Efficient Optosensing of Hippuric Acid in the Undiluted Human Urine with Hydrophilic “Turn-On”-Type Fluorescent Hollow Molecularly Imprinted Polymer Microparticles
Abstract
1. Introduction
2. Results and Discussion
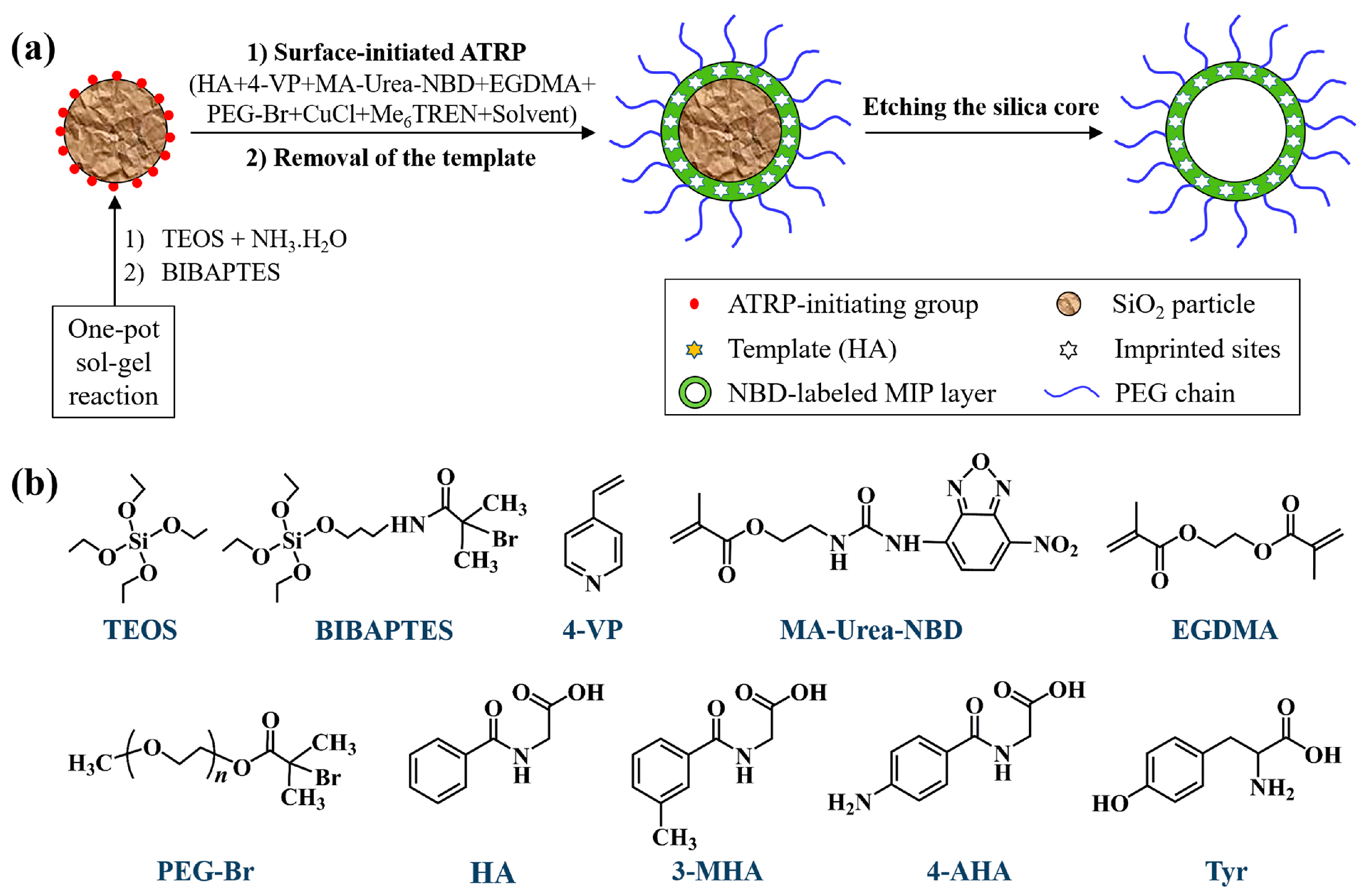
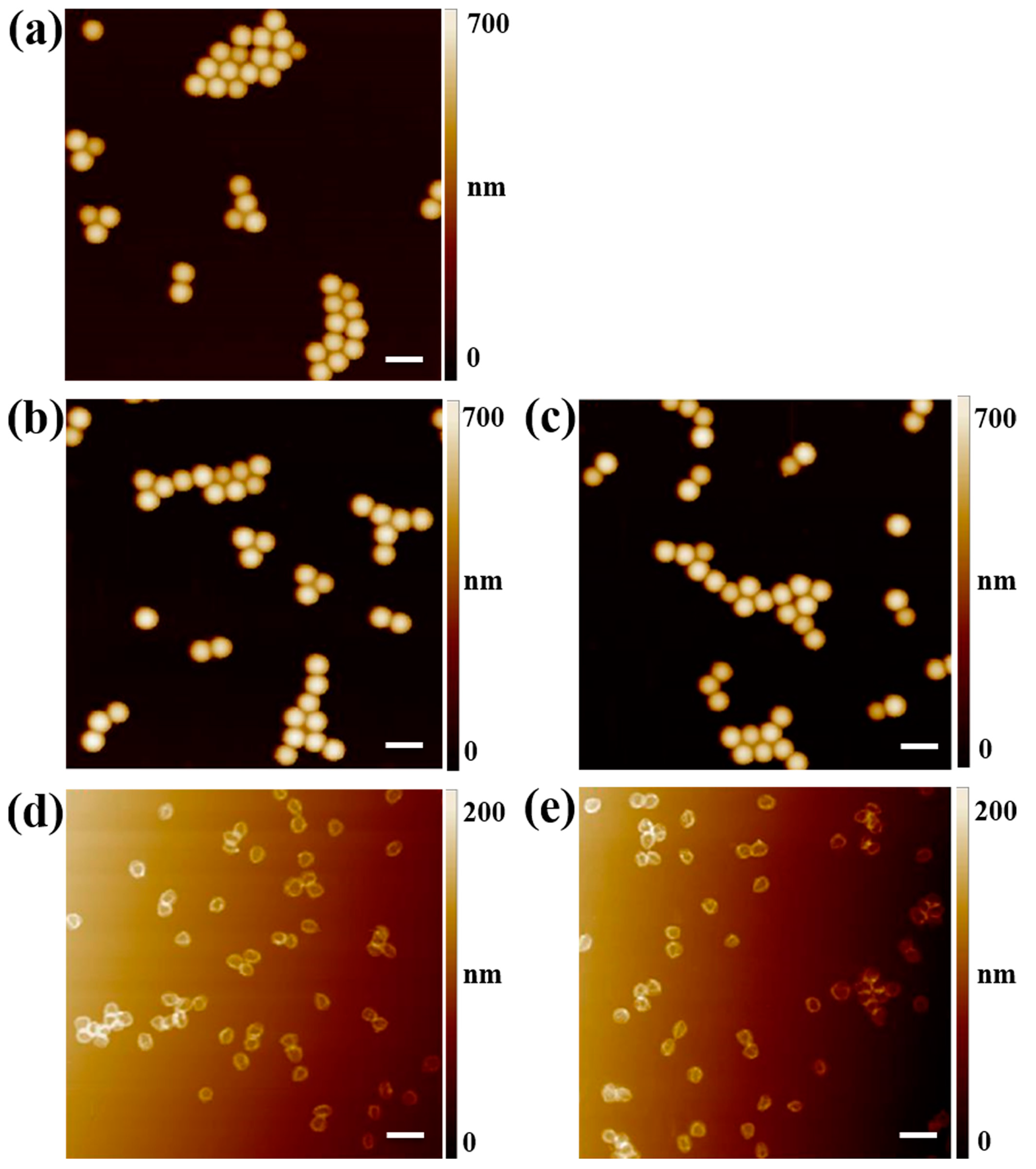
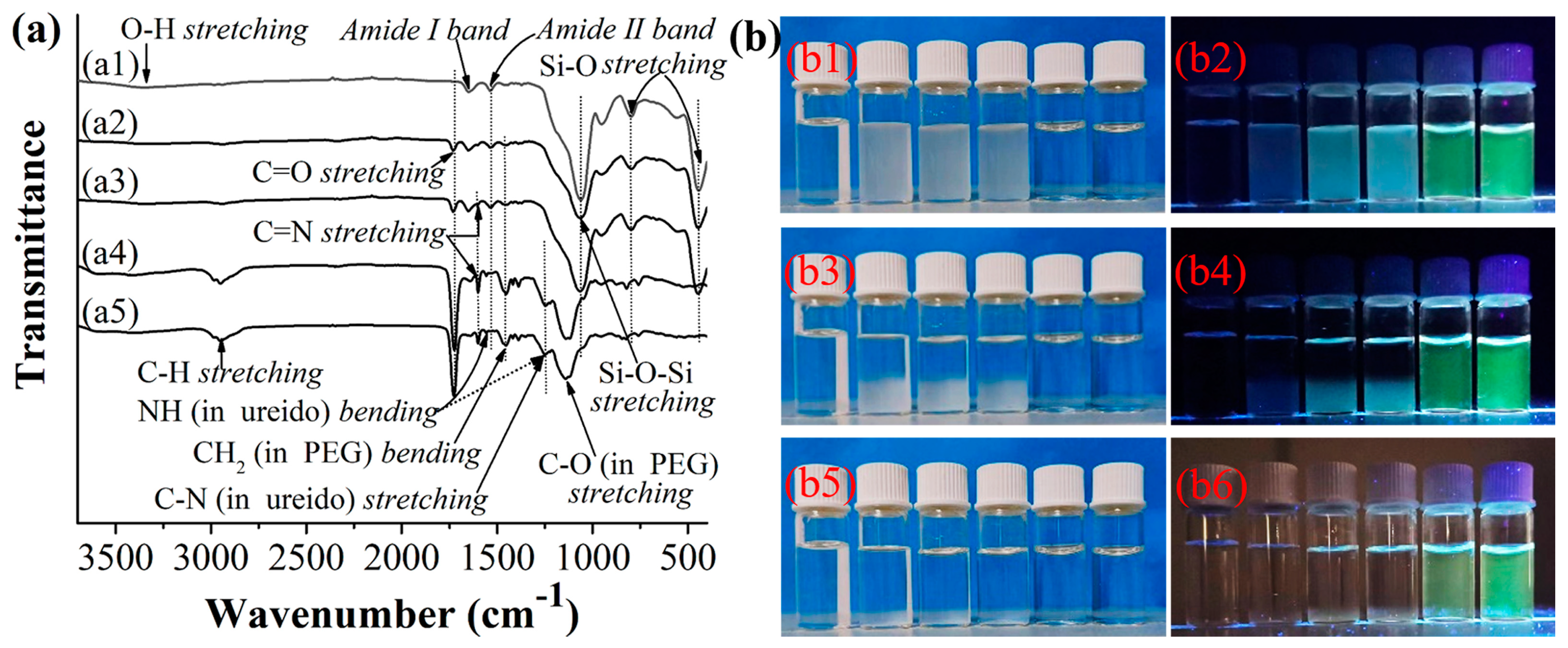
2.1. Synthesis and Characterization of the Hydrophilic Fluorescent Solid and Hollow HA-MIP/CP Microparticles
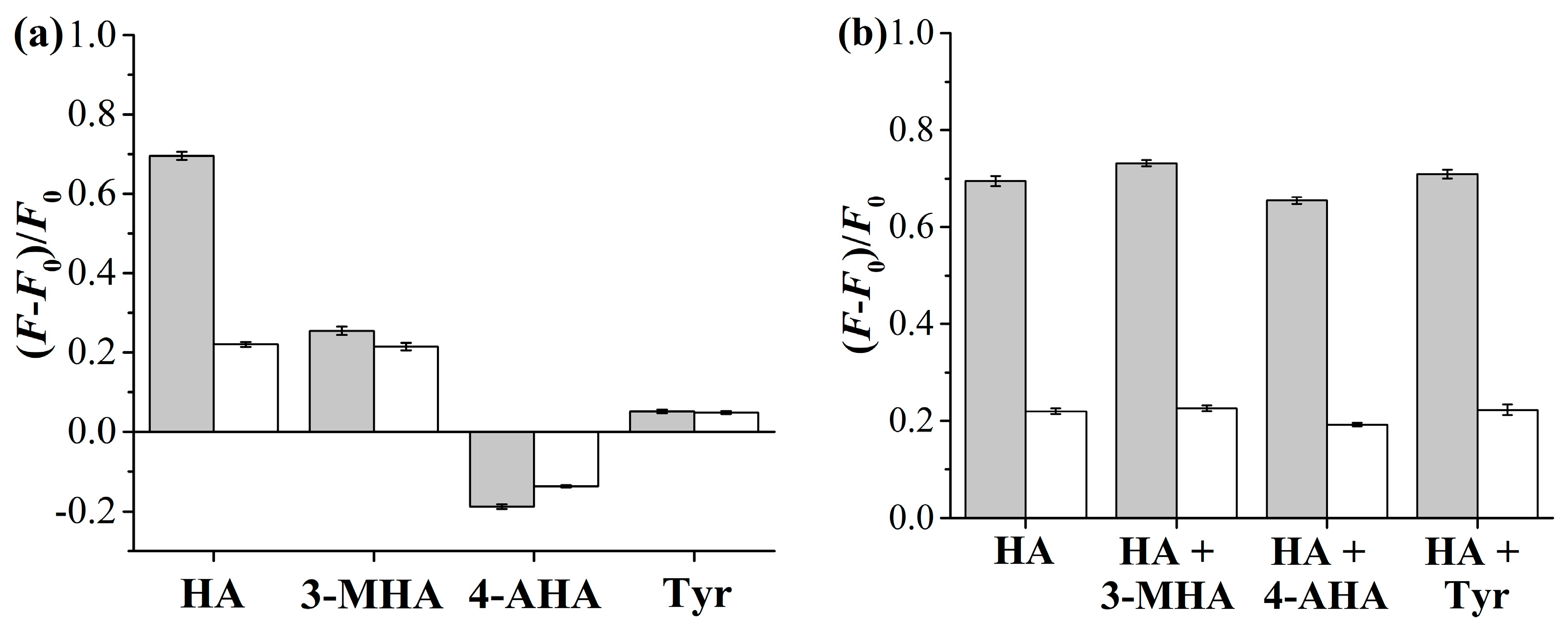
2.2. Equilibrium/Competitive Binding Properties of the Hydrophilic Fluorescent Solid and Hollow HA-MIPs/CPs in Different Media
2.3. Optosensing Properties of the Hydrophilic “Turn-On”-Type Fluorescent Solid and Hollow HA-MIP/CP Micropartilces in the Artificial Urine
2.4. Direct, Selective, Rapid, and Accurate Quantification of HA in the Undiluted Human Urine with the Hydrophilic “Turn-On”-Type Fluorescent Hollow HA-MIP
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of the Core-Shell-Corona-Structured “Turn-On”-Type Fluorescent HA-Imprinted Polymer (HA-MIP)/Control Polymer (CP) (or Non-Imprinted Polymer) Microspheres with Labeled Fluorescent Nitrobenzoxadiazole (NBD) Unit and Polyethylene Glycol (PEG) Brushes [Briefly Hydrophilic Fluorescent Solid HA-MIP/CP (i.e., SiO2@NBD-MIP@PEG and SiO2@NBD-CP@PEG, Entries 2 and 3 in Table 1)]
3.3. Preparation of the “Turn-On”-Type Fluorescent Hollow HA-MIP/CP Microparticles with PEG Brushes [Briefly Hydrophilic Fluorescent Hollow HA-MIP/CP (i.e., H@NBD-MIP@PEG and H@NBD-CP@PEG, Entries 4 and 5 in Table 1)]
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Ye, L.; Mosbach, K. Non-covalent molecular imprinting with emphasis on its application in separation and drug development. J. Mol. Recognit. 2006, 19, 248–259. [Google Scholar] [CrossRef]
- Hoshino, Y.; Shea, K.J. The evolution of plastic antibodies. J. Mater. Chem. 2011, 21, 3517–3521. [Google Scholar] [CrossRef]
- Takeuchi, T.; Sunayama, H. Beyond natural antibodies-a new generation of synthetic antibodies created by post-imprinting modification of molecularly imprinted polymers. Chem. Commun. 2018, 54, 6243–6251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Molecularly imprinted nanoparticles for biomedical applications. Adv. Mater. 2020, 32, 1806328. [Google Scholar] [CrossRef]
