Recent Progress on Green New Phase Extraction and Preparation of Polyphenols in Edible Oil
Abstract
:1. Introduction
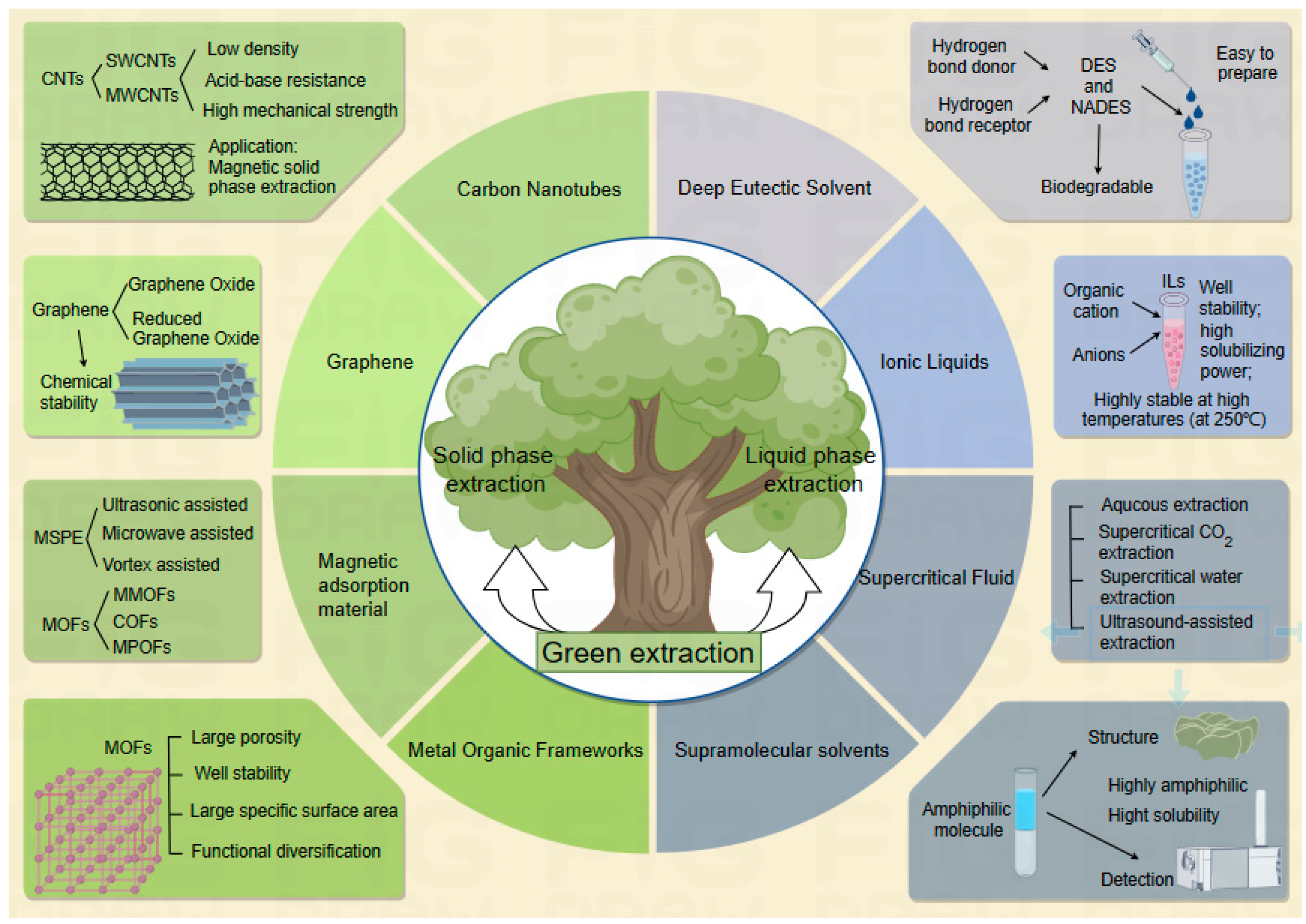
2. Research Progress on Green Extraction Techniques
2.1. Introduction to Green Extraction
2.2. Application of Green Extraction Technology
3. Application of Novel Materials in Solid-Phase Extraction
3.1. Carbon Nanotubes
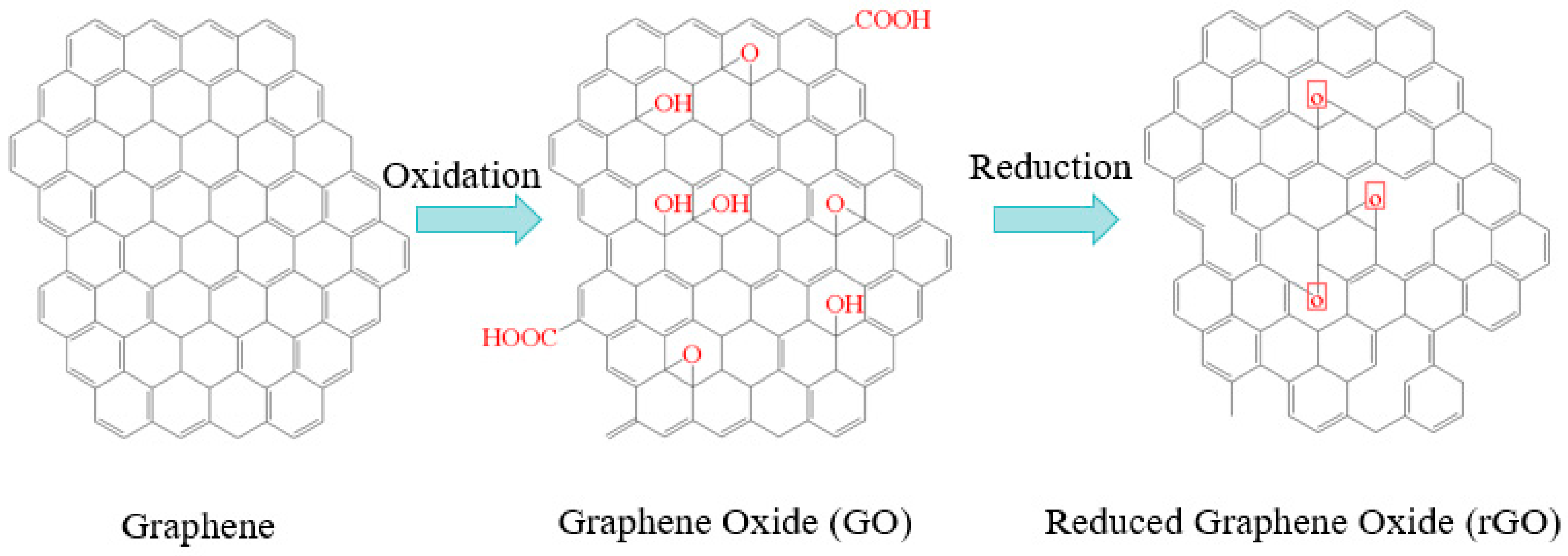
3.2. Graphene
3.3. Metal–Organic Frameworks
4. Application of Green Chemical Materials in Liquid-Phase Extraction
4.1. Deep Eutectic Solvent
4.2. Ionic Liquids
4.3. Supercritical Fluid Extraction
4.4. Supramolecular Solvents
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeb, A. A comprehensive review on different classes of polyphenolic compounds present in edible oils. Food Res. Int. 2021, 143, 110312. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. Adv. Food Nutr. Res. 2019, 90, 1–81. [Google Scholar] [PubMed]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Sebaaly, J.; Kelley, D. Direct Oral Anticoagulants in Obesity: An Updated Literature Review. Ann. Pharmacother. 2020, 54, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Ali Redha, A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Chang, S.H. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery-a review. Environ. Sci. Pollut. Res. Int. 2020, 27, 32371–32388. [Google Scholar] [CrossRef]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Han, X.; Chen, J.; Qiu, H.; Shi, Y.P. Solid/liquid phase microextraction of five bisphenol-type endocrine disrupting chemicals by using a hollow fiber reinforced with graphene oxide nanoribbons, and determination by HPLC-PDA. Mikrochim. Acta 2019, 186, 375. [Google Scholar] [CrossRef]
- Loussala, H.M.; Feng, J.; Han, S.; Sun, M.; Ji, X.; Li, C.; Fan, J.; Pei, M. Carbon nanotubes functionalized mesoporous silica for in-tube solid-phase microextraction of polycyclic aromatic hydrocarbons. J. Sep. Sci. 2020, 43, 3275–3284. [Google Scholar] [CrossRef]
- Pastor-Belda, M.; Navarro-Jiménez, T.; Garrido, I.; Viñas, P.; Campillo, N.; Fenoll, J.; Hernández-Córdoba, M. Magnetic solid-phase extraction or dispersive liquid-liquid microextraction for pyrethroid determination in environmental samples. J. Sep. Sci. 2018, 41, 2565–2575. [Google Scholar] [CrossRef]
- Pang, J.; Liao, Y.; Huang, X.; Ye, Z.; Yuan, D. Metal-organic framework-monolith composite-based in-tube solid phase microextraction on-line coupled to high-performance liquid chromatography-fluorescence detection for the highly sensitive monitoring of fluoroquinolones in water and food samples. Talanta 2019, 199, 499–506. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Deep eutectic solvent-based dispersive liquid-liquid micro-extraction of pesticides in food samples. Food Chem. 2021, 342, 127943. [Google Scholar] [CrossRef]
- Han, D.; Tang, B.; Lee, Y.R.; Row, K.H. Application of ionic liquid in liquid phase microextraction technology. J. Sep. Sci. 2012, 35, 2949–2961. [Google Scholar] [CrossRef]
- Han, W.C.; Shi, N.; Wang, X.Y.; Wang, Z.H.; Wang, K.L.; Gao, M.; Yu, L.; Chen, D.; Xu, X. Application of natural cotton fibers as an extraction sorbent for the detection of trans-resveratrol in adulterated peanut oils. Food Chem. 2021, 339, 127885. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Q.; Cao, L.; Li, S.; Zeng, X.; Zhou, W.; Zhang, C. Synthesis and Application of Porous Carbon Nanomaterials from Pomelo Peels: A Review. Molecules 2023, 28, 4429. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Di, X.; Wang, X.; Liu, Y.; Guo, X.; Di, X. Solid-phase extraction coupled with switchable hydrophilicity solvent-based homogeneous liquid-liquid microextraction for chloramphenicol enrichment in environmental water samples: A novel alternative to classical extraction techniques. Anal. Bioanal. Chem. 2019, 411, 803–812. [Google Scholar] [CrossRef]
