Abstract
Shigellosis is one of the major causes of death in children worldwide. Flavonoids and phenolic acids are expected to demonstrate anti-shigellosis activity and anti-diarrheal properties. The aerial part of A. integrifolia is commonly used against diarrhea. This study aimed to identify flavonoids and phenolic acids responsible for this therapeutic purpose. Antioxidant activity, total phenol content, and total flavonoid content were determined. The antibacterial activity of the aerial part against Shigella spp. was also tested using the agar well diffusion method. HPLC analysis was performed using UHPLC-DAD for different extracts of the aerial part. Autodock Vina in the PyRx platform was used to screen responsible components. Ciprofloxacin was used as a reference drug. An enzyme taking part in pyrimidine biosynthesis was used as a target protein. Molecular docking results were visualized using Discovery Studio and LigPlot1.4.5 software. Antioxidant activity, total phenol content, and total flavonoid content are more significant for the aerial part of A. integrifolia. From HPLC analysis, the presence of the flavonoids, quercetin, myricetin, and rutin and the phenolic acids gallic acid, chlorogenic acid, and syringic acid were identified from the aerial part of A. integrifolia. Regarding the antibacterial activity, the aerial part shows considerable activity against Shigella spp. Binding energies, RMSD and Ki values, interaction type, and distance are considered to identify the components most likely responsible for the therapeutic effects and observed activity. Antioxidant activity, total phenol content, and total flavonoid content of the aerial part are in line with anti-shigellosis activity. The top five components that are most likely potentially responsible for therapeutic purposes and anti-shigellosis activity are chlorogenic acid, rutin, dihydroquercetin, dihydromyricetin, and kaempferol.
1. Introduction
Ajuga integrifolia Buch.-Ham. Ex D. Don (Syn: A. remota; A. bracteosa) belonging to the family Lamiaceae is known by the names Armagussa, Etse Libawit, Medhanit, and Anamuro [1]. It is an evergreen, clump-forming flowering species of the genus Ajuga. The more than 300 species of the genus Ajuga have many variations [2]. Many biological activities were reported for the Ajuga spp., specifically for A. integrifolia and its synonyms. These include antioxidant and anti-inflammatory [3], antidiabetic [4], antibacterial, diuretic, stimulant, astringent, rheumatism, febrifuge, blood purification [5], and anticancer effects [6]. These activities are achieved in addition to the biological functions of phytochemicals in protecting plant species against pathogens and herbivores and acting as stress-protecting agents [7].
Diarrhea is a major cause of morbidity and mortality for children in developing countries, including Ethiopia [8,9]. It is among the ten major diseases identified and reported to kill human beings [10]. Shigellosis is a leading cause of dysentery and accounts for 5–10% of diarrheal diseases globally. Shigellosis is characterized by inflammation and ulcer formation on the large intestine, with signs such as fever and stomach pain [11]. It is caused by enteroinvasive E. coli (EIEC) and other species of Shigella, namely S. dysenteriae, S. flexneri, S. boydii, and S. sonnei [12]. Shigella spp. are easily differentiated from E. coli, but difficult to differentiate among the species due to similar biochemical traits and modes of invasion [13]. Shigella spp. invade the gut-lining epithelium and cause shigellosis [14]. The prevalence of Shigella spp. in Ethiopia was reported to be 6.6% and resistant to ampicillin (83.1%), amoxicillin (84.1%), erythromycin (86.5%), and multi-drugs (83.2%). Ciprofloxacin (8.9%) and norfloxacin (8.2%) were reported to have low resistance patterns [15].
Shigella spp. infections with increased levels of antimicrobial resistance in children are major public health problems in developing countries [16]. Poor sanitation practices and limited access to clean water are the main reasons for the spread of the disease through the fecal-oral route [17]. Shigella spp. is developing resistance to oral antibiotics [18,19,20,21,22]. Shigella enters the human body with contaminated food and water as part of its life cycle. It develops an acid-resistant system (glutamate decarboxylase system) which grants the ability to withstand the stomach’s acidic environment [17,23]. Passing through the stomach, the pathogen reaches the intestine and starts invasion. Type III Secretion System (T3SS) mediates Shigella invasion and leads to infection [17].
The proliferation of many pathogens, including Shigella spp., uses the pyrimidine biosynthesis pathway. N-carbamoyl-l-aspartate reversible interconversion to 4,5-dihydroorotate is catalyzed by dihydroorotase (DHO). This makes it a therapeutic target for inhibiting bacterial growth [24]. It is a crucial candidate for evaluating the response to antimicrobial resistance because there is considerable variation between bacterial and mammalian DHOs. Ethnobotanical studies indicate that the aerial part of A. integrifolia and its synonyms are used for hypertension [25,26,27,28], antimalarial and insecticidal activities [29,30,31], diuretic activity [32], tonsillitis [33], epilepsy [34], breast cancer [35], wound healing [36], abdominal pain and anthelmintic [37,38,39], and diarrhea [25,40,41,42,43,44]. Individual components have not been identified so far for anti-diarrhea; activity. Flavonoids and polyphenolics are known to have such activity [45,46].
Flavonoids have been discovered in vitro to be potent antibacterial agents against a variety of pathogens, which is not surprising since flavonoids are known to be produced by plants in response to microbial infection. There have been reports of the antibacterial properties of flavonoid-rich plant extracts from various species [47]. Apigenin, galangin, flavone and flavonol glycosides, isoflavones, flavanones, and chalcones are only a few flavonoids that have been proven to have strong antibacterial activity [48]. Rather than focusing on a single site of action, antibacterial flavonoids may have many cellular targets. Their ability to form complexes with proteins by non-specific forces including hydrogen bonds and hydrophobic effects, as well as by forming covalent bonds, is one of their molecular actions. Their capacity to inactivate microbial adhesins, enzymes, cell membrane transport proteins, etc., may therefore be related to their antibacterial activities. Microbial membranes may also be damaged by lipophilic flavonoids [49].
Despite the diversity of natural inhibitors, it is interesting to notice that many of them are phenolic in origin. Many researchers have thought of appropriate skeletons based on the architectures of natural compounds and developed fresh synthetic inhibitors. The previous analysis of tabulated compounds and the inhibitory outcomes point to the flavonol moiety as a potential scaffold, a key structural component, and a starting point for the continued development of novel inhibitors among all the natural and synthesized flavonoid derivatives [50]. Looking for potential inhibitors, in silico molecular docking studies are now becoming part of many pharmacological studies. This strategy produced a conceptual idea for a potential pharmacophore, followed by a targeted chemical synthesis. The application of a ligand-based method and focused chemical synthesis produced novel small synthetic molecules (MW < 500) [51]. After the virtual screening, the short-listed compounds were screened by the SwissADME modeling tool to discard any molecules with undesirable pharmacokinetics and therapeutic qualities. To identify the top binders of the target protein, iterative docking was also used for the drug-like compounds. For the evaluation of the dynamic behavior, stability of the protein–ligand complex, and binding affinity, binding free energy calculations were made, which led to the identification of possible inhibitors [52].