- Xu, S.; Wang, L.; Liu, Z. Molecularly imprinted polymer nanoparticles: An emerging versatile platform for cancer therapy. Angew. Chem. Int. Ed. 2020, 60, 3858–3869. [Google Scholar] [CrossRef] [PubMed]
- Tse Sum Bui, B.; Auroy, T.; Haupt, K. Fighting antibioticresistant bacteria: Promising strategies orchestrated by molecularly imprinted polymers. Angew. Chem. Int. Ed. 2022, 61, e202106493. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Canfarotta, F.; Whitcombe, M.J.; Piletsky, S.A. Polymeric nanoparticles for optical sensing. Biotechnol. Adv. 2013, 31, 1585–1599. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing-synthesis, characterisation and application. Sensor. Actuat. B-Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Wan, W.; Wagner, S.; Rurack, K. Fluorescent monomers: “Bricks” that make a molecularly imprinted polymer “bright”. Anal. Bioanal. Chem. 2016, 408, 1753–1771. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Wang, X.; Peng, H.; Xiong, H.; Chen, L. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron. 2018, 112, 54–71. [Google Scholar] [CrossRef]
- Zhang, H. Water-compatible fluorescent molecularly imprinted polymers. In Methods in Molecular Biology 2359: Molecularly Imprinted Polymers-Methods and Protocols; Martín-Esteban, A., Ed.; Humana Press (Springer Nature): New York, NY, USA, 2021; Chapter 8; pp. 97–108. [Google Scholar]
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar] [CrossRef]
- Niu, H.; Yang, Y.; Zhang, H. Efficient one-pot synthesis of hydrophilic and fluorescent molecularly imprinted polymer nanoparticles for direct drug quantification in real biological samples. Biosens. Bioelectron. 2015, 74, 440–446. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Niu, H.; Zhang, H. One-pot synthesis of quantum dot-labeled hydrophilic molecularly imprinted polymer nanoparticles for direct optosensing of folic acid in real, undiluted biological samples. Biosens. Bioelectron. 