Abstract
Transition metal organic framework materials and their selenides are considered to be one of the most promising cathode materials for nickel-zinc (denoted as Ni-Zn) batteries due to their low cost, environmental friendliness, and controllable microstructure. Yet, their low capacity and poor cycling performance severely restricts their further development. Herein, we developed a simple one-pot hydrothermal process to directly synthesize NiSe2 (denotes as NiSe2-X based on the molar amount of SeO2 added) stacked layered sheets. Benefiting from the peculiar architectures, the fabricated NiSe2−1//Zn battery based on NiSe2 and the Zn plate exhibits a high specific capacity of 231.6 mAh g−1 at 1 A g−1, and excellent rate performance (162.8 mAh g−1 at 10 A g−1). In addition, the NiSe2//Zn battery also presents a satisfactory cycle life at the high current density of 8 A g−1 (almost no decay compared to the initial specific capacity after 1000 cycles). Additionally, the battery device also exhibits a satisfactory energy density of 343.2 Wh kg−1 and a peak power density of 11.7 kW kg−1. This work provides a simple attempt to design a high-performance layered cathode material for aqueous Ni-Zn batteries.
1. Introduction
With the continuous consumption of conventional energy resources and the increasing demand for energy, the energy crisis and the consequent environmental problems have attracted extensive attention. A large quantity of energy storage devices have been developed in the past few decades, such as supercapacitors, Li-ion batteries, and aqueous rechargeable batteries [1,2,3,4,5,6,7]. Among those aforementioned, energy storage devices such as aqueous Zn-based batteries are attracting considerable attention due to their high theoretical capacity (5851 mAh cm−3 and 820 mAh g−1), low redox potential (0.763 V vs. the standard hydrogen electrode (SHE)), high output voltage (such as Ni-Zn battery about 1.8 V), good compatibility with aqueous electrolytes, eco-friendliness, low cost, and abundant source of the Zn anode [8,9,10,11,12,13,14,15,16]. Compared to the high theoretical capacities of zinc anode materials, most reported cathode materials have relatively low capacities (200–300 mAh g−1) [1,5,17,18,19,20,21,22]. In this scenario, the key to improving the capacity of Ni-Zn battery is to explore suitable cathode materials.
Among current cathode materials, transition metal selenides (TMSs) own higher conductivity than transition metal oxides and hydroxides. For example, the binding energy of Ni-Se is lower than that of Ni-O and Ni-S with weaker electronegativity, reducing the energy consumption of conversion reaction [23]. Additionally, due to the higher degree of reversibility of M-Se compared to M-O bonds (M = metal), TMSs always exhibit superior electrochemical performance such as high specific capacity, rate capability, and long-term cyclability [24,25,26,27,28]. Due to the porous structure and easy adjustment, MOFs are ideal precursor materials for the construction of TMSs, which have been identified as cathode candidates for zinc-based batteries. The synthesized TMSs through the above method not only own controllable nanostructures but also show greater electrochemical performance [6,7,29,30,31,32]. For instance, CoSe2-x-derived cobalt oxides nanoparticles synthesized by Tang et al. obtained a capacity of 7.42 mAh m−2 at 10 mA cm−2 with 0.02% cycle−1 capacity decay over 4200 cycles [19]. Porous NiCoSe2@NiOOH/CoOOH nanoflakes reported by Cui and his co-workers exhibited a capacity of 108.9 mAh g−1 at 1 A g−1 with 105.1% capacity retention after 4000 cycles [33]. MoSe2 decorated Ni/Co selenide complex hollow arrays presented a capacity of 142 mAh g−1 at 2 A g−1 with ~36% capacity loss after 1000 cycles [34]. However, the capacity and long cycle stability of most reported TMSs//Zn batteries have still fallen below expectations and cannot meet future practical applications. Moreover, the traditional method of constructing selenide by MOFs is relatively complicated, which requires high temperature carbonization followed by high-temperature selenization under Ar flow. Therefore, the investigation of new durable TMSs cathodes with high capacity is still highly favorable and challenging for achieving widely applicable aqueous rechargeable zinc-based batteries.
In this work, we propose a facile one-pot hydrothermal synthesis of NiSe2 stacked layered sheets without Ni-MOFs template and high temperature heat treatment. The NiSe2//Zn battery was assembled by using the synthesized NiSe2 nanosheet as the cathode and zinc as the anode of the aqueous Ni-Zn batteries, and the fabricated battery obtained a higher specific capacity of 231.6–162.8 mAh g−1 at various current densities of 1–10 A g−1, which indicated the good rate performance. Moreover, it shows excellent cycling performance with almost no decay compared to the initial specific capacity value after 1000 cycles at 8 A g−1. Impressively, the fabricated NiSe2//Zn battery shows outstanding performance with a maximum energy density of 343.2 Wh kg−1, and a maximum power density of 11.7 kW kg−1, substantially outstripping most of the recently reported Ni-Zn batteries.