Previous studies indicated that Shigella infections were more common in Ethiopia and that they tended to be more resistant to conventional medications such as ampicillin, amoxicillin, and erythromycin [15,21]. A considerable prevalence combined with higher levels of resistance to the currently used drugs brings a demand for a natural-based antibiotic. WHO identified Shigella as a marked pathogen against which new drugs need to be formulated and proposed in silico approach to identify drug targets [17]. Considering these, we aimed to justify the anti-shigellosis activity of the aerial part of A. integrifolia and identify responsible components for this activity using in silico molecular docking.
2. Results
2.1. Antioxidant Activity (DPPH Assay)
The DPPH antioxidant activities of the methanol extracts of the aerial of A. integrifolia (AIA) and root part of A. integrifolia (AIR) were analyzed using eight concentration points. The antioxidant activity was confirmed first by its color change, which indicated the scavenging activities of the parts of the target medicinal plant. The IC50 values of the extracts of AIA and AIR were calculated after being transformed and normalized to the minima (zero) to maxima (plateaus) from log [inhibitor conc.], which are variable responses. The IC50 value for the aerial part is significantly lower than the root sample with similar test conditions (Table 1). The antioxidant activity of the aerial part is also much closer to the reference ascorbic acid. The difference in the % of radical scavenging activity of the aerial versus the root sample is observed at lower concentrations (Figure 1). The regression constants (R2) for the sample extracts and the reference are similar and close to 1.

Table 1.
Result summary of IC50 and R2 values.
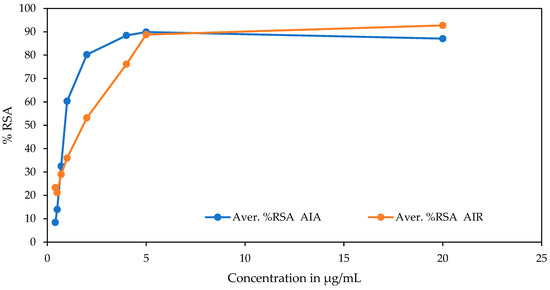
Figure 1.
Graph of % free radical scavenging activity of DPPH by the extracts vs. concentration of sample extracts.
2.2. Total Phenolic and Total Flavonoid Content
Total phenolic content (TPC) and total flavonoid content (TFC) of the extracts of AIA and AIR samples were determined using Folin–Ciocalteu and aluminum chloride methods, respectively. The formation of yellow and blue colors was observed after the addition of the respective reagents for TPC and TFC determinations, respectively. The equation of the standard curve for the determination of TPC was y = 0.0023x − 0.0693, where R2 = 0.9981, and for TFC, y = 0.002x + 0.0399, where R2 = 0.9971. Both the TPC and TFC of AIA are almost double that of AIR (Table 2).

Table 2.
Total phenol content and total flavonoid content for the sample extracts.
2.3. HPLC Analysis
HPLC analysis was carried out using the method mentioned above, and chromatograms were exported for qualitative identification of the presence of phenolic acids and flavonoids. The components from the sample extracts were identified using the reference standards’ peak shape, retention time, and UV-Vis spectra. As shown in Figure 2, the reference peaks were identical and overlaid, leading to the confirmation of the presence of the components. After the identification of the components in the sample extracts, the components were quantified using external calibration standards. From the HPLC chromatogram (Figure 2), gallic acid (RT: 4.450 min), chlorogenic acid (RT: 4.160 min), and syringic acid (RT: 5.897 min) were found in 75% methanol extract; myricetin (RT: 8.117 min) and quercetin (RT: 11.673 min) were found in the methanol extract; and quercetin (RT: 12.600 min) and rutin (RT: 19.797 min) were found in the acetone dip instant extract of aerial part of A. integrifolia. As shown in Table 3, the concentration of the phenolic acids and flavonoids ranges from 1.56 to 11.26 mg/100 g for AIR, from 7.97 to 58.23 mg/100 g for methanol extract of AIA (AIA MeOH), from 110 to 220 mg/100 g for acetone extract of AIA (AIA acet), and from 2.07 to 60.13 mg/100 g for 75% methanol extract of AIA. The concentrations are higher for acetone extract as the extraction method is mainly for flavonoids. AIR showed lower concentrations.
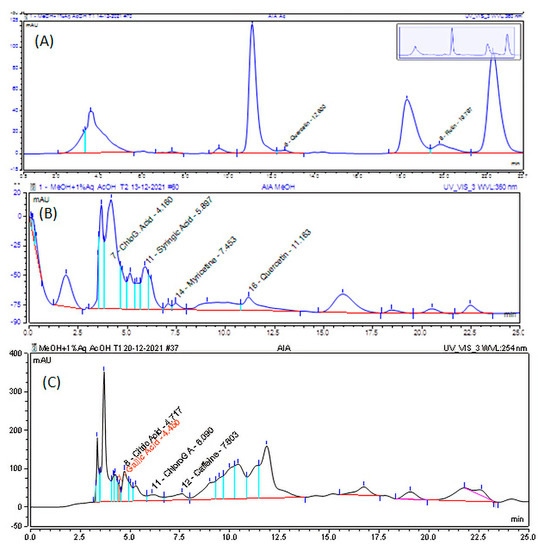
Figure 2.
HPLC chromatograms for (A) acetone, (B) methanol, and (C) 75% methanol extract of the aerial part of A. integrifolia.

Table 3.
HPLC quantitative result for A. integrifolia extracts.
2.4. Antibacterial Study
The antimicrobial susceptibility test was managed with four strains, including Shigella spp. The strains are two Gram-negative and one Gram-positive, chosen based on availability. Methanol extract of AIA showed smaller activity for E. coli and a greater zone of inhibition (17.67 ± 1.47) for Shigella spp. (Table 4). The ciprofloxacin reference is used following the moderate resistance of Shigella spp. for chloramphenicol. AIR does not show any activity for all strains considered.

Table 4.
Measurement of the zone of inhibition (mm) of test bacteria.
2.5. In silico Molecular Docking Study
Molecular and physicochemical characteristics, including molecular weight (MW), molecular refractivity (MR), count of particular atom kinds, and topological polar surface area (TPSA), can be obtained via the SwissADME online system. Lipophilicity is often described by the partition coefficient between n-octanol and water. A soluble molecule facilitates drug development activities, as handling and formulation problems can be managed easily in oral administration, influencing absorption. Lipinski’s Rule of Five and the Bioavailability Radar enable one to decide the drug-likeness of a molecule. Drug-likeness properties describe a molecule’s potential to be an oral drug, or its bioavailability. The resolution of VcDHO (PDB ID: 5vgm) was to be 1.95 Å for the full validation report for the target protein. The overall quality factor retrieved from the PROHECK online server and full validation report was 97.4026%. From the Ramchandran plot, residues in the most favored regions were found to be 90.5% (Supplementary Material S1, Figure S1). No residues were in the disallowed regions. The Q-mean value after preparation was also 0.17 (z-score) (Supplementary Material S1, Figure S2). The resolution, overall quality, and Q-mean values are acceptable for the intended activity.