2016, 86, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, H.; Zhang, H. Direct and highly selective drug optosensing in real, undiluted biological samples with quantum-dot-labeled hydrophilic molecularly imprinted polymer microparticles. ACS Appl. Mater. Interfaces 2016, 8, 15741–15749. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zou, Y.; Zhang, H. Well-defined hydrophilic “turnon”-type ratiometric fluorescent molecularly imprinted polymer microspheres for direct and highly selective herbicide optosensing in the undiluted pure milks. Talanta 2020, 211, 120711. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zou, Y.; Zhou, Y.; Zhang, H. Biological samplecompatible ratiometric fluorescent molecularly imprinted polymer microspheres by RAFT coupling chemistry. Langmuir 2020, 36, 12403–12413. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Liu, X.; Zhang, H. Preparation of complex biological sample-compatible “turn-on”-type ratiometric fluorescent molecularly imprinted polymer microspheres via one-pot surface-initiated ATRP. Microchim. Acta 2022, 189, 464. [Google Scholar] [CrossRef]
- Ton, X.A.; Tse Sum Bui, B.; Resmini, M.; Bonomi, P.; Dika, I.; Soppera, O.; Haupt, K. Versatile fiber-optic fluorescence sensor based on molecularly imprinted microstructures polymerized in situ. Angew. Chem. Int. Ed. 2013, 52, 8317–8321. [Google Scholar] [CrossRef]
- Ma, Y.; Pan, G.; Zhang, Y.; Guo, X.; Zhang, H. Narrowly dispersed hydrophilic molecularly imprinted polymer nanoparticles for efficient molecular recognition in real aqueous samples including river water, milk, and bovine serum. Angew. Chem. Int. Ed. 2013, 52, 1511–1514. [Google Scholar] [CrossRef]
- Zhang, H. Water-compatible molecularly imprinted polymers: Promising synthetic substitutes for biological receptors. Polymer 2014, 55, 699–714. [Google Scholar] [CrossRef]
- Zhang, H. Water-compatible molecularly imprinted polymers. In Polymer Chemistry Series: Molecularly Imprinted Polymers for Analytical Chemistry Applications; Kutner, W., Sharma, P.S., Eds.; Royal Society of Chemistry: Croydon, UK, 2018; Chapter 10; pp. 330–358. [Google Scholar]
- Ma, Y.; Gao, J.; Zheng, C.; Zhang, H. Well-defined biological sample-compatible molecularly imprinted polymer microspheres by combining RAFT polymerization and thiol-epoxy coupling chemistry. J. Mater. Chem. B 2019, 7, 2474–2483. [Google Scholar] [CrossRef]