2. Results and Discussions
The Ni-MOFs nanomaterials were first synthesized by a simple one-pot hydrothermal process. As shown in Figure 1a, the pristine Ni-MOFs nanomaterials own a cluster structure stacked with numerous layered sheets, and the self-assembled nanosheets can be better observed in the high magnification SEM images (Figure 1d). Based on the above synthesis process, NiSe2−X nanomaterials with different degrees of selenization can be obtained by adding different masses of SeO2 into the precursor solution of Ni-MOFs synthesis. Figure 1b–c and Figure S1 show the variation of the structure of NiSe2 nanosheets under different SeO2 addition conditions. It can be seen that NiSe2−0.5 still retains part of the original nanosheets due to the lack of SeO2 during the synthesis process. Meanwhile, in Figure 1f, the homogeneous growth and accumulation of thick nanosheets of NiSe2−1 can be observed. Through the high magnification SEM images of the corresponding area (Figure 1e, f and Figure S1b), it can be clearly observed that the thickness of nanosheets increased with the increased concentration of SeO2. Figure 1i–l shows the EDS images of NiSe2−1 nanomaterials, which indicates that Ni, Se, and C elements are uniformly distributed in the sample. In order to further explore the microstructure of Ni-MOFs and NiSe2-1 nanomaterials, we utilized TEM (Figure 1g and Figure S2). Figure 1g shows that the NiSe2-1 nanomaterials consisted of the square-shaped nanosheets. The inset picture in Figure 1g shows the corresponding selected area electron diffraction (SAED) pattern indicating the typical polycrystalline structure of NiSe2−1. The lattice fringes of the NiSe2−1 material were visible in Figure 1h, where the characteristic lattice spacing (0.269 nm) of NiSe2−1 was consistent with the (210) lattice plane of NiSe2 (JCPDS NO. 41-1495) [35].
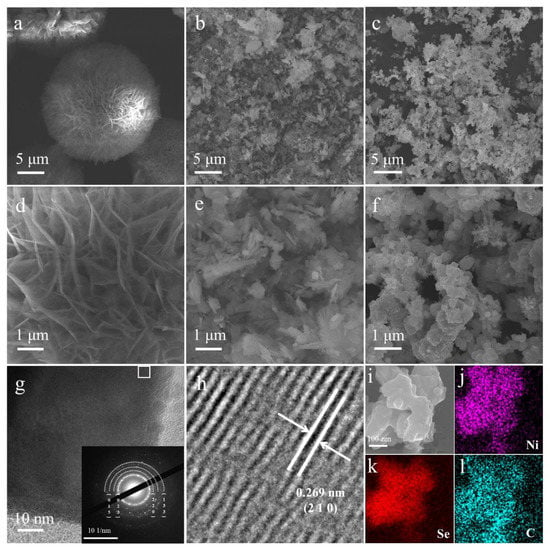
Figure 1.
SEM images of the (a) Ni-MOFs, (b) NiSe2−0.5, and (c) NiSe2−1 samples at low magnification. The SEM images of the corresponding area of (d) Ni-MOFs, (e) NiSe2−0.5, and (f) NiSe2−1 samples at high magnification. (g) TEM image of NiSe2−1 sample and corresponding SAED pattern (inset). (h) HRTEM image of NiSe2−1 sample. (i–l) EDS mapping.
Figure 2a shows the XRD spectra of the Ni-MOFs and NiSe2−X samples, which obviously demonstrates that the characteristic peaks of the four samples have changed significantly due to the different amount of SeO2 added during the synthesis process. The XRD diffraction patterns of NiSe2−1 sample, displaying six peaks at 2θ = 29.8°, 33.4°, 36.7°, 50.5°, 55.2° and 57.5° respectively, which are a right match (200), (210), (211), (311), (230), and (321) planes of NiSe2 (JCPDS NO. 41-1495), were also related to the lattice fringes of the NiSe2−1 material (Figure 1h). Additionally, the characteristic peaks of NiSe2−0.5 sample contain part characteristic peaks of Ni-MOFs, indicating incomplete transformation, which is consistent with the SEM results. To further explore the elemental composition and valence state of the samples, XPS was used to analyze the as-synthesized samples. Figure 2b is the survey spectra of Ni-MOFs and NiSe2−1 samples. It can be clearly seen that the NiSe2−1 sample synthesized by adding SeO2 has an obvious characteristic peak of Se, indicating the successful doping of Se. The C 1s spectrum of NiSe2−1 sample can be resolved into three characteristic peaks of sp2-C, sp3-C, C=O, and O-C=O bonds, respectively (Figure 2c) [22,23]. From the Ni 2p spectrum (Figure 2d), two peaks located at 855.9 and 873.7 eV were attributed to Ni2+ in Ni 2p3/2 and Ni 2p1/2 respectively. Meanwhile, the peaks located at 857.3 and 875.3 eV corresponded to the Ni3+ in Ni 2p3/2 and Ni 2p1/2, and the others were related to the satellite peaks [22,36]. As displayed in Figure 2e, the Se 3d5/2, 3d3/2, and SeOx level appeared at 55.7, 56.9 and 60.2 eV, respectively [35,37,38]. Additionally, the spectra of O 1s for Ni-MOFs and NiSe2−1 samples can be deconvoluted into three peaks assigned as Se-O (530.8 eV), OH- (533.6 eV) and adsorb water (532.5 eV), respectively (Figure S3) [35].