As shown in Table 5, Lipinski rule violations are tolerable for some molecules, and there were no violations for more than half of the ligands. Only rutin is beyond the tolerable limit of violation. The bioavailability score is above 0.5 for most and 0.1–0.2 for three molecules, including rutin. The synthetic accessibility is also >2.5 for most, including 6.52 for rutin. Medicinal chemistry friendliness is also reflected by lower pain alert and the absence of problematic fragments from Brenk filters [53].

Table 5.
Physicochemical and pharmacokinetic properties of ligands retrieved from SwissADME (Supplementary Material S2).
The Bioavailability Radar shown in Figure 3 is used to predict a molecule’s drug-likeness properties. The pink area on the radar represents the acceptable range for each property. The respective values are given as lipophilicity: −0.7 < XLOGP3 < +5.0, size: 150 < MW < 500 g/mol, polarity: 20 < TPSA < 130 Å2, solubility: log S < 6, saturation: fraction of sp3 hybridized carbons > 0.25, flexibility: number of rotatable bonds < 9. From the Bioavailability Radar (Figure 3), flexibility, lipophilicity, water insolubility, and size are in the acceptable range for most ligands. There exist some anomalies for saturation and polarity in some cases.
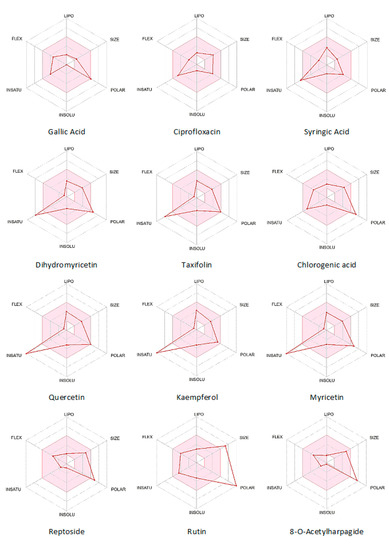
Figure 3.
Bioavailability radar for the ligand molecules from SwissADME.
The binding energies and interaction study shows a better binding affinity for most as observed to have lower binding energies than the reference drug (Table 6). In addition to the binding energy, the RMSD and Ki of individual ligands are also convincing to consider them for the good inhibitory activity of the target protein. Active site residues identified from the pdbsum online server (http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl; accessed 2 December 2022) considering the native ligands and the reference for the PDB data of the target protein [24] are His13, His15, His135, His173, and Asp246.

Table 6.
Result summary of molecular docking analysis of ligands with VcDHO protein (PDB ID: 5vgm).
The interaction diagrams from Discovery Studio and LigPlot software are similar. The interaction distance considered is less than or equal to 3 nm. Different interactions, including H-bonding, hydrophobic interactions or Van der Waals, Pi-alkyl, Pi-donor H-bond, and Pi-Pi stacked interactions shown in the interaction diagrams from Discovery Studio software in Figure 4.
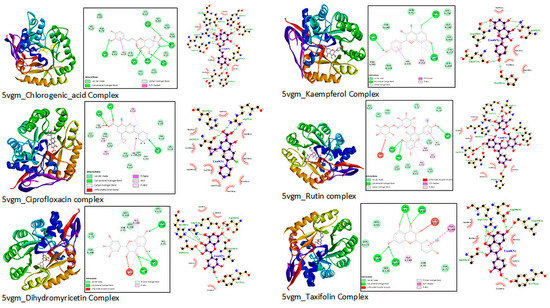
Figure 4.
The 3D and 2D views of interactions from Discovery Studio and LigPlot+1.4.5.
3. Discussion
The antioxidant activities of AIA were observed to be nearly double those of AIR extracts. The IC50 value for AIA was found to be lower than the value reported by Lere Keshebo et al. [54]. The computed smaller IC50 values of the plant parts considered in our study reveal that this medicinal plant is effective in treating many illnesses [55] due to its high antioxidant activities. This is also shown in various ethnomedicinal studies [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] and dictates the presence of polyphenols [45,46] in the extracts more significantly in the aerial part, as demonstrated by Banothu et al. [56]. Similarly, measured TPC and TFC values for AIA were twice those of AIR. This indicates that there is a significant correlation between antioxidant activity and TPC/TFC values [55], and their health benefits are strongly associated. In this study, both TPC and TFC are estimated to be higher, as described in prior investigations [57]. Following higher values of TPC/TFC and antioxidant activity, the antimicrobial activity of AIA against Shigella spp. was found to be significant and comparable with other herbs [54] and with ciprofloxacin (Table 4).
The antioxidant activity study result (Table 1) and the total phenol and total flavonoid contents determined (Table 2) indicate the presence of flavonoids and phenolic acids in the aerial part rather than in the root sample. This is in support of the absence of antibacterial activity in the root sample. Phenolics and flavonoids are among the most common components of medicinal plants responsible for antioxidant activities and thus their therapeutic benefits [58]. As per the availability of standards, we checked the presence of flavonoids such as quercetin, myricetin, and rutin and phenolic acids such as chlorogenic acid, syringic acid, and gallic acid. The higher concentration of phenolics in the acetone extract confirmed the presence of flavonoids, as the method [59] is developed for the extraction of flavonoids. HPLC analysis showed that there are also phenolic acid components in the acetone extract in comparable amounts. The identified components from HPLC analysis (Table 3) and others from the literature [60] were considered in the molecular docking study towards screening responsible components for the anti-shigellosis activity observed in the antibacterial study (Table 4) and the therapeutic purpose indicated. The absence of antimicrobial activity for the root part of the plant agrees with the lower concentrations of the phenolics, as shown in Table 3.
Most ligands, except rutin, are known to be Lipinski-compliant and can be considered drug-like molecules. The oral bioavailability test using the Lipinski rule complements the information from the bioavailability radar. Bioavailability and ADMET testing suggest the acceptability of these ligands as a drug [61]. Molecular docking of 5vgm with identified flavonoids and phenolic acids shows that most ligands have better binding affinity than the reference drug (Ciprofloxacin). Binding energy ranges from −8.1 kcal/mole to −5.4 kcal/mol, which is −7.0 kcal/mol for the reference drug. RMSD for the molecular docking for each ligand ranges from 0.1 to 3.4 Å (Table 5). Lower Ki values indicate lower inhibition concentrations for the intended activity and are thus significant. In addition, the lower RMSD values imply consistent docking into the referred active site bounded in the Vina search space indicated above.
This indicates that the environment and parameters set for the molecular docking were reasonably reliable [62]. More robust and relatively stable interactions were observed for most ligands considered. In general, H-bonding interactions dominate over other interactions, including hydrophobic interactions. The role of H-bonding for the overall binding energy is significantly higher [63]. The dihydro-flavonoids, rutin, and chlorogenic acid take the lead in terms of binding affinity. The number of H-bonding interactions is also considerable. Kaempferol and reptoside are comparable to the reference drug in binding energies and RMSD. Myricetin and quercetin also have a suitable binding affinity, slightly lower than the reference drug. Most ligands interact with two or three of the active site residues. Binding interactions with the active site residues also confirm the interaction to be on the right binding site. Common residues such as His250, Arg17, Tyr100, Pro101, and Leu218 appear in most interactions (Table 6 and Figure 4).