- Sajid, M.; Khaled Nazal, M.; Rutkowska, M.; Szczepańska, N.; Namieśnik, J.; Płotka-Wasylka, J. Solid Phase Microextraction: Apparatus, Sorbent Materials, and Application. Crit. Rev. Anal. Chem. 2019, 49, 271–288. [Google Scholar] [CrossRef]
- Zacharis, C.K.; Tzanavaras, P.D. Solid-Phase Microextraction. Molecules 2020, 25, 379. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Applications of Filled Single-Walled Carbon Nanotubes: Progress, Challenges, and Perspectives. Nanomaterials 2021, 11, 2863. [Google Scholar] [CrossRef]
- Spangler, E.J.; Olinger, A.D.; Kumar, P.B.S.; Laradji, M. Binding, unbinding and aggregation of crescent-shaped nanoparticles on nanoscale tubular membranes. Soft Matter 2021, 17, 1016–1027. [Google Scholar] [CrossRef]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An Overview on Recent Progress of Metal Oxide/Graphene/CNTs-Based Nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef]
- Ortiz-Morales, A.; Ortiz-López, J.; Leal-Acevedo, B.; Gómez-Aguilar, R. Thermoluminescence of single wall carbon nanotubes synthesized by hydrogen-arc-discharge method. Appl. Radiat. Isot. 2019, 145, 32–38. [Google Scholar] [CrossRef]
- Pérez Del Pino, Á.; Rodríguez López, M.; Ramadan, M.A.; García Lebière, P.; Logofatu, C.; Martínez-Rovira, I.; Yousef, I.; György, E. Enhancement of the supercapacitive properties of laser deposited graphene-based electrodes through carbon nanotube loading and nitrogen doping. Phys. Chem. Chem. Phys. 2019, 21, 25175–25186. [Google Scholar] [CrossRef]
- Wani, T.U.; Mohi-Ud-Din, R.; Wani, T.A.; Mir, R.H.; Itoo, A.M.; Sheikh, F.A.; Khan, N.A.; Pottoo, F.H. Green Synthesis, Spectroscopic Characterization and Biomedical Applications of Carbon Nanotubes. Curr. Pharm. Biotechnol. 2021, 22, 793–807. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Nag, A.; Alahi, M.E.E.; Mukhopadhyay, S.C.; Liu, Z. Multi-Walled Carbon Nanotubes-Based Sensors for Strain Sensing Applications. Sensors 2021, 21, 1261. [Google Scholar] [CrossRef]
- Arrigo, R.; Malucelli, G. Rheological Behavior of Polymer/Carbon Nanotube Composites: An Overview. Materials 2020, 13, 2771. [Google Scholar] [CrossRef]
- Ma, F.; Li, P.; Zhang, Q.; Yu, L.; Zhang, L. Rapid determination of trans-resveratrol in vegetable oils using magnetic hydrophilic multi-walled carbon nanotubes as adsorbents followed by liquid chromatography-tandem mass spectrometry. Food Chem. 2015, 178, 259–266. [Google Scholar] [CrossRef]
- Wu, R.; Ma, F.; Zhang, L.; Li, P.; Li, G.; Zhang, Q.; Zhang, W.; Wang, X. Simultaneous determination of phenolic compounds in sesame oil using LC–MS/MS combined with magnetic carboxylated multi-walled carbon nanotubes. Food Chem. 2016, 204, 334–342. [Google Scholar] [CrossRef]
- Zhao, Q.; Cheng, D.-Q.; Tao, M.; Ning, W.-J.; Yang, Y.-J.; Meng, K.-Y.; Mei, Y.; Feng, Y.-Q. Rapid magnetic solid-phase extraction based on alendronate sodium grafted mesoporous magnetic nanoparticle for the determination of trans-resveratrol in peanut oils. Food Chem. 2019, 279, 187–193. [Google Scholar] [CrossRef]