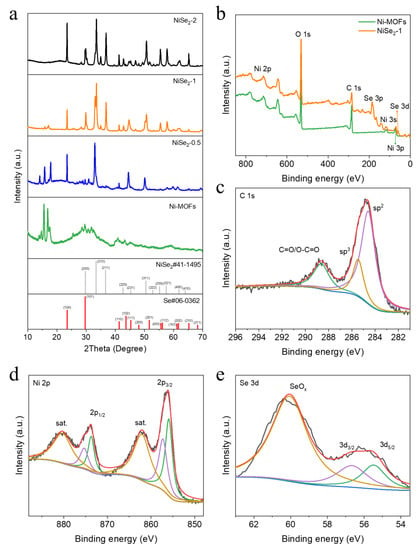
Figure 2.
(a) XRD patterns of Ni-MOFs and NiSe2−X (X = 0.5, 1, 2). (b) XPS survey of the Ni-MOFs and NiSe2−1 samples. (c) High resolution XPS spectra of C 1s. (d) Ni 2p and (e) Se 3d XPS spectra.
In order to evaluate the electrochemical performance of the prepared electrode materials, we assembled Ni-MOFs, NiSe2−X and Zinc plate into aqueous Ni-Zn batteries. Figure 3a shows different cyclic voltammetry (CV) curves of NiSe2-1 in the initial five cycles. It can be clearly observed that the current response signal increases with the number of scans, indicating the improvement of its electrochemical performance. This phenomenon can be attributed to the continuous activation of the electrode as a result of the surface chemical reaction between NiSe2−1 and KOH electrolyte [39,40]. Figure 3b shows the CV curves of the fabricated batteries with the working window from 1.6-2.0 V, which exhibit typical battery behavior with obvious redox peaks. As illustrated in Figure 3c, the NiSe2−1 displays the highest specific capacity, while the GCD curves of the as-prepared Ni-MOFs and NiSe2−X samples are at the same current density (3 A g−1). The corresponding GCD curves of NiSe2-1 at different current densities are depicted in Figure S4, and it can be clearly seen that the discharge specific capacity reached a remarkable 231.6 mAh g−1 at 1 A g−1, and the discharge specific capacity was still maintained at 162.8 mAh g−1 with a high current density of 10 A g−1, proving its excellent rate performance. Meanwhile, the obvious charge/discharge voltage plateaus appear in the GCD curves at different current densities, and the voltage range of the plateau (at 1.85–1.95 V) matches the position of the redox peaks in the cyclic voltammetry curves correspondingly. This result further confirms that the NiSe2-1 electrode undergoes a redox reaction, which has the typical characteristics of a battery-type material. The rate performance of the original Ni-MOFs and NiSe2−1 electrodes applied to Ni-Zn batteries is shown in Figure 3d. It is noteworthy that the NiSe2−1 exhibits an apparent increase in specific capacity during the initial cycles, which follows the same trend as that in Figure 3a. Both rate capability curves can illustrate that there is no significant decay after the rate cycling due to the good rate capability of Ni-based materials [23]. However, when the current density falls back to 1 A g−1 after 40 cycles, the average capacity retention of the NiSe2−1 electrode can even reach 117% compared to the initial average capacity, which is better than that of the Ni-MOFs electrode (96%) [41].
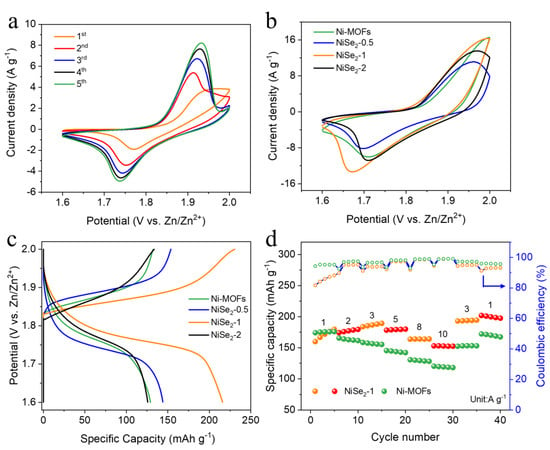
Figure 3.