Reptoside and 8-O-acetylharpagide were considered in the study only for comparison. The antibacterial study shows that the root does not show any significant activity for Shigella spp. However, regarding iridoid glycoside composition, both the root and aerial samples were compared when studied using TLC. Iridoid glycosides may not be responsible for the anti-shigellosis activity of the aerial part of A. integrifolia. From HPLC analysis, the root sample was found to have an insignificant concentration of flavonoids and phenolic acids. The top five responsible components for anti-shigellosis activity via inhibiting VcDHO are chlorogenic acid, rutin, taxifolin (dihydroquercetin), dihydromyricetin, and kaempferol. The 3-OH and 4-carbonyl functionalities are known to enhance antimicrobial activity. The number of hydroxyl groups is associated with hydrophobicity. Additive hydroxyl groups may lower hydrophobicity, but C3 charges may be increased, which is a clear sign of pharmacological activity [64,65]. The OH groups on the flavonoids are essential for bioactivity, and the change in the position or number of such groups affects biological potency. The plant-derived flavonoid quercetin is a broad-spectrum protein inhibitor [66]. The role of myricetin and quercetin is also not to be undermined following their lower binding energies and lower RMSD and Ki values. The synergic effect will also be considered as there is more than one binding active site for the target proteins and the varied composition of natural products. This demands further study and the activity study of the individual flavonoids and phenolic acids. The observed activity justifies the ethnomedical use of Ajuga spp. in traditional medicine. The minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) could also be determined to complement the Ki values from the molecular docking study.
4. Materials and Methods
4.1. Chemicals and Reagents
All chemicals and reagents needed for the extraction, total phenolic content, and total flavonoid content determination and antibacterial activity were AR-grade. Standard references (phenolic acid standards: syringic acid, chlorogenic acid, and gallic acid; flavonoid standards: myricetin, quercetin, rutin, and kaempferol), DPPH, and ascorbic acid for the antioxidant test were all from Sigma (>99.9%, Sigma, Shanghai, China), While for HPLC analysis, all HPLC-grade reagents were used.
4.2. Plant Material Collection
The aerial part of A. integrifolia was collected from the Addis Ababa Science and Technology University campus and the nearby area of Koye Feche (VRP5 + 3W6, 8.8852 °N 38.8098 °E, elevation: 2840). The plant was identified and an herbarium sample was deposited (voucher number: FB-004/11) in the national herbarium at the College of Science, Addis Ababa University, Ethiopia.
4.3. Extraction
Methanol extract of the aerial parts was obtained via Soxhlet extraction using a method by Imoru et al. [67] with slight modifications. Using an ultrasonic probe (SJIA-950W, Sjiolab, Ningbo, China) 75% methanol (aqueous) extraction was performed. The method optimization of UAE followed a method reported by Zakaria et al. [68] with minor modifications. Acetone extract from dip instant extraction, specifically attempted for the extraction of flavonoids, was carried out using the method employed by Mawela [69].
4.4. Antioxidant Test—DPPH Assay Calorimetric Method
Modified protocol for the free radical scavenging effect using DPPH assay from Banothu et al. [56] was used, and Soxhlet methanol extracts were tested for antioxidant activity using DPPH as a reagent and ascorbic acid as a standard. We prepared 1000 ppm DPPH and 1000 ppm (mg/L) standard. Ascorbic acid was prepared by dissolving 0.10 g in 100 mL of methanol, and concentrations of 25, 50, 100, 150, 200, 250, 300, 350, 400, 450, and 500 mg/L were prepared for calibration. Absorbance was measured using a Jasco V-770 spectrophotometer (Jasco, Easton, MD, USA), using 1 mm path length in a rectangular cell holder. Sample extracts (0.1 g/mL) of the aerial part and root samples were diluted with CH3OH using 5, 10, 25, 50, 100, 150, and 200 dilution factors. Absorbance was measured and recorded in triplicate after 30 min incubation time in a dark place at 517 nm. The proportion of sample to DPPH was 1:3, i.e., 750 mL of DPPH added to 250 mL of sample, which was modified for some cases. DPPH scavenging capacity was computed by using the following formula:
IC50 values were computed from the relation log [sample] vs. absorbance (normalized) using Graph pad prism 8 software, as suggested for better EC50 estimation [70].
4.5. Total Phenolic Content (TPC) Determination: FC Method
The total phenolic content of aerial and root samples of A. integrifolia was determined by a colorimetric method Folin–Ciocalteu (FC) assay, as described by McDonald et al. [71] with some modifications. First, 0.4 mL of sample extract and 0.4 mL of FC reagent (10× diluted) were mixed, and then 0.2 mL of 2% Na2CO3 was added after 5 min. Using a V-770 UV-Vis spectrophotometer, absorbance at 765 nm was measured after the mixture had been incubated at room temperature for 35 min. The control was methanol. A standard solution of gallic acid for the calibration curve was prepared in 1000 ppm (mg/L) and serially diluted to 6.25, 12.5, 25, 50, 100, 150, 200, 250, and 500 mg/L standard solutions. The calibration curve was created using the average absorbance values at the appropriate concentrations from each experiment performed in triplicate. TPC was calculated as the milligrams of gallic acid equivalent (GAE) for each extracted material gram (dry weight).
4.6. Total Flavonoid Content (TFC): Aluminum Chloride Method
With slight adjustments, the aluminum chloride colorimetric method reported by Chang et al. [72] was used to measure the extracts’ total flavonoid concentration. Briefly, the mixture of 0.3 mL sample extract, 0.3 mL of 2% AlCl3, 0.3 mL 1% sodium nitrite, and 0.3 mL 5% NaOH was mixed. The mixture was incubated at room temperature for a total of 30 min. Methanol was used as a control. Absorbance was measured at λ 314 nm using the spectrophotometer mentioned above. Quercetin prepared at 1000 ppm (mg/L) was used as a standard and calibration concentrations were prepared for TPC determination. TFC was computed as mg of quercetin equivalent (QE) per gram (dry weight) of sample extract.
4.7. HPLC (UHPLC-DAD) Analysis
Ultra-high-performance liquid chromatography coupled with a diode array detector (Ultimate-3000 UHPLC-DAD, Thermo Scientific Dionex, Sunnyvale, CA, USA) was used to determine the presence of flavonoids and phenolic acids. The column was in reverse phase with Fortis 5 mm C18 (4.6 × 250 mm column dimension). Methanol-acidified (1% acetic acid) ultra-pure water (60/40, v/v) at the flow rate of 0.8 mL/min was used as the mobile phase. Autosamplers and column temperature were set at 25 °C and 35 °C, respectively. Then, 10 μL of the purified sample extracts dissolved in the mobile phase mixture was injected into the column, and UV-Vis detection was attained at 254 nm, 272 nm, 360 nm, and 372 nm. The standard mixture of the phenolic acids syringic acid, chlorogenic acid, and gallic acid, and the flavonoids quercetin, myricetin, and rutin at the concentrations of 2.5, 10, 20, 40, 50 mg/mL were used as the external reference standard mixtures.