- Badjah Hadj Ahmed, A.Y.; Obbed, M.S.; Wabaidur, S.M.; AlOthman, Z.A.; Al-Shaalan, N.H. High-performance liquid chromatography analysis of phenolic acid, flavonoid, and phenol contents in various natural Yemeni honeys using multi-walled carbon nanotubes as a solid-phase extraction adsorbent. J. Agric. Food Chem. 2014, 62, 5443–5450. [Google Scholar] [CrossRef]
- De Luca, P.; Macario, A.; Siciliano, C.; Janos, B.N. Recovery of Biophenols from Olive Vegetation Waters by Carbon Nanotubes. Materials 2022, 15, 2893. [Google Scholar] [CrossRef]
- Sánchez-Arévalo, C.M.; Olmo-García, L.; Fernández-Sánchez, J.F.; Carrasco-Pancorbo, A. Polycyclic aromatic hydrocarbons in edible oils: An overview on sample preparation, determination strategies, and relative abundance of prevalent compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3528–3573. [Google Scholar] [CrossRef]
- Yao, L.; Chen, A.; Li, L.; Liu, Y. Preparation, properties, applications and outlook of graphene-based materials in biomedical field: A comprehensive review. J. Biomater. Sci. 2023, 34, 1121–1156. [Google Scholar] [CrossRef]
- Raslan, A.; Saenz Del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Hui, K.H.; Ambrosi, A.; Pumera, M.; Bonanni, A. Improving the Analytical Performance of Graphene Oxide towards the Assessment of Polyphenols. Chemistry 2016, 22, 3830–3834. [Google Scholar] [CrossRef]
- Şahin, S.; Ciğeroğlu, Z.; Özdemir, O.K.; Bilgin, M.; Elhussein, E.; Gülmez, Ö. Recovery of hydroxytyrosol onto graphene oxide nanosheets: Equilibrium and kinetic models. J. Mol. Liq. 2019, 285, 213–222. [Google Scholar] [CrossRef]
- Şahin, S.; Abdelillah Ali Elhussein, E.; Abdel Salam, M.; Bayazit, Ş.S. Recovery of polyphenols from water using Zr-based metal-organic frameworks and their nanocomposites with graphene nanoplatelets. J. Ind. Eng. Chem. 2019, 78, 164–171. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S. An efficient ZrO2/Co3O4/reduced graphene oxide nanocomposite electrochemical sensor for simultaneous determination of gallic acid, caffeic acid and protocatechuic acid natural antioxidants. Electrochim. Acta 2016, 211, 273–288. [Google Scholar] [CrossRef]
- Henna, T.K.; Pramod, K. Graphene quantum dots redefine nanobiomedicine. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110651. [Google Scholar] [CrossRef]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene Quantum Dots for Optical Bioimaging. Small 2019, 15, e1902136. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, Applications, and Prospects of Graphene Quantum Dots: A Comprehensive Review. Small 2022, 18, e2102683. [Google Scholar] [CrossRef]
- Benítez-Martínez, S.; Valcárcel, M. Graphene quantum dots as sensor for phenols in olive oil. Sens. Actuators B Chem. 2014, 197, 350–357. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Pan, M.; Xie, X.; Liu, K.; Hong, L.; Wang, S. Synthesis of Magnetic Metal-Organic Frame Material and Its Application in Food Sample Preparation. Foods 2020, 9, 1610. [Google Scholar] [CrossRef]
- Firooz, S.K.; Armstrong, D.W. Metal-organic frameworks in separations: A review. Anal. Chim. Acta 2022, 1234, 340208. [Google Scholar] [CrossRef]
- Huang, C.; Liu, C.; Chen, X.; Xue, Z.; Liu, K.; Qiao, X.; Li, X.; Lu, Z.; Zhang, L.; Lin, Z.; et al. A Metal-Organic Framework Nanosheet-Assembled Frame Film with High Permeability and Stability. Adv. Sci. 2020, 7, 1903180. [Google Scholar] [CrossRef]
- Jia, C.; Bueken, B.; Cirujano, F.G.; Van Geem, K.M.; De Vos, D. Phenolics isolation from bio-oil using the metal-organic framework MIL-53(Al) as a highly selective adsorbent. Chem. Commun. 2019, 55, 6245–6248. [Google Scholar] [CrossRef]