(a) CV curves of NiSe2-1 at a scan rate of 1 mV s−1 for initial preactivation. (b) CV curves of Ni-MOFs and NiSe2−X (X = 0.5, 1, 2) at the same scan rate of 5 mV s−1. (c) GCD curves of Ni-MOFs and NiSe2−X (X = 0.5, 1, 2) at the same current density of 3 A g−1. (d) Rate performances and CE of Ni-MOFs and NiSe2−1.
In order to further understand the storage mechanism of Zn2+, we used different electrochemical methods to explore its kinetics. Figure 4a shows the cyclic voltammetry (CV) curves of NiSe2−1 electrode at different scan rates. It is obvious that with the increase of the scanning rate, the peak pattern of the redox peaks does not change, but the position of the redox shifts, which is mainly attributed to polarization. Theoretically, the peak current (i) and the scan rate (v) of the CV curves follow the equation: i = avb, where a and b are variable parameters. A b value of 0.5 indicates a diffusion-controlled behavior of the Zn-ion extract/insert electrode material, and a b value of 1 indicates a control by the capacitive process. The b values can be obtained by fitting a linear relationship between log i and log v [42,43]. As exhibited in Figure 4b, the b values at each redox peak of the NiSe2−1 electrode are 0.74 and 0.76, respectively, between 0.5 and 1, suggesting the presence of both diffusion-controlled and capacity-based kinetic processes. The pseudocapacitive contributions of NiSe2−1 at 0.2, 0.4, 0.6, 0.8, 1 mV s−1, presented in Figure 4c, based on the pseudocapacity calculation equation i = k1v + k2v1/2, where k1 and k2 are constant values, and the calculated result of it is the theoretical value of the pseudocapacitive contribution [44,45]. For the as-prepared NiSe2-1 electrode, the k1v value ranges from 67% to 98% at the scan range of 0.2–1 mV s−1. Typically, the original CV curve and fitted pseudocapacitive contribution (the shaded area) at 1 mV s−1 are shown in Figure S5. In addition, the capacity contribution still dominates at the lowest scan rate. Under the influence of pseudocapacitance behavior, the active material NiSe2−1 tends to undergo redox reaction on the electrode surface to store charge, which can effectively protect the structure of the NiSe2−1 and improve the cyclic stability of the electrode material. More importantly, the near-surface pseudocapacitance effect can obtain a shorter ion transport path and a faster electron transport rate, which is conducive to enhancing the electrochemical performance of NiSe2−1 [23]. The electrochemical impedance spectroscopy (EIS) in Figure 4d, where the equivalent circuit for simulation was inset, shows that the Nyquist plot consists of the semicircle in the high-frequency range related to the charge-transfer resistance and the low-frequency regions related to a diffusion-limited electron transfer process of an electrode [46,47]. It can be clearly seen that with the addition of SeO2 (Figure S6), the structure of the material gradually becomes thicker and reacts completely. The corresponding EIS of Ni-MOFs and NiSe2−X (X = 0.5, 1, 2) are depicted in Figure S7. Apparently, in the high frequency region, the Rct of NiSe2-1 electrode is 34.31 Ω, while the Rct of Ni-MOFs electrode is 40.6 Ω; in the low frequency region, NiSe2-1 electrode has a higher slope of the line than Ni-MOFs electrode, which means a faster electron diffusion. Therefore, the NiSe2−1 electrode exhibits better impedance performance than the pristine Ni-MOFs in both low and high frequency regions.
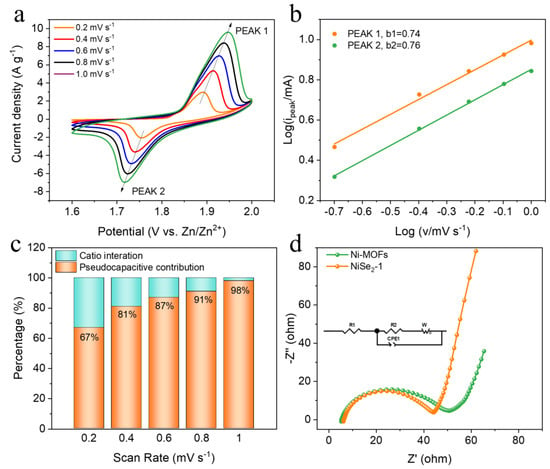
Figure 4.
(a) CV scans of NiSe2−1 at various rates. (b) Correspondingly fitted b-value at respective redox peaks. (c) Relative contribution of the capacitive and diffusion-controlled charge storage at different scan rates. (d) Nyquist plots of Ni-MOFs and NiSe2−1.
