Situ Embedment of CoOx on G-C3N4 as Z Scheme Heterojunction for Efficient Photocatalytic Degradation of Methyl Orange and Phenol under Visible Light. J. Alloys Compd. 2022, 927, 167047. [Google Scholar] [CrossRef]
- de Oliveira, R.L.; Anderson, M.A.; de Aragão Umbuzeiro, G.; Zocolo, G.J.; Zanoni, M.V.B. Assessment of By-Products of Chlorination and Photoelectrocatalytic Chlorination of an Azo Dye. J. Hazard. Mater. 2012, 205–206, 1–9. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Fathinia, M. Heterogeneous Photocatalytic Ozonation of Ciprofloxacin Using Synthesized Titanium Dioxide Nanoparticles on a Montmorillonite Support: Parametric Studies, Mechanistic Analysis and Intermediates Identification. RSC Adv. 2016, 6, 87569–87583. [Google Scholar] [CrossRef]
- Hasan, I.; Bhatia, D.; Walia, S.; Singh, P. Removal of Malachite Green by Polyacrylamide-g-Chitosan γ-Fe2O3 Nanocomposite-an Application of Central Composite Design. Groundw. Sustain. Dev. 2020, 11, 100378. [Google Scholar] [CrossRef]
- Hasan, I.; Ahamd, R. A Facile Synthesis of Poly (Methyl Methacrylate) Grafted Alginate@Cys-Bentonite Copolymer Hybrid Nanocomposite for Sequestration of Heavy Metals. Groundw. Sustain. Dev. 2019, 8, 82–92. [Google Scholar] [CrossRef]
- Hasan, I.; Alharthi, F.A. Caffeine-Alginate Immobilized CeTiO4 Bionanocomposite for Efficient Photocatalytic Degradation of Methylene Blue. J. Photochem. Photobiol. A Chem. 2022, 433, 114126. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A Review on UV/TiO2 Photocatalytic Oxidation Process. Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar] [CrossRef]
- Muthukumaran, M.; Gnanamoorthy, G.; Varun Prasath, P.; Abinaya, M.; Dhinagaran, G.; Sagadevan, S.; Mohammad, F.; Oh, W.C.; Venkatachalam, K. Enhanced Photocatalytic Activity of Cuprous Oxide Nanoparticles for Malachite Green Degradation under the Visible Light Radiation. Mater. Res. Express. 2020, 7, 015038. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO Photocatalyst for the Efficient and Rapid Photocatalytic Degradation of Azo Dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, R.; Thayumanavan, A.; Sriram, S. Photocatalytic Activity of Zn-Doped Fe 2O 3 Nanoparticles: A Combined Experimental and Theoretical Study. Bull. Mater. Sci. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Mushtaq, F.; Chen, X.; Hoop, M.; Torlakcik, H.; Pellicer, E.; Sort, J.; Gattinoni, C.; Nelson, B.J.; Pané, S. Piezoelectrically Enhanced Photocatalysis with BiFeO3 Nanostructures for Efficient Water Remediation. iScience 2018, 4, 236–246. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, X.; Liu, H.; Qu, J.; Huang, C.P. Synthesis of Visible-Light Sensitive M-BiVO4 (M = Ag, Co, and Ni) for the Photocatalytic Degradation of Organic Pollutants. Sep. Purif. Technol. 2009, 77, 275–282. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable Synthesis of Bi2MoO6 and Effect of Morphology and Variation in Local Structure on Photocatalytic Activities. Appl. Catal. B 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Babu, M.J.; Botsa, S.M.; Rani, S.J.; Venkateswararao, B.; Muralikrishna, R. Enhanced Photocatalytic Degradation of Cationic Dyes under Visible Light Irradiation by CuWO4-RGO Nanocomposite. Adv. Compos. Hybrid Mater. 2020, 3, 205–212. [Google Scholar] [CrossRef]