- Ma, F.; Cai, X.; Mao, J.; Yu, L.; Li, P. Adsorptive removal of aflatoxin B1 from vegetable oils via novel adsorbents derived from a metal-organic framework. J. Hazard. Mater. 2021, 412, 125170. [Google Scholar] [CrossRef]
- Terzopoulou, A.; Wang, X.; Chen, X.Z.; Palacios-Corella, M.; Pujante, C.; Herrero-Martín, J.; Qin, X.H.; Sort, J.; deMello, A.J.; Nelson, B.J.; et al. Biodegradable Metal-Organic Framework-Based Microrobots (MOFBOTs). Adv. Healthc. Mater. 2020, 9, e2001031. [Google Scholar] [CrossRef]
- Lv, D.; Nong, W.; Guan, Y. Edible ligand-metal-organic frameworks: Synthesis, structures, properties and applications. Coord. Chem. Rev. 2022, 450, 214234. [Google Scholar] [CrossRef]
- Kang, W.; Lin, H.; Jiang, R.; Yan, Y.; Ahmad, W.; Ouyang, Q.; Chen, Q. Emerging applications of nano-optical sensors combined with near-infrared spectroscopy for detecting tea extract fermentation aroma under ultrasound-assisted sonication. Ultrason. Sonochem. 2022, 88, 106095. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, T.; Hao, L.; Guo, Y.; Liu, W.; Guo, L.; Wang, C.; Wang, Z.; Wu, Q. Advances in magnetic porous organic frameworks for analysis and adsorption applications. TrAC Trends Anal. Chem. 2020, 132, 116048. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Granados, M.; Sentellas, S.; Saurina, J. Microwave-assisted extraction with natural deep eutectic solvents for polyphenol recovery from agrifood waste: Mature for scaling-up? Sci. Total Environ. 2023, 912, 168716. [Google Scholar] [CrossRef]
- Fernandez, E.; Vidal, L.; Canals, A. Rapid determination of hydrophilic phenols in olive oil by vortex-assisted reversed-phase dispersive liquid-liquid microextraction and screen-printed carbon electrodes. Talanta 2018, 181, 44–51. [Google Scholar] [CrossRef]
- Ranjha, M.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Bettaieb Rebey, I.; Dakhlaoui, S.; Msaada, K.; Saidani Tounsi, M.; Ksouri, R.; Fauconnier, M.L.; Hamrouni-Sellami, I. Green extraction of oil from Carum carvi seeds using bio-based solvent and supercritical fluid: Evaluation of its antioxidant and anti-inflammatory activities. Phytochem. Anal. 2020, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.A.; Barreto, J.A.; Santos, L.B.; de Assis, R.D.S.; Novaes, C.G.; Cassella, R.J. In-syringe dispersive liquid-liquid microextraction. Talanta 2022, 238, 123002. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, N.; Cao, W.; Hong, Z. Advances in dispersive liquid-liquid microextraction and its application to analysis of biological samples. Se Pu 2020, 38, 491–501. [Google Scholar] [PubMed]
- Thomas, P.A.; Marvey, B.B. Room Temperature Ionic Liquids as Green Solvent Alternatives in the Metathesis of Oleochemical Feedstocks. Molecules 2016, 21, 184. [Google Scholar] [CrossRef]
- Zhao, Z.; Ji, Y.; Liu, X.; Zhao, L. Progress in the application of deep eutectic solvents to extraction and separation technology. Se Pu 2021, 39, 152–161. [Google Scholar]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents. Molecules 2020, 25, 1077. [Google Scholar] [CrossRef]
- Şahin, S. Tailor-designed deep eutectic liquids as a sustainable extraction media: An alternative to ionic liquids. J. Pharm. Biomed. Anal. 2019, 174, 324–329. [Google Scholar] [CrossRef]