In order to further explore the practicability of the Ni-Zn batteries prepared, we investigated the cycle performance, energy density, and power density. Figure 5a displays the comparison of the long cycle performance of Ni-MOFs and NiSe2−1 as the cathode electrode of Ni-Zn batteries. It can be seen that the specific capacity of Ni-MOFs is slightly higher than that of NiSe2−1 during the very initial cycles, corresponding to the initial rate capability curve in Figure 3e. However, it is worth noting that the specific capacity of NiSe2−1 has a rather high percentage increase compared to the pristine Ni-MOFs, and the increase in capacity over about 100 cycles is attributed to the surface chemistry between NiSe2−1 and the KOH electrolyte, which activates the electrode [39,40]. After reaching the peak specific capacity value, both materials began to decay slowly, and after 1000 cycles, NiSe2-1 decayed by 50% of the peak capacity (Ni-MOFs, 61%), as shown within Figure 5a. Additionally, based on the GCD curves, the energy density and power density of the NiSe2−1//Zn battery were calculated to obtain the Ragone plots (Figure 5b). Noteworthily, the NiSe2−1//Zn battery obtained a maximum energy density of 343.2 Wh kg−1 and a peak power density of 11.7 kW kg−1, which is superior to most of the previously reported rechargeable batteries, including Ni//Bi battery (1.4 kW kg−1, 70.9 Wh kg−1) [9], Zn//OD-ZMO@PEDOT battery (273.4 Wh kg−1, 2.6 kW kg−1) [21], NiCoSe2@NiOOH/CoOOH//Zn battery (0.72 kW kg−1, 194.2 Wh kg−1) [33], Co-doped Ni(OH)2//Zn battery (1.725 kW kg−1, 148.54 Wh kg−1) [48], Ni//Fe battery (11.8 kW kg−1, 94.5 Wh kg−1) [49] and flexible Ni/Fe battery (1.02 kW kg−1, 85.8 Wh kg−1) [50].
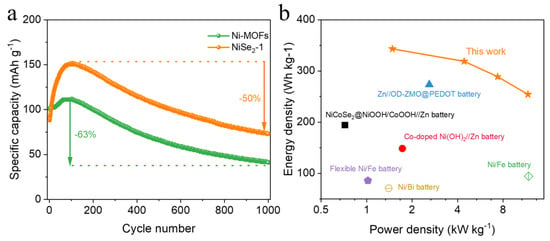
Figure 5.
(a) Long-term cycling stability of Ni-MOFs and NiSe2−1 at 8 A g−1 over 1000 cycles. (b) Ragone plot of the NiSe2−1//Zn battery and other reported rechargeable batteries [9,21,33,48,49,50].
3. Experimental Section
3.1. Synthesis of Ni-MOFs
All chemicals in this work were utilized as received without any purification (the purity of chemicals was 98%). 1 mmol Ni(NO3)2·6H2O and 1 mmol PTA were dissolved in a mixed solution consisting of 20 mL of DMF and 5 mL of 0.4 M NaOH, stirred well at room temperature and transferred into a 50 mL Teflon-lined stainless steel reactor, which was subjected to hydrothermal reaction at 160 °C for 12 h in an oven. After the reaction, the solution was cooled down to room temperature, centrifugally, and dried at 60 °C for 12 h to obtain Ni-MOFs.
3.2. Synthesis of NiSe2 Architectures
The synthesis of NiSe2 is based on the above synthesis method. It only needs to add different masses of SeO2 to the precursor solution for the synthesis of Ni-MOFs. The resulting products were named as NiSe2−X (X = 0.5,1,2) according to the molar amount of SeO2 added.
3.3. Fabrication of Aqueous Ni-Zn Batteries and Electrochemical Measurements
The aqueous Ni-Zn batteries were fabricated by a piece of Zn plate, the synthesized NiSe2−X (X = 0.5, 1, 2) electrode, Zn flakes, and a mixed aqueous consisting of 3 M KOH and 0.2 M Zn(CH3COO)2 as the anode, cathode, and electrolyte. The mass loading was about 2.4 mg cm−2. The electrochemical performances (CV, CP, EIS) of the fabricated batteries were characterized by an electrochemical workstation (CHI 660E) and Land 2001A.
3.4. Materials Characterization
The surface morphology, microstructure, crystalline structure, chemical composition, and elemental valence of the synthesized samples were investigated by the field-emission scanning electron microscope (SEM, Sigma500, ZEISS, Oberkochen, Germany) equipped with an energy dispersive X-ray element analysis system (EDS), transmission electron microscope (TEM, JEM-F200, JEOL, Tokyo, Japan), X-ray diffraction (XRD, X ’Pert Pro MPD, PANalytical, Malvern, UK) patterns, X-ray photoelectron spectroscopy (XPS, NEXSA, Thermo VG, Waltham, MA, USA), and Raman spectroscopy (LabRAM HR Evolution, HORIBA, Kyoto, Japan).