- Mohamed Jaffer Sadiq, M.; Krishna Bhat, D. Novel NiWO4-ZnO-NRGO Ternary Nanocomposites with Enhanced Photocatalytic Activity. Mater. Today Proc. 2018, 5, 22412–22420. [Google Scholar] [CrossRef]
- Muthuraj, V. Superior Visible Light Driven Photocatalytic Degradation of Fluoroquinolone Drug Norfloxacin over Novel NiWO4 Nanorods Anchored on G-C 3 N 4 Nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 43–54. [Google Scholar] [CrossRef]
- Rosal, F.J.O.; Gouveia, A.F.; Sczancoski, J.C.; Lemos, P.S.; Longo, E.; Zhang, B.; Cavalcante, L.S. Electronic Structure, Growth Mechanism, and Sonophotocatalytic Properties of Sphere-like Self-Assembled NiWO4 Nanocrystals. Inorg. Chem. Commun. 2018, 98, 34–40. [Google Scholar] [CrossRef]
- Hosseini, S.; Farsi, H.; Moghiminia, S.; Zubkov, T.; Lightcap, I.V.; Riley, A.; Peters, D.G.; Li, Z. Nickel Tungstate (NiWO4) Nanoparticles/Graphene Composites: Preparation and Photoelectrochemical Applications. Semicond. Sci. Technol. 2018, 33, 055008. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Rahimi-Nasrabadi, M.; Karimi, M.S.; Mirsadeghi, S. Evaluation of Photocatalytic and Supercapacitor Potential of Nickel Tungstate Nanoparticles Synthesized by Electrochemical Method. New J. Chem. 2018, 42, 19934–19944. [Google Scholar] [CrossRef]
- Liaquat, H.; Imran, M.; Latif, S.; Iqbal, S.; Hussain, N.; Bilal, M. Citric Acid-Capped NiWO4/Bi2S3 and RGO-Doped NiWO4/Bi2S3 Nanoarchitectures for Photocatalytic Decontamination of Emerging Pollutants from the Aqueous Environment. Environ. Res. 2022, 212, 113276. [Google Scholar] [CrossRef]
- Hao, M.; Meng, X.; Miao, Y. Synthesis of NiWO4 Powder Crystals of Polyhedron for Photocatalytic Degradation of Rhodamine. Solid State Sci. 2017, 72, 103–108. [Google Scholar] [CrossRef]
- Li, Z.; Shen, Y.; Yang, C.; Lei, Y.; Guan, Y.; Lin, Y.; Liu, D.; Nan, C.W. Significant Enhancement in the Visible Light Photocatalytic Properties of BiFeO3–Graphene Nanohybrids. J. Mater. Chem. A Mater. 2012, 1, 823–829. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Wang, X.; Xiao, X.; Dai, Z.; Wu, W.; Zheng, J.; Ren, F.; Jiang, C. Preparation of M@BiFeO3 Nanocomposites (M = Ag, Au) Bowl Arrays with Enhanced Visible Light Photocatalytic Activity. J. Am. Ceram. Soc. 2015, 98, 2255–2263. [Google Scholar] [CrossRef]
- Irfan, S.; Li, L.; Saleemi, A.S.; Nan, C.W. Enhanced Photocatalytic Activity of La3+ and Se4+ Co-Doped Bismuth Ferrite Nanostructures. J. Mater. Chem. A Mater. 2017, 5, 11143–11151. [Google Scholar] [CrossRef]
- Ge, J.; Sun, Y.; Chen, W.; Song, F.; Xie, Y.; Zheng, Y.; Rao, P. Z-Scheme Heterojunction Based on NiWO4/WO3 Microspheres with Enhanced Photocatalytic Performance under Visible Light. Dalton Trans. 2021, 50, 13801–13814. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xi, Y.; Huang, Q. A Novel p–n Mn0.2Cd0.8S/NiWO4 Heterojunction for Highly Efficient Photocatalytic H2 Production. Dalton Trans. 2020, 49, 12242–12248. [Google Scholar] [CrossRef] [PubMed]
- Hitha, H.; John, M.; Jose, A.; Kuriakose, S.; Varghese, T. Influence of Bi3+ Doping on Structural, Optical and Photocatalytic Degradation Properties of NiWO4 Nanocrystals. J. Solid State Chem. 2021, 295, 121892. [Google Scholar] [CrossRef]