- Niu, M.C.; Chuong, P.H.; He, H. Core-shell nanoparticles coated with molecularly imprinted polymers: A review. Microchim. Acta 2016, 183, 2677–2695. [Google Scholar] [CrossRef]
- Wan, L.B.; Chen, Z.L.; Huang, C.X.; Shen, X.T. Core-shell molecularly imprinted particles. TRAC-Trend Anal. Chem. 2017, 95, 110–121. [Google Scholar] [CrossRef]
- Bhogal, S.; Kaur, K.; Malik, A.K.; Sonne, C.; Lee, S.S.; Kim, K.H. Core-shell structured molecularly imprinted materials for sensing applications. TRAC-Trend Anal. Chem. 2020, 133, 116043. [Google Scholar] [CrossRef]
- Bhogal, S.; Kaur, K.; Mohiuddin, I.; Kumar, S.; Lee, J.; Brown, R.J.C.; Kim, K.-H.; Malik, A.K. Hollow porous molecularly imprinted polymers as emerging adsorbents. Environ. Pollut. 2021, 288, 117775. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Zhang, Z.; Wang, Z.; Liu, B.; Gao, D.; Xie, C. Single-hole hollow polymer microspheres toward specific high-capacity uptake of target species. Adv. Mater. 2007, 19, 2370–2374. [Google Scholar] [CrossRef]
- Chen, W.; Xue, M.; Xue, F.; Mu, X.; Xu, Z.; Meng, Z.; Zhu, G.; Shea, K.J. Molecularly imprinted hollow spheres for the solid phase extraction of estrogens. Talanta 2015, 140, 68–72. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, H.; Shen, H.; Pan, J.; Dai, X.; Yan, Y.; Pan, G.; Sellergren, B. Molecularly imprinted fluorescent hollow nanoparticles as sensors for rapid and efficient detection λ-cyhalothrin in environmental water. Biosens. Bioelectron. 2016, 85, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.W. Health consequences of catabolic synthesis of hippuric acid in humans. Curr. Clin. Pharmacol. 2010, 5, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-adergani, B.; Abdel-Rehim, M. Three-phase molecularly imprinted sol–gel based hollow fiber liquid-phase microextraction combined with liquid chromatography–tandem mass spectrometry for enrichment and selective determination of a tentative lung cancer biomarker. J. Chromatogr. B. 2015, 995–996, 38–45. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Niu, H.; Zhang, H. Hydrophilic hollow molecularly imprinted polymer microparticles with photo- and thermoresponsive template binding and release properties in aqueous media. ACS Appl. Mater. Interfaces 2015, 7, 27340–27350. [Google Scholar] [CrossRef]