All electrochemical characterization was carried out in a two-electrode system with a potential window of 1.6 to 2.0 V. Galvanostatic charge/discharge (GCD), Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were performed using an electrochemical workstation (CHI 660E). The cycle life of the Ni-Zn batteries was tested by using a Land 2001A.
4. Conclusions
In summary, based on the synthesis of Ni-MOF, we have developed a simple one-pot synthesis method of NiSe2 with a layered structure. Benefiting from the unique nanosheet structure, the assembled aqueous NiSe2−1//Zn battery exhibits remarkable specific capacity (231.6 mAh g−1 at 1 A g−1), excellent rate performance (162.8 mAh g−1 at 10 A g−1), and prominent energy density (343.2 Wh kg−1) as well as excellent long-term cycling stability (almost no decay after 1000 cycles at 8 A g−1 compared to the initial specific capacity value). Moreover, NiSe2 can be easily prepared by a one-pot hydrothermal synthesis method, which facilitates the widespread and commercial application of the energy storage devices and lays the foundation for future research and development of such materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031098/s1, Figure S1: SEM images of the NiSe2−2 sample (a) at low magnification, (b) at high magnification; Figure S2: TEM image of (a) Ni-MOFs sample. (b) NiSe2−1 sample; Figure S3: High resolution O 1s XPS spectra of (a) Ni-MOFs and (b) NiSe2−1; Figure S4: GCD curves of NiSe2−1 at different current densities; Figure S5: Ratio of pseudo-capacitive capacity for NiSe2−1 at 1 mV s−1; Figure S6: SEM images of (a) Ni-MOFs, (b) NiSe2−0.5, (c) NiSe2−1 and (d) NiSe2−2 samples; Figure S7: Nyquist plots of Ni-MOFs and NiSe2−X (X = 0.5, 1, 2).
Author Contributions
Conceptualization, F.W. and X.L.; Material Synthesis, S.C. and Y.H.; Characterization of morphological components, S.C. and H.L.; Electrochemical data, S.C. and W.X.; Data Processing, D.Z. and S.C.; Writing Original Draft Preparation, S.C.; Writing-Review& Editing, F.W. and X.L.; Funding Acquisition, F.W. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (22005222), Guangdong province innovation and strong school project (2020ZDZX2004, and 2020KQNCX087), Joint Science Foundation of Wuyi University and HK and Macao (2019WGALH14), Science Foundation for High-Level Talents of Wuyi University (2019AL022, and 5041700133), Innovation Foundation of Educational Com-mission Guangdong Province (2020KQNCX090), Guangdong Basic and Applied Basic Research Foundation (2022A1515110136), and Basic and Applied Basic Research Foundation of Jiangmen (JZ202212).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Zhang, H.; Wang, R.; Lin, D.; Zeng, Y.; Lu, X. Ni-based nanostructures as high-performance cathodes for rechargeable Ni−Zn battery. ChemNanoMat 2018, 4, 525–536. [Google Scholar] [CrossRef]
- Gui, Q.; Ba, D.; Li, L.; Liu, W.; Li, Y.; Liu, J. Recent advances in materials and device technologies for aqueous hybrid supercapacitors. Sci. China Mater. 2022, 65, 10–31. [Google Scholar] [CrossRef]
- Mustaqeem, M.; Naikoo, G.A.; Yarmohammadi, M.; Pedram, M.Z.; Pourfarzad, H.; Dar, R.A.; Chen, Y.F. Rational design of metal oxide based electrode materials for high performance supercapacitors–A review. J. Energy Storage 2022, 55, 105419. [Google Scholar] [CrossRef]
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage Mater. 2022, 15, e339. [Google Scholar] [CrossRef]
- Liang, G.; Zhi, C. A reversible Zn-metal battery. Nat. Nanotechnol. 2021, 16, 854–855. [Google Scholar] [CrossRef]
- Wu, D.; Yu, H.; Hou, C.; Du, W.; Song, X.; Shi, T.; Sun, X.; Wang, B. NiS nanoparticles assembled on biological cell walls-derived porous hollow carbon spheres as a novel battery-type electrode for hybrid supercapacitor. J. Mater. Sci. 2020, 55, 14431–14446. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, J.; Li, J.; Huang, W.; Hou, K.; Zhao, Y.; Guan, L. Yolk–shell NiSx@C nanosheets as K-ion battery anodes with high rate capability and ultralong cycle life. J. Mater. Chem. A 2019, 7, 18932–18939. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, L.; Wu, Z.; Guo, X.; Fang, G.; Liang, S. Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode. Chem. Commun. 2018, 54, 4457–4460. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, Z.; Meng, Y.; Wang, Y.; Yu, M.; Lu, X.; Tong, Y. Flexible ultrafast aqueous rechargeable Ni//Bi battery based on highly durable single-crystalline bismuth nanostructured anode. Adv. Mater. 2016, 28, 9188–9195. [Google Scholar] [CrossRef]