- Cui, Y.P.; Shang, Y.R.; Shi, R.X.; Che, Q.D.; Wang, J.P. Pt-Decorated NiWO4/WO3 Heterostructure Nanotubes for Highly Selective Sensing of Acetone. Trans. Nonferrous Met. Soc. China 2022, 32, 1981–1993. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Seifzadeh, D. Novel Ternary G-C3N4/Fe3O4/MnWO4 Nanocomposites: Synthesis, Characterization, and Visible-Light Photocatalytic Performance for Environmental Purposes. J. Mater. Sci. Technol. 2018, 34, 1638–1651. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the Size and Internal Structure of Colloidal Particles by Means of Rontgen Rays. Nachr. Von Der Ges. Der Wiss. Zu Göttingen 1918, 26, 98–100. [Google Scholar]
- Dutta, D.P.; Raval, P. Effect of Transition Metal Ion (Cr3+, Mn2+ and Cu2+) Doping on the Photocatalytic Properties of ZnWO4 Nanoparticles. J. Photochem. Photobiol. A Chem. 2018, 357, 193–200. [Google Scholar] [CrossRef]
- Azizi, S.; Asadpour-Zeynali, K. Electrochemical Synthesis of Tungstate Bimetallic Nanoparticles with Application in Electrocatalytic Determination of Paracetamol. ChemistrySelect 2022, 7, e202104548. [Google Scholar] [CrossRef]
- Leeladevi, K.; Arunpandian, M.; Vinoth Kumar, J.; Chellapandi, T.; Mathumitha, G.; Lee, J.W.; Nagarajan, E.R. CoWO4 Decorated ZnO Nanocomposite: Efficient Visible-Light-Activated Photocatalyst for Mitigation of Noxious Pollutants. Phys. B Condens. Matter 2022, 626, 413493. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Solids; Ables, A., Ed.; American Elsevier: Amsterdam, The Netherlands, 1970. [Google Scholar]
- Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Grumezescu, A.M.; Purcar, V.; Radițoiu, V.; Fierascu, R.C. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. [Google Scholar] [CrossRef] [PubMed]
- Harish, S.; Navaneethan, M.; Archana, J.; Silambarasan, A.; Ponnusamy, S.; Muthamizhchelvan, C.; Hayakawa, Y. Controlled Synthesis of Organic Ligand Passivated ZnO Nanostructures and Their Photocatalytic Activity under Visible Light Irradiation. Dalton Trans. 2015, 44, 10490–10498. [Google Scholar] [CrossRef] [PubMed]
- Kalikeri, S.; Kamath, N.; Gadgil, D.J.; Shetty Kodialbail, V. Visible Light-Induced Photocatalytic Degradation of Reactive Blue-19 over Highly Efficient Polyaniline-TiO2 Nanocomposite: A Comparative Study with Solar and UV Photocatalysis. Environ. Sci. Pollut. Res. 2017, 25, 3731–3744. [Google Scholar] [CrossRef]
- Elgorban, A.M.; al Kheraif, A.A.; Syed, A. Construction of Ag2WO4 Decorated CoWO4 Nano-Heterojunction with Recombination Delay for Enhanced Visible Light Photocatalytic Performance and Its Antibacterial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127416. [Google Scholar] [CrossRef]
- Anis, S.F.; Lalia, B.S.; Palmisano, G.; Hashaikeh, R. Photoelectrochemical Activity of Electrospun WO3/NiWO4 Nanofibers under Visible Light Irradiation. J. Mater. Sci. 2018, 53, 2208–2220. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Z. Accelerated Charge Transfer via a Nickel Tungstate Modulated Cadmium Sulfide p–n Heterojunction for Photocatalytic Hydrogen Evolution. Catal. Sci. Technol. 2019, 9, 1944–1960. [Google Scholar] [CrossRef]
- Tri, N.L.M.; Duc, D.S.; van Thuan, D.; Al Tahtamouni, T.; Pham, T.D.; Tran, D.T.; Le Chi, N.T.P.; Nguyen, V.N. Superior Photocatalytic Activity of Cu Doped NiWO4 for Efficient Degradation of Benzene in Air Even under Visible Radiation. Chem. Phys. 2019, 525, 110411. [Google Scholar] [CrossRef]