- Sperry, J.; García-Álvarez, J. Special Issue: “Organic Reactions in Green Solvents”. Molecules 2016, 21, 1527. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Volodina, N.; Gagarionova, S.; Shilovskikh, V.; Bulatov, A. A rotating disk sorptive extraction based on hydrophilic deep eutectic solvent formation. Anal. Chim. Acta 2021, 1141, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Carmona, I.; Aguirre, I.; Griffith, D.M.; García-Borrego, A. Towards a circular economy in virgin olive oil production: Valorization of the olive mill waste (OMW) “alpeorujo” through polyphenol recovery with natural deep eutectic solvents (NADESs) and vermicomposting. Sci. Total Environ. 2023, 872, 162198. [Google Scholar] [CrossRef]
- Rodríguez-Juan, E.; Rodríguez-Romero, C.; Fernández-Bolaños, J.; Florido, M.C.; Garcia-Borrego, A. Phenolic compounds from virgin olive oil obtained by natural deep eutectic solvent (NADES): Effect of the extraction and recovery conditions. J. Food Sci. Technol. 2021, 58, 552–561. [Google Scholar] [CrossRef]
- Fanali, C.; Posta, S.D.; Dugo, L.; Russo, M.; Gentili, A.; Mondello, L.; De Gara, L. Application of deep eutectic solvents for the extraction of phenolic compounds from extra-virgin olive oil. Electrophoresis 2020, 41, 1752–1759. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Squeo, G.; Pasqualone, A.; Caponio, F.; Summo, C. An easy and green tool for olive oils labelling according to the contents of hydroxytyrosol and tyrosol derivatives: Extraction with a natural deep eutectic solvent and direct spectrophotometric analysis. Food Chem. 2019, 291, 1–6. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Extraction of phenolic compounds from extra virgin olive oil by a natural deep eutectic solvent: Data on UV absorption of the extracts. Data Brief 2016, 8, 553–556. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197 Pt A, 554–561. [Google Scholar] [CrossRef]
- Flieger, J.; Feder-Kubis, J.; Tatarczak-Michalewska, M. Chiral Ionic Liquids: Structural Diversity, Properties and Applications in Selected Separation Techniques. Int. J. Mol. Sci. 2020, 21, 4253. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Kumar, A.; Misra, N. Superhalogens as Building Blocks of Ionic Liquids. J. Phys. Chem. A 2021, 125, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Recent advances on ionic liquid uses in separation techniques. J. Chromatogr. A 2018, 1559, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Zandu, S.K.; Chopra, H.; Singh, I. Ionic Liquids for Therapeutic and Drug Delivery Applications. Curr. Drug Res. Rev. 2020, 12, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.L.C.; Sousa, E.; Saraiva, M. Immobilized imidazolium-based ionic liquids in C18 for solid-phase extraction. Analyst 2020, 145, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, Y.; Cao, J.; Li, M.; Yang, C.; Yan, H. Imidazolium ionic-liquid-modified phenolic resin for solid-phase extraction of thidiazuron and forchlorfenuron from cucumbers. J. Chromatogr. A 2020, 1623, 461192. [Google Scholar] [CrossRef]
- Boon, Y.H.; Mohamad Zain, N.N.; Mohamad, S.; Osman, H.; Raoov, M. Magnetic poly(β-cyclodextrin-ionic liquid) nanocomposites for micro-solid phase extraction of selected polycyclic aromatic hydrocarbons in rice samples prior to GC-FID analysis. Food Chem. 2019, 278, 322–332. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L. Research progress in application of immobilized ionic liquid materials to separation by solid-phase extraction. Se Pu 2021, 39, 241–259. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, W.; Zhang, T.; Yao, S. Systematic investigation for extraction and separation of polyphenols in tea leaves by magnetic ionic liquids. J. Sci. Food Agric. 2018, 98, 4550–4560. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.; Su, A.; Zhang, H. Dispersive liquid-liquid microextraction of phenolic compounds from vegetable oils using a magnetic ionic liquid. J. Sep. Sci. 2017, 40, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, H.; Zhang, Z.H.; Wu, X.L.; Chen, W.G.; Zhu, Y.; Fang, C.F.; Zhao, Y.G. Three-dimensional ionic liquid functionalized magnetic graphene oxide nanocomposite for the magnetic dispersive solid phase extraction of 16 polycyclic aromatic hydrocarbons in vegetable oils. J. Chromatogr. A 2017, 1489, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Ayub, H.; Sehrish, A.; Ambreen, S.; Khan, F.A.; Itrat, N.; Nazir, A.; Shoukat, A.; Shoukat, A.; Ejaz, A.; et al. Essential Components from Plant Source Oils: A Review on Extraction, Detection, Identification, and Quantification. Molecules 2023, 28, 6881. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Du, M.; Luo, F.; Jin, Y. Physicochemical Properties and Lipid Composition of Camellia Seed Oil (Camellia oleifera Abel.) Extracted Using Different Methods. Food Sci. Technol. Res. 2015, 21, 779–785. [Google Scholar] [CrossRef]
- Koubaa, M.; Mhemdi, H.; Barba, F.J.; Angelotti, A.; Bouaziz, F.; Chaabouni, S.E.; Vorobiev, E. Seed oil extraction from red prickly pear using hexane and supercritical CO2: Assessment of phenolic compound composition, antioxidant and antibacterial activities. J. Sci. Food Agric. 2017, 97, 613–620. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Smith, S.A.; Chen, M.; Liu, H.; Zhang, J.; Tang, L.; Li, J.; Liu, Q.; Wu, X. Optimization of Supercritical CO2 Extraction of Moringa oleifera Seed Oil Using Response Surface Methodological Approach and Its Antioxidant Activity. Front. Nutr. 2021, 8, 829146. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, H.; Ma, T. Optimization of Supercritical Fluid CO2 Extraction from Yellow Horn Seed and Its Anti-Fatigue and Antioxidant Activity. Molecules 2023, 28, 4853. [Google Scholar] [CrossRef] [PubMed]
- Buelvas-Puello, L.M.; Franco-Arnedo, G.; Martínez-Correa, H.A.; Ballesteros-Vivas, D.; Sánchez-Camargo, A.D.P.; Miranda-Lasprilla, D.; Narváez-Cuenca, C.E.; Parada-Alfonso, F. Supercritical Fluid Extraction of Phenolic Compounds from Mango (Mangifera indica L.) Seed Kernels and Their Application as an Antioxidant in an Edible Oil. Molecules 2021, 26, 7516. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Han, Q.; Yang, Y.; Wang, H.; Wang, S.; Li, G. Characterization and Biological Activities of Seed Oil Extracted from Berberis dasystachya Maxim. by the Supercritical Carbon Dioxide Extraction Method. Molecules 2020, 25, 1836. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, P.; Wang, S.; Ruan, Y.; Zhong, W.; Hu, C.; He, D. Comparison of Different Extraction Processes on the Physicochemical Properties, Nutritional Components and Antioxidant Ability of Xanthoceras sorbifolia Bunge Kernel Oil. Molecules 2022, 27, 4185. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhou, L.; Kong, F.; Wang, S.; Hong, K.; Lei, F.; He, D. Effects of Extraction Strategies on Yield, Physicochemical and Antioxidant Properties of Pumpkin Seed Oil. Foods 2023, 12, 3351. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Gómez, A.; Serrano-Crespín, A.; Rubio, S. Supramolecular-solvent based extraction of hydroxytyrosol from brines of the processing of table olives. Sep. Purif. Technol. 2023, 322, 124351. [Google Scholar] [CrossRef]
- Carasek, E.; Bernardi, G.; Morelli, D.; Merib, J. Sustainable green solvents for microextraction techniques: Recent developments and applications. J. Chromatogr. A 2021, 1640, 461944. [Google Scholar] [CrossRef]