- Qi, J.Q.; Chang, Y.; He, Y.Z.; Sui, Y.W.; Wei, F.X.; Meng, Q.K.; Wei, Z.J. Effect of Zr, Mo and TiC on microstructure and high-temperature tensile strength of cast titanium matrix composites. Mater. Design 2016, 99, 421–426. [Google Scholar] [CrossRef]
- Dong, N.; Zhang, F.; Pan, H. Towards the practical application of Zn metal anodes for mild aqueous rechargeable Zn batteries. Chem. Sci. 2022, 13, 8243–8252. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.X.; Li, J.Y.; Wang, S.H.; Guo, Y.G.; Wan, L.J. Uniform lithium nucleation/growth induced by lightweight nitrogen-doped graphitic carbon foams for high-performance lithium metal anodes. Adv. Mater. 2018, 30, 1706216. [Google Scholar] [CrossRef]
- Kumar, P.; Narayan Maiti, U.; Sikdar, A.; Kumar Das, T.; Kumar, A.; Sudarsan, V. Recent advances in polymer and polymer composites for electromagnetic interference shielding: Review and future prospects. Polym. Rev. 2019, 59, 687–738. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Huang, Y.; Yang, Q.; Li, X.; Huang, Z.; Zhi, C. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 2020, 49, 180–232. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. Pseudo-Zn–Air and Zn-ion intercalation dual mechanisms to realize high-areal capacitance and long-life energy storage in aqueous Zn battery. Adv. Energy Mater. 2019, 9, 1901480. [Google Scholar] [CrossRef]
- Wang, H.; Liang, M.; Duan, D.; Shi, W.; Song, Y.; Sun, Z. Rose-like Ni3S4 as battery-type electrode for hybrid supercapacitor with excellent charge storage performance. Chem. Eng. J. 2018, 350, 523–533. [Google Scholar] [CrossRef]
- Wang, K.; Fan, X.; Chen, S.; Deng, J.; Zhang, L.; Jing, M.; Li, J.; Gou, L.; Li, D.; Ma, Y. 3D Co-doping α-Ni(OH)2 nanosheets for ultrastable, high-rate Ni-Zn battery. Small 2022, 9, 2206287. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Wang, D.; Ma, L.; Liang, G.; Huang, Z.; Dong, B.; Huang, Q.; Zhi, C. Vertically aligned Sn4+ preintercalated Ti2CTX MXene sphere with enhanced Zn ion transportation and superior cycle lifespan. Adv. Energy Mater. 2020, 10, 2001394. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Lv, H.; Xie, D.; Wang, W.; Zhi, C.; Li, H. Stabilized Co3+/Co4+ redox pair in in situ produced CoSe2−x-derived cobalt oxides for alkaline Zn batteries with 10 000-cycle lifespan and 1.9-V voltage plateau. Adv. Energy Mater. 2020, 10, 2000892. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Liang, G.; Li, H.; Liu, Z.; Tang, Z.; Liang, J.; Zhi, C. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano 2019, 13, 10643–10652. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, Q.; He, W.; Lai, Z.; Zhang, X.; Yu, M.; Tong, Y.; Lu, X. Extracting oxygen anions from ZnMn2O4: Robust cathode for flexible all-solid-state Zn-ion batteries. Energy Storage Mater. 2019, 21, 154–161. [Google Scholar] [CrossRef]
- Zhou, L.; Zeng, S.; Zheng, D.; Zeng, Y.; Wang, F.; Xu, W.; Liu, J.; Lu, X. NiMoO4 nanowires supported on Ni/C nanosheets as high-performance cathode for stable aqueous rechargeable nickel-zinc battery. Chem. Eng. J. 2020, 400, 125832. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Xie, L.; Zhu, L.; Cao, X. An enabling strategy for ultra-fast lithium storage derived from micro-flower-structured NiX (X=O, S, Se). Electrochim. Acta 2020, 343, 136138. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Lee, Y. High-performance Li–Se battery enabled via a one-piece cathode design. Adv. Energy Mater. 2020, 10, 1903477. [Google Scholar] [CrossRef]
- Yang, J.; Gao, H.; Ma, D.; Zou, J.; Lin, Z.; Kang, X.; Chen, S.J.E.A. High-performance Li-Se battery cathode based on CoSe2-porous carbon composites. Electrochim. Acta 2018, 264, 341–349. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Fu, T.; Hwang, B.-J.; Li, X.; Zhao, J. One-dimensional Cu2–xSe nanorods as the cathode material for high-performance aluminum-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 17942–17949. [Google Scholar] [CrossRef]