- Boonpangrak, S.; Whitcombe, M.J.; Prachayasittikul, V.; Mosbach, K.; Ye, L. Preparation of molecularly imprinted polymers using nitroxide-mediated living radical polymerization. Biosens. Bioelectron. 2006, 22, 349–354. [Google Scholar] [CrossRef]
- Hishiya, T.; Shibata, M.; Kakazu, M.; Asanuma, H.; Komiyama, M. Molecularly imprinted cyclodextrins as selective receptors for steroids. Macromolecules 1999, 32, 2265–2269. [Google Scholar] [CrossRef]
- Apodaca, D.C.; Pernites, R.B.; Ponnapati, R.R.; Del Mundo, F.R.; Advincula, R.C. Electro polymerized molecularly imprinted polymer films of a bis-terthiophene dendron: Folic acid quartz crystal microbalance sensing. ACS Appl. Mater. Interfaces 2011, 3, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.; Wu, X.; Fu, J.; Kang, Q.; Shen, D.; Li, J.; Chen, L. A molecular imprinting-based turn-on ratiometric fluorescence sensor for highly selective and sensitive detection of 2,4-dichlorophenoxyacetic acid (2,4-D). Biosens. Bioelectron. 2016, 81, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Inoue, O.; Seiji, K.; Suzuki, T.; Watanabe, T.; Nakatsuka, H.; Satoh, H.; Ikeda, M. Simultaneous determination of hippuric acid, o-, m-, and p-methylhippuric acid, phenylglyoxylic acid, and mandelic acid by HPLC. Bull. Environ. Contam. Toxicol. 1991, 47, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Taguchi, T. Quantitative analysis of urinary glycine conjugates by high performance liquid chromatography: Excretion of hippuric acid and methylhippuric acids in the urine of subjects exposed to vapours of toluene and xylenes. Int. Arch. Occup. Environ. Health 1986, 58, 121–129. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Z.; Yan, F.; Sun, B. Extraction of the toluene exposure biomarkers hippuric acid and methylhippuric acid using a magnetic molecularly imprinted polymer and their quantitation by LC-MS/MS. Microchim. Acta 2019, 186, 135. [Google Scholar] [CrossRef]