4.8. Antibacterial Tests
An antimicrobial efficacy study was conducted in the Ethiopian Biotechnology Institute, Ethiopia Microbiology Laboratory.
4.8.1. Test Organisms
Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 25923) were obtained from the Ethiopian Public Health Institute. Shigella spp. was obtained from clinical isolates selected by a researcher at Bio and Emerging Technology Institute (formerly EBTi), Ethiopia.
4.8.2. Antibacterial Activity
Selected test organisms were subjected to susceptibility tests for each extract using the agar well diffusion method as used by Lulekal et al. [73] with slight modifications. The susceptibility test was performed in triplicate for the methanol extract of the root and aerial part of A. integrifolia. Test organisms were swabbed onto sterile Mueller Hinton agar (HIMEDIA, Mumbai, India) plates using sterile cotton swabs. Mueller Hinton was swabbed, the agar was allowed to dry, and the blue points were used to drill 6 evenly spaced holes. Then, 100 μL of one sample extract (with a concentration of 0.1 g/mL) was placed in three holes, while 100 μL of the other extract was placed in the remaining three holes. For the negative and positive controls, equal volumes of sterile distilled water, methanol (diluent), chloramphenicol, and ciprofloxacin suspensions were used. The zones of inhibition (ZOI) (susceptibility or resistance) of the extracts and control for each test organism were then measured with a ruler and reported in millimeters after incubation at 37 °C for 24 h.
4.9. In Silico Study
4.9.1. Physicochemical and Pharmacokinetic Properties
The SwissADME web tool (http://www.swissadme.ch; accessed 2 December 2022), with free access to a pool of fast and robust predictive models including built-in methods such as the BOILEDEgg, iLOGP, and Bioavailability Radar, was used to retrieve properties such as physicochemical, pharmacokinetics, and drug-likeness. Medicinal chemistry friendliness was checked using two models: PAINS alert and the Brenk filter models [53]. Easy, efficient input formats and interpretation are made possible through a user-friendly interface.
4.9.2. Molecular Docking: Interaction Study
For the selection and preparation of target protein and ligand structures, DHO is preferred for its essential role in the proliferation of pathogens and as one of the key enzymes in the aforementioned pathway [24]. Lipowska and coworkers proposed two structures of DHOs, the plague-causing pathogen from Yersinia pestis (YpDHO) (PDB ID: 6CTY), and the causative agent of cholera from Vibrio cholerae (VcDHO) (PDB ID: 5VGM). We selected the latter one after checking the crystal structure resolution, total quality factor, and Q-mean values [74] and testing for docking with the reference drug on the respective active site. The ligands used in this study were the flavonoids and phenolic acids identified in the HPLC analysis and from the literature [75]. The structure of these ligands is shown in Figure 5. Ciprofloxacin was used as a reference after its first-line treatment for shigellosis [8,76]. Preparations of the receptor protein and ligands were managed after the structure retrieved from pdb (https://www.rcsb.org/structure/5VGM; accessed 2 December 2022) and PubChem (https://pubchem.ncbi.nlm.nih.gov/; accessed 2 December 2022) online servers using standard procedure [77]. The structures were minimized using USCF Chimera 1.15 software (Resource for Biocomputing, Visualization, and Informatics University of California, San Francisco, CA, USA) and ChemDraw 3D software (Version 12.0, PerkinElmer, Waltham, MA, USA). The prepared structures were saved as pdb files and made ready for molecular docking.
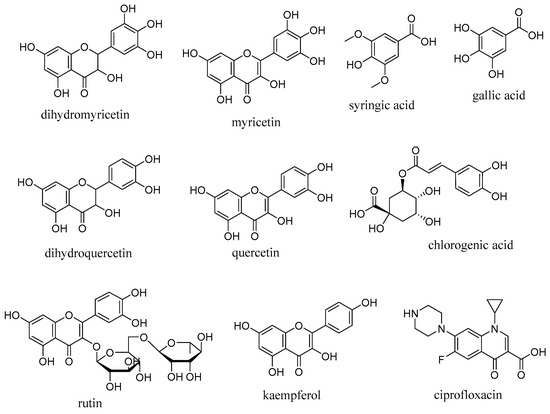
Figure 5.
Structures of ligands selected for the in silico study.
4.9.3. Molecular Docking and Visualization
Prepared structures of the receptor macromolecule and ligand molecules were loaded in PyRx 8.0 software [63] and converted to the respective pdbqt files. Using the information about the residues of the active site, Vina search parameters were set as follows: exhaustiveness = 8; center x = 21.5380864417, y = 18.4743434105, z = 82.1235234094; dimensions x = 30.1832504022, y = 30.810349529, z = 30.3537531811. After completing the Vina, a CSV output file with pose binding energies and respective RMSD values was generated for each ligand on an Excel sheet and output for further interaction study using visualizing software such as PyMol 2.5 (Schrödinger, Mannheim, Germany), Discovery Studio (Dassault Systèmes, Vélizy-Villacoublay, France) [78], and LiPlot+1.4.5 (European Bioinformatics Institute, Cambridge, United Kingdom) [79]. From the visualizing software’s interaction types, their distance and interacting residues were extracted and used for the discussion and conclusions.
5. Conclusions
The search for novel and effective therapeutic targets has become necessary due to the rise in organisms’ antimicrobial resistance tendencies. The antioxidant activity of the aerial part of A. integrifolia was more significant than the root sample. This indicates the presence of phenolics and flavonoids in the aerial part rather than in the root sample, as was also confirmed for the antibacterial activity. Following the considerable activity of the aerial part against Shigella spp., potential anti-shigellosis activity was screened. VcDHO was selected as a drug target for its role in the proliferation of pathogenic bacteria. As responsible components, flavonoids and phenolic acids identified from HPLC analysis and others from the previous study in the aerial sample of A. integrifolia were considered. Most likely, chlorogenic acid, rutin, taxifolin (dihydroquercetin), dihydromyricetin, and kaempferol are potentially responsible for the anti-shigellosis activity and therapeutic potential. The synergic effect and specific activity of the compounds identified as potential inhibitors of VcDHO are essential to reaching a more robust conclusion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031111/s1, Supplementary Material S1: Figure S1: Main Ramachandran plot for 5VGM; Supplementary Material S1: Figure S2 Quality comparison for 5VGM; Supplementary Material S2: SwissADME data for ligands.