- Huang, D.; Tan, S.; Li, M.; Wang, D.; Han, C.; An, Q.; Mai, L. Highly efficient non-nucleophilic Mg (CF3SO3) 2-based electrolyte for high-power Mg/S battery. ACS Appl. Mater. Interfaces 2020, 12, 17474–17480. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Q.; Chou, S.L.; Dou, S.X. Advances and challenges in metal sulfides/selenides for next-generation rechargeable sodium-ion batteries. Adv. Mater. 2017, 29, 1700606. [Google Scholar] [CrossRef]
- Yin, X.; Chen, H.; Zhi, C.; Sun, W.; Lv, L.P.; Wang, Y. Functionalized graphene quantum dot modification of yolk-shell NiO microspheres for superior lithium storage. Small 2018, 14, e1800589. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.M.; Pumera, M. 2D-Pnictogens: Alloy-based anode battery materials with ultrahigh cycling stability. Chem. Soc. Rev. 2018, 47, 6964–6989. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Wang, X.; Lou, X.W.D. Hierarchical microboxes constructed by SnS nanoplates coated with nitrogen-doped carbon for efficient sodium storage. Angew. Chem. Int. Ed. 2019, 58, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Bai, X.; Zhu, J.; Han, C.; Huang, Y.; Kang, L.; Zhi, C.; Li, H. Electrochemically induced NiCoSe2@NiOOH/CoOOH heterostructures as multifunctional cathode materials for flexible hybrid zn batteries. Energy Storage Mater. 2021, 36, 427–434. [Google Scholar] [CrossRef]
- Cai, D.; Wang, Y.; Fei, B.; Chao Li, C.; Zhang, C.; Sa, B.; Chen, Q.; Zhan, H. Engineering of MoSe2 decorated Ni/Co selenide complex hollow arrayed structures with dense heterointerfaces for high-performance aqueous alkaline Zn batteries. Chem. Eng. J. 2022, 450, 138341. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Liu, H.; Feng, L. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl. Catal. B Environ. 2020, 276, 119165. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Xin, L.; Wu, M.; Sun, W.; Lou, X. Pollen-inspired synthesis of porous and hollow NiO elliptical microstructures assembled from nanosheets for high-performance electrochemical energy storage. Chem. Eng. J. 2017, 321, 546–553. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Xu, G.; Zhang, C.; Song, H.; He, Y.; Zhang, C.; Jia, D. Facile synthesis of NiS hierarchical hollow cubes via Ni formate frameworks for high performance supercapacitors. Chem. Eng. J. 2017, 320, 22–28. [Google Scholar] [CrossRef]
- Guo, K.; Yang, F.; Cui, S.; Chen, W.; Mi, L. Controlled synthesis of 3D hierarchical NiSe microspheres for high-performance supercapacitor design. RSC Adv. 2016, 6, 46523–46530. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Zhang, H.; Yang, Z.; Shi, X.; Liu, Q.; Lu, X. Enhancing Zn-ion storage capability of hydrated vanadium pentoxide by the strategic introduction of La3+. ChemSusChem 2020, 13, 1568–1574. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Cover picture: Highly stable aqueous zinc-ion storage using a layered calcium vanadium oxide bronze cathode. Angew. Chem. Int. Ed. 2018, 57, 3837. [Google Scholar] [CrossRef]
- Chen, H.; Shen, Z.; Pan, Z.; Kou, Z.; Liu, X.; Zhang, H.; Gu, Q.; Guan, C.; Wang, J. Hierarchical micro-nano sheet arrays of nickel–cobalt double hydroxides for high-rate Ni–Zn batteries. Adv. Sci. 2019, 6, 1802002. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3-x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Zeng, Y.; Han, Y.; Zhao, Y.; Zeng, Y.; Yu, M.; Liu, Y.; Tang, H.; Tong, Y.; Lu, X. Advanced Ti-doped Fe2O3@PEDOT core/shell anode for high-energy asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, K.; Chen, Y. Wide voltage aqueous asymmetric supercapacitors: Advances, strategies, and challenges. Adv. Funct. Mater. 2022, 32, 2108107. [Google Scholar] [CrossRef]
- Xu, C.; Liao, J.; Yang, C.; Wang, R.; Wu, D.; Zou, P.; Lin, Z.; Li, B.; Kang, F.; Wong, C.-P. An ultrafast, high capacity and superior longevity Ni/Zn battery constructed on nickel nanowire array film. Nano Energy 2016, 30, 900–908. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.; Ke, Q.; Li, X.; Zhang, H.; Wang, J. High-performance flexible solid-state Ni/Fe battery consisting of metal oxides coated carbon cloth/carbon nanofiber electrodes. Adv. Energy Mater. 2016, 6, 1601034. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Zhang, L.; Jiang, J.; Yan, J.; Huang, Y.; Lin, J.; Fan, H.J.; Shen, Z.X. A flexible alkaline rechargeable Ni/Fe battery based on graphene foam/carbon nanotubes hybrid film. Nano Lett. 2014, 14, 7180–7187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).