- Saito, T.; Takeichi, S. Simultaneous detection of hippuric acid and methylhippuric acid in urine by empore (TM) disk and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2002, 30, 365–370. [Google Scholar] [CrossRef]
- Hao, J.N.; Yan, B. Recyclable lanthanide-functionalized MOF hybrids to determine hippuric acid in urine as a biological index of toluene exposure. Chem. Commun. 2015, 51, 14509–14512. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Water compatible molecularly imprinted nanoparticles as a restricted access material for extraction of hippuric acid, a biological indicator of toluene exposure, from human urine. Microchim. Acta 2017, 184, 879–887. [Google Scholar] [CrossRef]
- Boscari1, C.N.; Mazzuia, G.R.; Wisniewski, C.; Borges, K.B.; Figueiredo, E.C. Molecularly imprinted probe for solid-phase extraction of hippuric and 4-methylhippuric acids directly from human urine samples followed by MEKC analysis. Electrophoresis 2017, 38, 1083–1090. [Google Scholar] [CrossRef]
- Moein, M.M.; El-Beqqali, A.; Javanbakht, M.; Karimi, M.; Akbari-adergani, B.; Abdel-Rehim, M. On-line detection of hippuric acid by microextraction with a molecularly-imprinted polysulfone membrane sorbent and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1372, 55–62. [Google Scholar] [CrossRef]
- Karazan, Z.M.; Roushani, M. A new method for electrochemical determination of Hippuric acid based on molecularly imprinted copolymer. Talanta 2022, 246, 123491. [Google Scholar] [CrossRef]
- Zhang, H.; Klumperman, B.; Ming, W.; Fischer, H.; van der Linde, R. Effect of Cu(II) on the kinetics of the homogeneous atom transfer radical polymerization of methyl methacrylate. Macromolecules 2001, 34, 6169–6173. [Google Scholar] [CrossRef]
- Unsal, E.; Elmas, B.; Çağlayan, B.; Tuncel, M.; Patir, S.; Tuncel, A. Preparation of an ion-exchange chromatographic support by a “grafting from” strategy based on atom transfer radical polymerization. Anal. Chem. 2006, 78, 5868–5875. [Google Scholar] [CrossRef]
- Ciampolini, M.; Nardi, N. Five-coordinated high-spin complexes of bivalent cobalt, nickel, and copper with tris(2-dimethylaminoethyl)amine. Inorg. Chem. 1966, 5, 41–44. [Google Scholar] [CrossRef]