Author Contributions
Conceptualization, F.B.T.; Formal analysis, F.B.T. and T.B.A.; Funding acquisition, S.M.W. and K.A.D.; Investigation, T.G.T.; Methodology, F.B.T., T.B.A. and T.G.T.; Resources, M.G.T. and R.K.B.; Supervision, M.G.T. and R.K.B.; Validation, Y.H.G., T.B.A., A.B., D.P.P., S.M.W., K.A.D., I.Š., P.K., V.K. and S.A.F.; Writing—original draft, F.B.T.; Writing—review and editing, Y.H.G., T.B.A., A.B., D.P.P., S.M.W., K.A.D., I.Š., P.K., V.K., S.A.F. and R.K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project Number (RSP2023R388) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This article and its supplemental information files contain all the data created or analyzed during this investigation.
Acknowledgments
This work was funded by the Researchers Supporting Project Number (RSP2023R388) King Saud University, Riyadh, Saudi Arabia. The authors acknowledge Belete Adefris for providing standard references and Addis Ababa Science and Technology University, Industrial Chemistry Department, and EBTi for the facilities to conduct the investigations. All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Fichtl, R.; Adi, A. Honeybee Flora of Ethiopia; Edwards, S., Kelbessa, E., Eds.; Margraf Verlag: Weikersheim, Germany, 1994. [Google Scholar]
- Hedberg, I. Flora of Ethiopia and Eritrea. Vol. 5, Gentianaceae to Cyclocheilaceae; Hedberg, I., Kelbessa, E., Edwards, S., Demissew, S., Persson, E., Eds.; The National Herbarium, Biology Department, Science Faculty, Addis Ababa University: Addis Ababa, Ethiopia, 2006. [Google Scholar]
- Ullah, M.A.; Gul, F.Z.; Khan, T.; Bajwa, M.N.; Drouet, S.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Liu, C.; Hano, C.; Abbasi, B.H. Differential Induction of Antioxidant and Anti-Inflammatory Phytochemicals in Agitated Micro-Shoot Cultures of Ajuga Integrifolia Buch. Ham. Ex D.Don with Biotic Elicitors. AMB Express 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alene, M.; Abdelwuhab, M.; Belay, A.; Yazie, T.S. Evaluation of Antidiabetic Activity of Ajuga Integrifolia (Lamiaceae) Root Extract and Solvent Fractions in Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.H. Review of the Active Principles of Medicinal and Aromatic Plants and Their Disease Fighting Properties. In Medicinal and Aromatic Plants: Expanding their Horizons through Omics; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–36. ISBN 9780128195901. [Google Scholar]
- Ferrari, B.; Castilho, P.; Tomi, F.; Rodrigues, A.I.; Do Ceu Costa, M.; Casanova, J. Direct Identification and Quantitative Determination of Costunolide and Dehydrocostuslactone in the Fixed Oil of Laurus Novocanariensis By13C-NMR Spectroscopy. Phytochem. Anal. 2005, 16, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Magozwi, D.K.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.W.M.; Sonopo, M.; McGaw, L.J.; Augustyn, W.A.; Tembu, V.J. Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships. Pharmaceuticals 2021, 14, 428. [Google Scholar] [CrossRef]
- Mulatu, G.; Beyene, G.; Zeynudin, A. Prevalence of Shigella, Salmonella and Campylobacter Species and Their Susceptibility Patterns among under Five Children with Diarrhea in Hawassa Town, South Ethiopia. Ethiop. J. Health Sci. 2014, 24, 101–108. [Google Scholar] [CrossRef]
- Tosisa, W.; Mihret, A.; Ararsa, A.; Eguale, T.; Abebe, T. Prevalence and Antimicrobial Susceptibility of Salmonella and Shigella Species Isolated from Diarrheic Children in Ambo Town. BMC Pediatr. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Hunde, D.; Asfaw, Z.; Kelbessa, E. Use of Traditional Medicinal Plants by People of “Boosat” Sub District, Central Eastern Ethiopia. Ethiop. J. Health Sci. 2006, 16, 141–155. [Google Scholar]
- Clarkson, K.A.; Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.; Porter, C.K.; Chakraborty, S.; Brubaker, J.; Elwood, D.; et al. Immune Response Characterization in a Human Challenge Study with a Shigella Flexneri 2a Bioconjugate Vaccine. EBioMedicine 2021, 66, 103308. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Furuta, K.; Shimomura, K.; Kasama, Y.; Shimamoto, T. Genetic Characterization of Multidrug Resistance in Shigella Spp. from Japan. J. Med. Microbiol. 2006, 55, 1685–1691. [Google Scholar] [CrossRef]
- Ud-Din, A.; Wahid, S. Relationship among Shigella Spp. and Enteroinvasive Escherichia Coli (EIEC) and Their Differentiation. Braz. J. Microbiol. 2014, 45, 1131–1138. [Google Scholar] [CrossRef]
- Qasim, M.; Wrage, M.; Nüse, B.; Mattner, J. Shigella Outer Membrane Vesicles as Promising Targets for Vaccination. Int. J. Mol. Sci. 2022, 23, 994. [Google Scholar] [CrossRef] [PubMed]
- Hussen, S.; Mulatu, G.; Yohannes Kassa, Z. Prevalence of Shigella Species and Its Drug Resistance Pattern in Ethiopia: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Debas, G.; Kibret, M.; Biadglegne, F.; Abera, B. Prevalence and Antimicrobial Susceptibility Patterns of Shigella Species at Felege Hiwot Referral Hospital, Northwest Ethiopia. Ethiop. Med. J 2011, 49, 249–256. [Google Scholar] [PubMed]
- Mukhopadhyay, S.; Ganguli, S.; Chakrabarti, S. Shigella Pathogenesis: Molecular and Computational Insights. AIMS Mol. Sci. 2020, 7, 99–121. [Google Scholar] [CrossRef]
- Beyene, G.; Tasew, H. Prevalence of Intestinal Parasite, Shigella and Salmonella Species among Diarrheal Children in Jimma Health Center, Jimma Southwest Ethiopia: A Cross Sectional Study. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, G.; Mulugeta, G.; Lema, T.; Aseffa, A. Prevalence and Antimicrobial Susceptibility Patterns of Salmonella Serovars and Shigella Species. J. Microb. Biochem. Technol. 2014, 6, S2-006. [Google Scholar] [CrossRef]
- Lamboro, T.; Ketema, T.; Bacha, K. Prevalence and Antimicrobial Resistance in Salmonella and Shigella Species Isolated from Outpatients, Jimma University Specialized Hospital, Southwest Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 4210760. [Google Scholar] [CrossRef] [PubMed]