- Thilagavathi, T.; Venugopal, D.; Thangaraju, D.; Marnadu, R.; Palanivel, B.; Imran, M.; Shkir, M.; Ubaidullah, M.; AlFaify, S. A Facile Co-Precipitation Synthesis of Novel WO3/NiWO4 Nanocomposite with Improved Photocatalytic Activity. Mater. Sci. Semicond. Process. 2021, 133, 105970. [Google Scholar] [CrossRef]
- Botsa, S.M.; Jagadeesh Babu, M.; Suresh, P.; Kalyani, P.; Venkateswararao, B.; Muralikrishna, R. Spherical NiWO4-Reduced Graphene Oxide Nanocomposite for Effective Visible Light Driven Photocatalytic Activity for the Decolourisation of Organic Pollutants. Arab. J. Chem. 2020, 13, 8489–8497. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Shekofteh-Gohari, M. Novel Magnetic Fe3O4/ZnO/NiWO4 Nanocomposites: Enhanced Visible-Light Photocatalytic Performance through p-n Heterojunctions. Sep. Purif. Technol. 2017, 184, 334–346. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, Y.; Shad, N.A.; Sajid, M.M.; Irfan, M.; Munawar, A.; Hussain, T.; Imran, M.; Hussain, D. Facile Hydrothermal Synthesis of Nickel Tungstate (NiWO4) Nanostructures with Pronounced Supercapacitor and Electrochemical Sensing Activities. J. Alloys Compd. 2021, 878, 160314. [Google Scholar] [CrossRef]


| Catalyst | kapp (min−1) | Error | t1/2 (min) | R2 |
|---|---|---|---|---|
| NiWO4 | 0.063 | 6.16 × 10−4 | 11.00 | 0.99 |
| Ni1−xMnxWO4 NC | 0.067 | 1.31 × 10−3 | 10.34 | 0.99 |
| Catalysts | Irradiation Time (min) | Light Source | Organic Pollutant | % Degradation | References |
|---|---|---|---|---|---|
| Cu-NiWO4 | 180 | Visible light | Benzene | 96.50 | [47] |
| Bi-doped NiWO4 | 90 | UV Irradiation | Rhodamine | 86.71 | [33] |
| rGO-NiWO4/Bi2S3 | 40 | Visible light | Methyl Orange | 72.00 | [26] |
| WO3/NiWO4 | 80 | UV Irradiation | Methylene blue | 90.63 | [48] |
| NiWO4-RGO | 240 | Visible light | o-Nitrophenol | 82.00 | [49] |
| Fe3O4/ZnO/NiWO4 | 300 | Visible light | Rhodmaine B | 97.90 | [50] |
| Ni1−xMnxWO4 | 70 | Visible light | Methyl Orange | 99.06% | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, I.; Albaeejan, M.A.; Alshayiqi, A.A.; Al-Nafaei, W.S.; Alharthi, F.A. In Situ Hydrothermal Synthesis of Ni1−xMnxWO4 Nanoheterostructure for Enhanced Photodegradation of Methyl Orange. Molecules 2023, 28, 1140. https://doi.org/10.3390/molecules28031140
Hasan I, Albaeejan MA, Alshayiqi AA, Al-Nafaei WS, Alharthi FA. In Situ Hydrothermal Synthesis of Ni1−xMnxWO4 Nanoheterostructure for Enhanced Photodegradation of Methyl Orange. Molecules. 2023; 28(3):1140. https://doi.org/10.3390/molecules28031140
Chicago/Turabian StyleHasan, Imran, Mohammed Abdullah Albaeejan, Alanoud Abdullah Alshayiqi, Wedyan Saud Al-Nafaei, and Fahad A. Alharthi. 2023. "In Situ Hydrothermal Synthesis of Ni1−xMnxWO4 Nanoheterostructure for Enhanced Photodegradation of Methyl Orange" Molecules 28, no. 3: 1140. https://doi.org/10.3390/molecules28031140
APA StyleHasan, I., Albaeejan, M. A., Alshayiqi, A. A., Al-Nafaei, W. S., & Alharthi, F. A. (2023). In Situ Hydrothermal Synthesis of Ni1−xMnxWO4 Nanoheterostructure for Enhanced Photodegradation of Methyl Orange. Molecules, 28(3), 1140. https://doi.org/10.3390/molecules28031140