- Wan, W.; Biyilal, M.; Wagner, R.; Sellergren, B.; Rurack, K. Fluorescent sensory microparticles that “light-up” consisting of a silica core and a molecularly imprinted polymer (MIP) shell. Angew. Chem. Int. Ed. 2013, 52, 7023–7027. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Thongboonkerd, V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010, 402, 110–112. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, Y.; Jiao, Y.; Zhang, H. Narrow or monodisperse, physically cross-linked, and “living” spherical polymer particles by one-stage RAFT precipitation polymerization. Macromolecules 2019, 52, 143–156. [Google Scholar] [CrossRef]


| Entry | Sample | ΔW (%) a | Dn,AFM (nm) b | U b | Dn,DLS (nm) c | PDI c | Contact Angle (°) d |
|---|---|---|---|---|---|---|---|
| 1 | SiO2-Br | - | 496 | 1.004 | 529 | 0.112 | 78.2 ± 2.6 |
| 2 | SiO2@NBD-MIP@PEG | 18.4 | 525 | 1.005 | 573 | 0.134 | 64.6 ± 2.7 |
| 3 | SiO2@NBD-CP@PEG | 17.5 | 523 | 1.010 | 570 | 0.115 | 64.9 ± 2.1 |
| 4 | H@NBD-MIP@PEG | 83.4 | - | - | 568 | 0.129 | - |
| 5 | H@NBD-CP@PEG | 83.8 | - | - | 566 | 0.158 | - |
| Entry | Analyte(s) | Spiked Analyte(s) (μM) | Detected by MIP Optosensor HA (μM) | Optosensing Recovery ± RSD (%) (n = 3) b | Detected by HPLC HA (μM) | HPLC Recovery ± RSD (%) (n = 3) b,c |
|---|---|---|---|---|---|---|
| 1 | HA | 0 (Blank urine) | 1.53 | - | 1.56 | - |
| 2 | HA | 0.5 | 2.01 ± 0.02 | 96.0 ± 4.0 | 2.08 ± 0.02 | 103.7 ± 4.4 |
| 3 | HA | 5 | 6.52 ± 0.06 | 99.8 ± 1.2 | 6.61 ± 0.09 | 101.0 ± 1.8 |
| 4 | HA | 10 | 11.64 ± 0.06 | 101.1 ± 0.6 | 11.50 ± 0.15 | 99.4 ± 1.5 |
| 5 | HA + 3-MHA + 4-AHA + Tyr | 0.5 HA + 0.5(3-MHA) + 0.5(4-AHA) + 0.5 Tyr | 2.02 ± 0.01 | 98.0 ± 2.0 | 2.07 ± 0.02 | 102.3 ± 4.1 |
| 6 | HA + 3-MHA + 4-AHA + Tyr | 5 HA + 5(3-MHA) + 5(4-AHA) + 5 Tyr | 6.63 ± 0.08 | 102.0 ± 1.6 | 6.74 ± 0.07 | 103.5 ± 1.5 |
| 7 | HA + 3-MHA + 4-AHA + Tyr | 10 HA + 10(3-MHA) + 10(4-AHA) + 10 Tyr | 11.69 ± 0.09 | 101.6 ± 0.9 | 11.62 ± 0.23 | 100.6 ± 2.3 |
| Analytical Method a | Sample | Linear Range | LOD | Recovery (%) | RSD (%) | Ref. |
|---|---|---|---|---|---|---|
| Solid-phase extraction (SPE)/HPLC-UV | Human urine (filtered through Whatman paper No. 42) | 0.3–7500 μg/L (0.0017–41.86 μM) | 0.15 μg/L (0.84 nM) | 88.0–104.0 | <6.1 | [44] |