- Keddar, M.N.; Ballesteros-Gómez, A.; Amiali, M.; Siles, J.A.; Zerrouki, D.; Martín, M.A.; Rubio, S. Efficient extraction of hydrophilic and lipophilic antioxidants from microalgae with supramolecular solvents. Sep. Purif. Technol. 2020, 251, 117327. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, L.; Li, S.; Li, S.; Wu, Y.; Li, Z.; Qiu, H. Green materials with promising applications: Cyclodextrin-based deep eutectic supramolecular polymers. Green Chem. 2023, 25, 4180–4195. [Google Scholar] [CrossRef]
- Shi, F.; Hai, X.; Zhu, Y.; Ma, L.; Wang, L.; Yin, J.; Li, X.; Yang, Z.; Yuan, M.; Xiong, H.; et al. Ultrasonic assisted extraction of polyphenols from bayberry by deep eutectic supramolecular polymer and its application in bio-active film. Ultrason. Sonochem. 2023, 92, 106283. [Google Scholar] [CrossRef]

| Number | NADES | Extraction Method | Source | Target Compound | Detection Method | Reference |
|---|---|---|---|---|---|---|
| 1 | Lactic acid:Glucose:Water 6:1:6 | Liquid–liquid extraction | Olive oil | Total phenol content | Direct Spectrophotometric Detection | [72] |
| 2 | Apply choline chloride to the surface of the rotating disc | Rotary disk adsorption extraction | Olive oil | Gallic acid, protocatechuic acid, tyrosol, vanillic acid, p-coumarinic acid, syringaldehyde, thymol | HPLC-FLD | [73] |
| 3 | Citric acid:Fructose 1:1 (19% water) | Liquid–liquid extraction | Olive oil | 3,4 dihydroxyphenyl glycol, hydroxytyrosol glucoside, hydroxytyrosol, tyrosol glucoside (salidroside), verbascoside, dialdehydic form of decarboxymethyl oleuropein aglycon (3,4-DHPEA-EDA), caffeoil-6′-secologanoside, comselogoside | HPLC-ESI-MS | [74] |
| 4 | Choline chloride:Xylitol:Water 2:1:3 | Liquid–liquid extraction | Olive oil | Hydroxytyrosol, Oleacin, oleocanthal, oleuropein, aglycone, ligstroside, aglycone, 1-acetoxypinoresinol, tyrosol, luteolin, apigenin, pinoresinol | HPLC-DAD | [75] |
| 5 | Betaine:Glycerol 1:2 (30% water) | Liquid–liquid extraction | Olive oil | Hydroxytyrosol, tyrosol, dialdehydic form of oleuropein aglycon, oleuropein aglycon isomer, lygstroside aglycon | HPLC-DAD/ESI-MS | [76] |
| 6 | Lactic acid:Glucose:Water 3:1:3 | Liquid–liquid extraction | Olive oil | Hydroxytyrosol, tyrosol derivatives | Direct Spectrophotometric Detection | [77] |
| 7 | Lactic acid:Glucose:Water 6:1:6 | Liquid–liquid extraction | Olive oil | Benzoic acid derivatives (hydroxybenzoic acid, protocathecuic acid, vanillic acid), cinnamic acid derivatives (p-coumaric acid, caffeic acid), phenyl-ethyl alcohols (tyrosol), flavonoids (apigenin), lignans (pinoresinol) | Direct Spectrophotometric Detection | [78] |
| 8 | Choline chloride:Ethylene glycol 1:2, Choline chloride:Glycerol 1:2 | Ultrasonic-assisted liquid–liquid microextraction method | Olive oil; Sesame oil; Cinnamon oil; Almond oil; | Ferulic, caffeic, cinnamic | HPLC-UV | [79] |
| 9 | Choline chloride:xylitol Choline chloride/1,2-propanediol | Liquid–liquid microextraction | Olive oil | Oleacein, Oleocanthal, | UPLC-DAD/MS | [80] |
| Cation | Anion | Cation + Anion | Example |
|---|---|---|---|
| Pyridine cation | Halogenation Non-Halogenated | Pyrazole Halogenated Pyrazole Non-Halogenated | N-Butylpyrazole bromide N-Butylpyrazole tetrafluoroborate |
| Imidazole cation | Halogenation Non-halogenated | Imidazole halogenated Imidazole Non-Halogenated | 1-Butyl-3-methylpyrazole bromide 1-Butyl-3-methyl tetrafluoroborate |
| Quaternary ammonium cation | Halogenation Non-halogenated | Quaternary ammonium halogenated Quaternary ammonium non-halogenated | Tetraethylammonium bromide Tetrabutyl tetrafluoroborate |