- Marami, D.; Hailu, K.; Tolera, M. Prevalence and Antimicrobial Susceptibility Pattern of Salmonella and Shigella Species among Asymptomatic Food Handlers Working in Haramaya University Cafeterias, Eastern Ethiopia. BMC Res. Notes 2018, 11, 7–12. [Google Scholar] [CrossRef]
- Terfassa, A.; Jida, M. Prevalence and Antibiotics Susceptibility Pattern of Salmonella and Shigella Species among Diarrheal Patients Attending Nekemte Referral Hospital, Oromia, Ethiopia. Int. J. Microbiol. 2018, 2018, 9214689. [Google Scholar] [CrossRef]
- Yang, G.; Wang, L.; Wang, Y.; Li, P.; Zhu, J.; Qiu, S.; Hao, R.; Wu, Z.; Li, W.; Song, H. Hfq Regulates Acid Tolerance and Virulence by Responding to Acid Stress in Shigella Flexneri. Res. Microbiol. 2015, 166, 476–485. [Google Scholar] [CrossRef]
- Lipowska, J.; Miks, C.D.; Kwon, K.; Shuvalova, L.; Zheng, H.; Lewiński, K.; Cooper, D.R.; Shabalin, I.G.; Minor, W. Pyrimidine Biosynthesis in Pathogens— Structures and Analysis of Dihydroorotases from Yersinia Pestis and Vibrio Cholerae. Int. J. Biol. Macromol. 2019, 136, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Asres, K.; Bucar, F.; Kartnig, T.; Witvrouw, M.; Pannecouque, C.; Clercq, E. De Antiviral Activity against Human Immunodeficiency Virus Type 1 (HIV-1) and Type 2 (HIV-2) of Ethnobotanically Selected Ethiopian Medicinal Plants. Phytochem. Res. 2001, 15, 62–69. [Google Scholar]
- Fullas, F. Ethiopian Traditional Medicine: Common Medicinal Plants in Perspective, 1st ed.; F. Fullas: Sioux City, IA, USA, 2001. [Google Scholar]
- Suleman, S.; Alemu, T. A Survey on Utilization of Ethnomedicinal Plants in Nekemte Town, East Wellega (Oromia), Ethiopia. J. Herbs Spices Med. Plants 2012, 18, 37–41. [Google Scholar] [CrossRef]
- El-Hilaly, J.; Amarouch, M.Y.; Morel, N.; Lyoussi, B.; Quetin-Leclercq, J. Ajuga Iva Water Extract Antihypertensive Effect on Stroke-Prone Spontaneously Hypertensive Rats, Vasorelaxant Effects Ex Vivo and in Vitro Activity of Fractions. J. Ethnopharmacol. 2021, 270, 113791. [Google Scholar] [CrossRef] [PubMed]
- Bekele, D.; Asfaw, Z.; Petros, B.; Tekie, H. Ethnobotanical Study of Plants Used for Protection against Insect Bite and for the Treatment of Livestock Health Problems in Rural Areas of Akaki District, Eastern Shewa, Ethiopia. Topclass. J. Herb. Med. 2012, 1, 12–24. [Google Scholar]
- Meragiaw, M.; Asfaw, Z. Review of Antimalarial, Pesticidal and Repellent Plants in the Ethiopian Traditional Herbal Medicine. Res. Rev. J. Herb. Sci. 2014, 3, 21–25. [Google Scholar]
- Asnake, S.; Teklehaymanot, T.; Hymete, A.; Erko, B.; Giday, M. Survey of Medicinal Plants Used to Treat Malaria by Sidama People of Boricha District, Sidama Zone, South Region of Ethiopia. Evid.-Based Complement. Altern. Med. 2016, 2016, 9690164. [Google Scholar] [CrossRef]
- Hailu, W.; Engidawork, E. Evaluation of the Diuretic Activity of the Aqueous and 80 % Methanolic Extracts of the Leaves of Ajuga Remota B. (Lamiaceae) in Mice. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2011. [Google Scholar]
- Chekole, G. Ethnobotanical Study of Medicinal Plants Used against Human Ailments in Gubalafto. J. Ethnobiol. Ethnomed. 2017, 13, 55. [Google Scholar] [CrossRef]
- Abera, B. Medicinal Plants Used in Traditional Medicine by Oromo People, Ghimbi District, Southwest Ethiopia. J. Ethnobiol. Ethnomed. 2014, 10, 1–15. [Google Scholar] [CrossRef]
- Tuasha, N.; Petros, B.; Asfaw, Z. Plants Used as Anticancer Agents in the Ethiopian Traditional Medical Practices: A Systematic Review. Evid.-Based Complement. Altern. Med 2018, 2018, 6274021. [Google Scholar] [CrossRef]
- Gebrehiwot, M. An Ethnobotanical Study of Medicinal Plants in Seru Wereda, Arsi Zone of Oromia Region, Ethiopia. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2010. [Google Scholar]
- Regassa, R. Assessment of Indigenous Knowledge of Medicinal Plant Practice and Mode of Service Delivery in Hawassa City, Southern Ethiopia. J. Med. Plants Res. 2013, 7, 517–535. [Google Scholar] [CrossRef]
- Teklay, A.; Abera, B.; Giday, M. An Ethnobotanical Study of Medicinal Plants Used in Kilte Awulaelo District, Tigray Region of Ethiopia. J. Ethnobiol. Ethnomed. 2013, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Maryo, M.; Nemomissa, S.; Bekele, T. An Ethnobotanical Study of Medicinal Plants of the Kembatta Ethnic Group in Enset-Based Agricultural Landscape of Kembatta Tembaro (KT) Zone, Southern Ethiopia. Pelagia Res. Libr. Asian J. Plant Sci. Res. 2015, 5, 42–61. [Google Scholar] [CrossRef]
- Gedif, T.; Hahn, H. The Use of Medicinal Plants in Self-Care in Rural Central Ethiopia. J. Ethnopharmacol. 2003, 87, 155–161. [Google Scholar] [CrossRef]
- Parvez, N.; Yadav, S. Ethnopharmacology of Single Herbal Preparations of Medicinal Plants in Asendabo District, Jimma, Ethiopia. Indian J. Tradit. Knowl. 2010, 9, 724–729. [Google Scholar]
- Gabriel, T.; Guji, T. Ethnopharmacological Survey of Medicinal Plants in Agaro District, Jimma Zone, South West Ethiopia. Int J. Pharm. Sci. Res. 2014, 5, 3551–3559. [Google Scholar] [CrossRef]
- Tafesse, T.B.; Hymete, A.; Mekonnen, Y.; Tadesse, M. Antidiabetic Activity and Phytochemical Screening of Extracts of the Leaves of Ajuga Remota Benth on Alloxan-Induced Diabetic Mice. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Rubnawaz, S.; Okla, M.K.; Akhtar, N.; Khan, I.U.; Bhatti, M.Z.; Duong, H.Q.; El-tayeb, M.A.; Elbadawi, Y.B.; Almaary, K.S.; Moussa, I.M.; et al. Antibacterial, Antihemolytic, Cytotoxic, Anticancer, and Antileishmanial Effects of Ajuga Bracteosa Transgenic Plants. Plants 2021, 10, 1894. [Google Scholar] [CrossRef]
- Meite, S.; N’Guessan, J.D.; Bahi, C.; Yapi, H.F.; Djaman, A.J.; Guede Guina, F. Antidiarrheal Activity of the Ethyl Acetate Extract of Morinda Morindoides in Rats. Trop. J. Pharm. Res. 2009, 8, 201–207. [Google Scholar] [CrossRef]
- Asrie, A.B.; Abdelwuhab, M.; Shewamene, Z.; Gelayee, D.A.; Adinew, G.M.; Birru, E.M. Antidiarrheal Activity of Methanolic Extract of the Root Bark of Cordia Africana. J. Exp. Pharm. 2016, 8, 53–59. [Google Scholar] [CrossRef]