| SPE/LC-MS/MS | Human urine (filtered through a 0.22 μm PTFE membrane) | 0.5–10,000 μg/L (0.0028–55.81 µM) | 89 ng/L (0.50 nM) | 91.4–109.1 | 6.4–9.6 (intra-day) 9.2–11.5 (inter-day) | [41] |
| SPE/micellar electrokinetic chromatography (MEKC) | Human urine (without pretreatment) | 0.5–5.0 g/L (2.79–27.91 mM) | 0.15 g/L (0.84 mM) | - | <16 | [45] |
| Micro-extraction by packed sorbent (MEPS)/LC-MS/MS | Plasma and urine (pretreated to remove proteins with acetonitrile) | 1–1000 nM | 0.3 nM | 91–96 | 1.1–7.1 | [46] |
| Hollow fiber based liquid-phase microextraction/ LC-MS/MS | Human plasma and urine [pretreated to remove proteins with 25 mM ammonium acetate (pH 5.0)] | 1–2000 nM | 0.3 nM | 97–104 | 1.2–4.1 | [33] |
| Electrochemical sensing | Human serum (pretreated to remove proteins with methanol) and diluted human urine | 0.05–40 nM and 40–500 nM | 0.012 nM | 96.0–105.0 | 1.2–3.2 | [47] |
| Direct fluorescent optosensing | Human urine (without any pretreatment) | 0–20 µM | 0.097 µM | 96.0–102.0 | 0.6–4.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, Q.; Zhang, H. Efficient Optosensing of Hippuric Acid in the Undiluted Human Urine with Hydrophilic “Turn-On”-Type Fluorescent Hollow Molecularly Imprinted Polymer Microparticles. Molecules 2023, 28, 1077. https://doi.org/10.3390/molecules28031077
Zhang W, Li Q, Zhang H. Efficient Optosensing of Hippuric Acid in the Undiluted Human Urine with Hydrophilic “Turn-On”-Type Fluorescent Hollow Molecularly Imprinted Polymer Microparticles. Molecules. 2023; 28(3):1077. https://doi.org/10.3390/molecules28031077
Chicago/Turabian StyleZhang, Wanlan, Qun Li, and Huiqi Zhang. 2023. "Efficient Optosensing of Hippuric Acid in the Undiluted Human Urine with Hydrophilic “Turn-On”-Type Fluorescent Hollow Molecularly Imprinted Polymer Microparticles" Molecules 28, no. 3: 1077. https://doi.org/10.3390/molecules28031077
APA StyleZhang, W., Li, Q., & Zhang, H. (2023). Efficient Optosensing of Hippuric Acid in the Undiluted Human Urine with Hydrophilic “Turn-On”-Type Fluorescent Hollow Molecularly Imprinted Polymer Microparticles. Molecules, 28(3), 1077. https://doi.org/10.3390/molecules28031077