| Quaternary phosphoric cation | Halogenation Non-halogenated | Quaternary phosphine halogenated Quaternary phosphine non-halogenated | Tetrabutylphosphonium bromide Tetrabutylphosphonium tetrafluoroborate |
| Number | Supercritical Fluid Extraction | Source | Target Compound | Optimum Extraction Condition | Reference |
|---|---|---|---|---|---|
| 1 | Supercritical fluid extractio, subcritical fluid extraction, | Camellia oleifera oil | squalene, tea polyphenols, tocopherol, phytosterol. | extraction temperature of 45 °C | [99] |
| 2 | Supercritical CO2 | Moringa oleifera seed oi | unsaturated fatty acids, including oleic acid, octadecanoic acid, palmitic acid, stearic acid, eicosanoic acid. | extraction temperature of 45 °C; extraction time of 2.5 h; extraction pressure of 50 MPa; CO2 flow rate of 240 L/h; resulting in a maximum yield of 38.54%. | [100] |
| 3 | Supercritical CO2 | Yellow horn seed oil | oleic 33.40%, linoleic acids 44.81%, nervonic acid 4.06%. | extraction temperature of 50 °C; extraction time of 2 h; extraction pressure of 40 MPa; resulting in a maximum yield of 31.61%. | [101] |
| 4 | Supercritical CO2 and EtOH as co-Solvent | Sunflower edible oil | total polyphenol content, total flavonoids content, | extraction pressure 21 MPa; extraction temperature of 60 °C; co-solvent contribution 15% w/w EtOH | [102] |
| 5 | Supercritical CO2 | Berberis dasystachya Maxim Seed Oil | unsaturated fatty acids (85.62%) and polyunsaturated fatty acids (57.90%), aldehydes, esters. | extraction pressure 25.00 Mpa; extraction temperature of 59.03 °C; CO2 flow rate of 2.25 SL/min; maximum yield of 12.54 ± 0.56 g/100 g | [103] |
| 6 | Hexane extraction (HE), aqueous enzymatic extraction (AEE), supercritical fluid extraction (SFE). | Xanthoceras sorbifolia Bunge Kernel Oil | monounsaturated fatty acids (49.31–50.38%), oleic acid (30.73–30.98%), nervonic acid (2.73–3.09%), HE: resulted in the highest oil yield (98.04%), tocopherol content (530.15 mg/kg), sterol content (2104.07 mg/kg). | HE: extraction temperature of 50 °C; extraction time of 4 h. SFE: CO2 flow rate of 18 L/h; extraction pressure of 28 MPa; extraction temperature of 42 °C. | [104] |
| 7 | Cold pressing (CP), microwave pretreatment pressing (MP), supercritical fluid extraction (SFE) | Pumpkin Seed Oil | total polyphenol content, squalene, tocopherols, phytosterols. | SFE > MP > CP | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, F.; Li, X.; Zhang, Y.; Wu, Y.; Bai, K.; Agusti, R.; Soleimani, A.; Wang, W.; Yi, S. Recent Progress on Green New Phase Extraction and Preparation of Polyphenols in Edible Oil. Molecules 2023, 28, 8150. https://doi.org/10.3390/molecules28248150
Liang F, Li X, Zhang Y, Wu Y, Bai K, Agusti R, Soleimani A, Wang W, Yi S. Recent Progress on Green New Phase Extraction and Preparation of Polyphenols in Edible Oil. Molecules. 2023; 28(24):8150. https://doi.org/10.3390/molecules28248150
Chicago/Turabian StyleLiang, Feng, Xue Li, Yu Zhang, Yi Wu, Kaiwen Bai, Romero Agusti, Ali Soleimani, Wei Wang, and Shumin Yi. 2023. "Recent Progress on Green New Phase Extraction and Preparation of Polyphenols in Edible Oil" Molecules 28, no. 24: 8150. https://doi.org/10.3390/molecules28248150
APA StyleLiang, F., Li, X., Zhang, Y., Wu, Y., Bai, K., Agusti, R., Soleimani, A., Wang, W., & Yi, S. (2023). Recent Progress on Green New Phase Extraction and Preparation of Polyphenols in Edible Oil. Molecules, 28(24), 8150. https://doi.org/10.3390/molecules28248150