- Mishra, M.P.; Rath, S.; Swain, S.S.; Ghosh, G.; Das, D.; Padhy, R.N. In Vitro Antibacterial Activity of Crude Extracts of 9 Selected Medicinal Plants against UTI Causing MDR Bacteria. J. King Saud. Univ. Sci. 2017, 29, 84–95. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents. 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and Synthetic Flavonoid Derivatives as New Potential Tyrosinase Inhibitors: A Systematic Review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Kranich, R.; Busemann, A.S.; Bock, D.; Schroeter-Maas, S.; Beyer, D.; Heinemann, B.; Meyer, M.; Schierhorn, K.; Zahlten, R.; Wolff, G.; et al. Rational Design of Novel, Potent Small Molecule Pan-Selectin Antagonists. J. Med. Chem. 2007, 50, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Mughal, E.U.; Zia, K.; Sadiq, A.; Naeem, N.; Javid, A.; Ul-Haq, Z.; Saeed, M. Synthetic Flavonoids as Potential Antiviral Agents against SARS-CoV-2 Main Protease. J. Biomol. Struct. Dyn. 2022, 40, 3777–3788. [Google Scholar] [CrossRef] [PubMed]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons Learnt from Assembling Screening Libraries for Drug Discovery for Neglected Diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Lere Keshebo, D.; Washe, A.P.; Alemu, F. Determination of Antimicrobial and Antioxidant Activities of Extracts from Selected Medicinal Plants. Am Sci Res J Eng 2016, 16, 212–222. [Google Scholar]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant Activity and Protecting Health Effects of Common Medicinal Plants. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 67, pp. 75–135. ISBN 9780123945983. [Google Scholar]
- Banothu, V.; Neelagiri, C.; Adepally, U.; Lingam, J.; Bommareddy, K. Phytochemical Screening and Evaluation of in Vitro Antioxidant and Antimicrobial Activities of the Indigenous Medicinal Plant Albizia Odoratissima. Pharm. Biol. 2017, 55, 1155–1161. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Bhatt, I.D.; Rawat, S.; Rawal, R.S. Antioxidants in Medicinal Plants. In Biotechnology for Medicinal Plants; Chandra, S., Lata, H., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642299742. [Google Scholar]
- Omosa, L.K.; Amugune, B.; Ndunda, B.; Milugo, T.K.; Heydenreich, M.; Yenesew, A.; Midiwo, J.O. Antimicrobial Flavonoids and Diterpenoids from Dodonaea Angustifolia. S. Afr. J. Bot. 2014, 91, 58–62. [Google Scholar] [CrossRef]
- Israili, Z.H.; Lyoussi, B. Ethnopharmacology of the Plants of Genus Ajuga. Pak. J. Pharm. Sci. 2009, 22, 425–462. [Google Scholar] [PubMed]
- Alam, J.; Roy, T. In Silico Screening for Novel Inhibitors of Invasion Protein Antigen IpaD of Shigella Flexneri—An Agent of Shigellosis. Int. J. Comput. Bioinform. Silico Model 2015, 3, 497–501. [Google Scholar]
- Rabbi, M.F.; Akter, S.A.; Hasan, M.J.; Amin, A. In Silico Characterization of a Hypothetical Protein from Shigella Dysenteriae ATCC 12039 Reveals a Pathogenesis-Related Protein of the Type-VI Secretion System. Bioinform. Biol. Insights 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2012, 31, 455–461. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K. shun Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Aderogba, M.A.; Ndhlala, A.R.; Rengasamy, K.R.R.; Staden, J. Van Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton Menyharthii. Molecules 2013, 18, 12633–12644. [Google Scholar] [CrossRef]
- Imoru, A.; Onibi, G.E.; Osho, I.B. Nutritional and Biochemical Compositions of Turmeric (Curcuma Longa Linn) Rhizome Powder—A Promising Animal Feed Additive. Int. J. Sci. Eng. Res. 2018, 9, 424–429. [Google Scholar]
- Zakaria, F.; Tan, J.-K.; Mohd Faudzi, S.M.; Rahman, M.; Ashari, E. Ultrasound-Assisted Extraction Condition Optimization Using Response Surface Methodology from Mitragyna Speciosa (Korth.) Havil Leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef]
- Mawela, K.G. The toxicity and repellent properties of plant extracts used in ethnoveterinary medicine to control ticks. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2008; pp. 72–82. Available online: https://repository.up.ac.za/handle/2263/29224 (accessed on 11 December 2022).
- Chen, Z.; Bertin, R.; Froldi, G. EC50 Estimation of Antioxidant Activity in DPPH Assay Using Several Statistical Programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Lulekal, E.; Rondevaldova, J.; Bernaskova, E.; Cepkova, J.; Asfaw, Z.; Kelbessa, E.; Kokoska, L.; Van Damme, P. Antimicrobial Activity of Traditional Medicinal Plants from Ankober District, North Shewa Zone, Amhara Region, Ethiopia. Pharm. Biol. 2014, 52, 614–620. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the Estimation of the Absolute Quality of Individual Protein Structure Models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Han, K.; Li, M.; Zhang, T.; Liu, D.; Yu, L.; Lv, H. Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Species from the Genus Ajuga L.: A Systematic Review. Am. J. Chin. Med. 2019, 47, 959–1003. [Google Scholar] [CrossRef] [PubMed]
- Wambe, H.; Noubissi, P.A.; Fokam Tagne, M.A.; Foyet Fondjo, A.; Fankem, G.O.; Kamtchouing, I.; Ngakou Mukam, J.; Nguelefack, T.B.; Kamgang, R. Anti-Shigellosis Activity of Cola Anomala Water/Ethanol Pods Extract on Shigella Flexneri- Induced Diarrhea in Rats. Biomed. Res. Int. 2019, 2019, 6706230. [Google Scholar] [CrossRef]
- Fitriah, A.; Holil, K.; Syarifah, U.; Fitriyah; Utomo, D.H. In Silico Approach for Revealing the Anti-Breast Cancer and Estrogen Receptor Alpha Inhibitory Activity of Artocarpus Altilis. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018; p. 070003. [Google Scholar]
- Utami, W.; Aziz, H.A.; Fitriani, I.N.; Zikri, A.T.; Mayasri, A.; Nasrudin, D. In Silico Anti-Inflammatory Activity Evaluation of Some Bioactive Compound from Ficus Religiosa through Molecular Docking Approach. J. Phys. Conf. Ser. 2020, 1563, 1–9. [Google Scholar] [CrossRef]
- Tanuja, J.; Priyanka, S.; Tushar, J.; Subhash, C. In Silico Screening of Anti-Inflammatory Compounds from Lichen by Targeting Cyclooxygenase-2. J. Biomol. Struct. Dyn. 2019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).