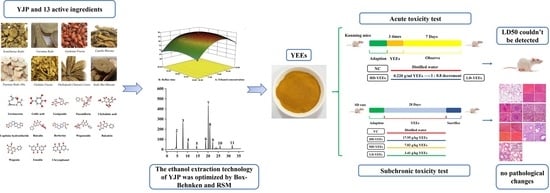
Optimization of Ethanol Extraction Technology for Yujin Powder Using Response Surface Methodology with a Box–Behnken Design Based on Analytic Hierarchy Process–Criteria Importance through Intercriteria Correlation Weight Analysis and Its Safety Evaluation
Abstract
:1. Introduction
2. Results
2.1. Method Validation of HPLC
2.1.1. Linear Relationships
2.1.2. Precision, Stability and Repeatability
2.1.3. Sample Recovery Rate
2.2. AHP Weight
2.3. CRITIC Weight
2.4. Weight Determination by AHP-CRITIC Mixed Weighting Method
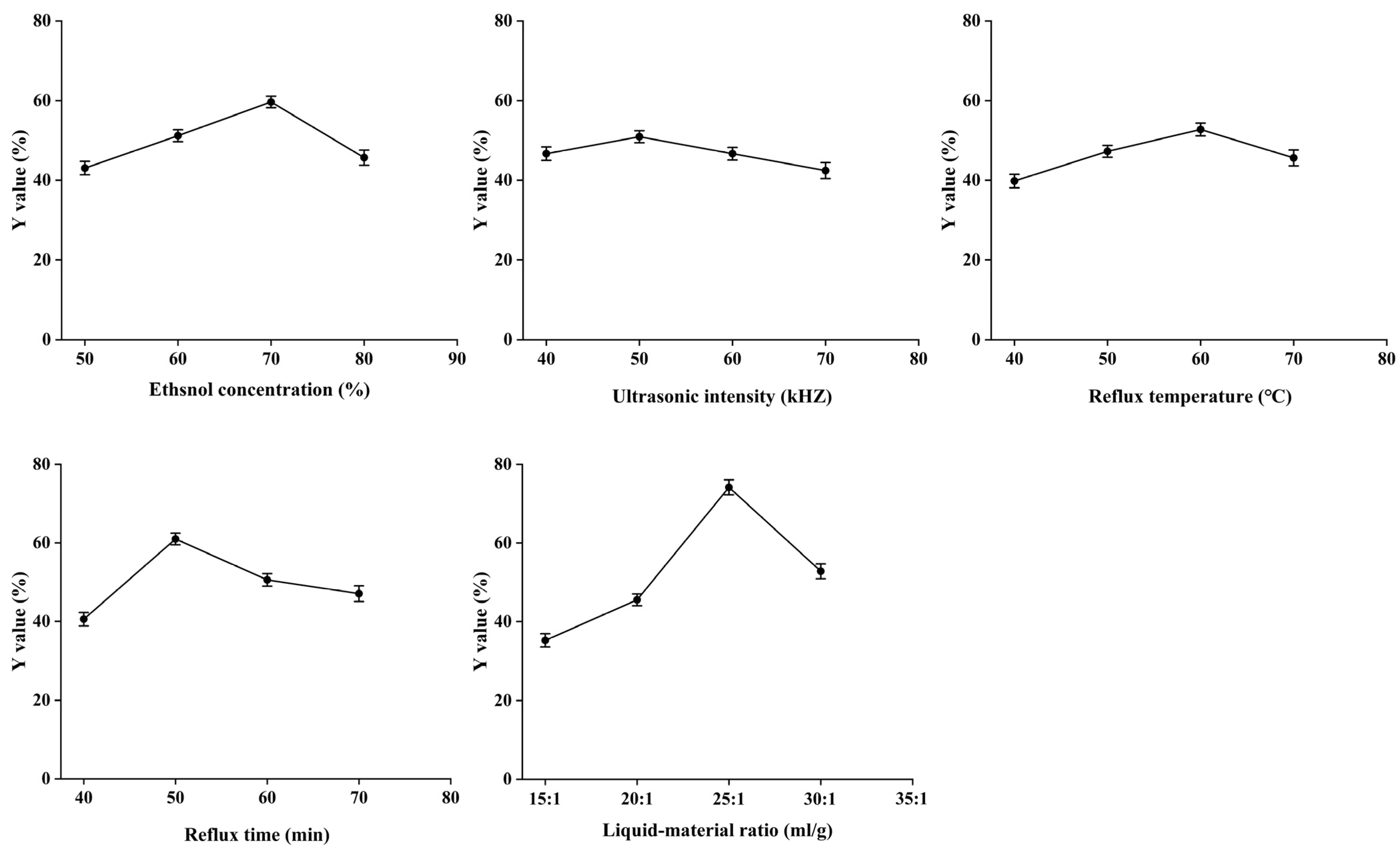
2.5. Single-Factor Experiments
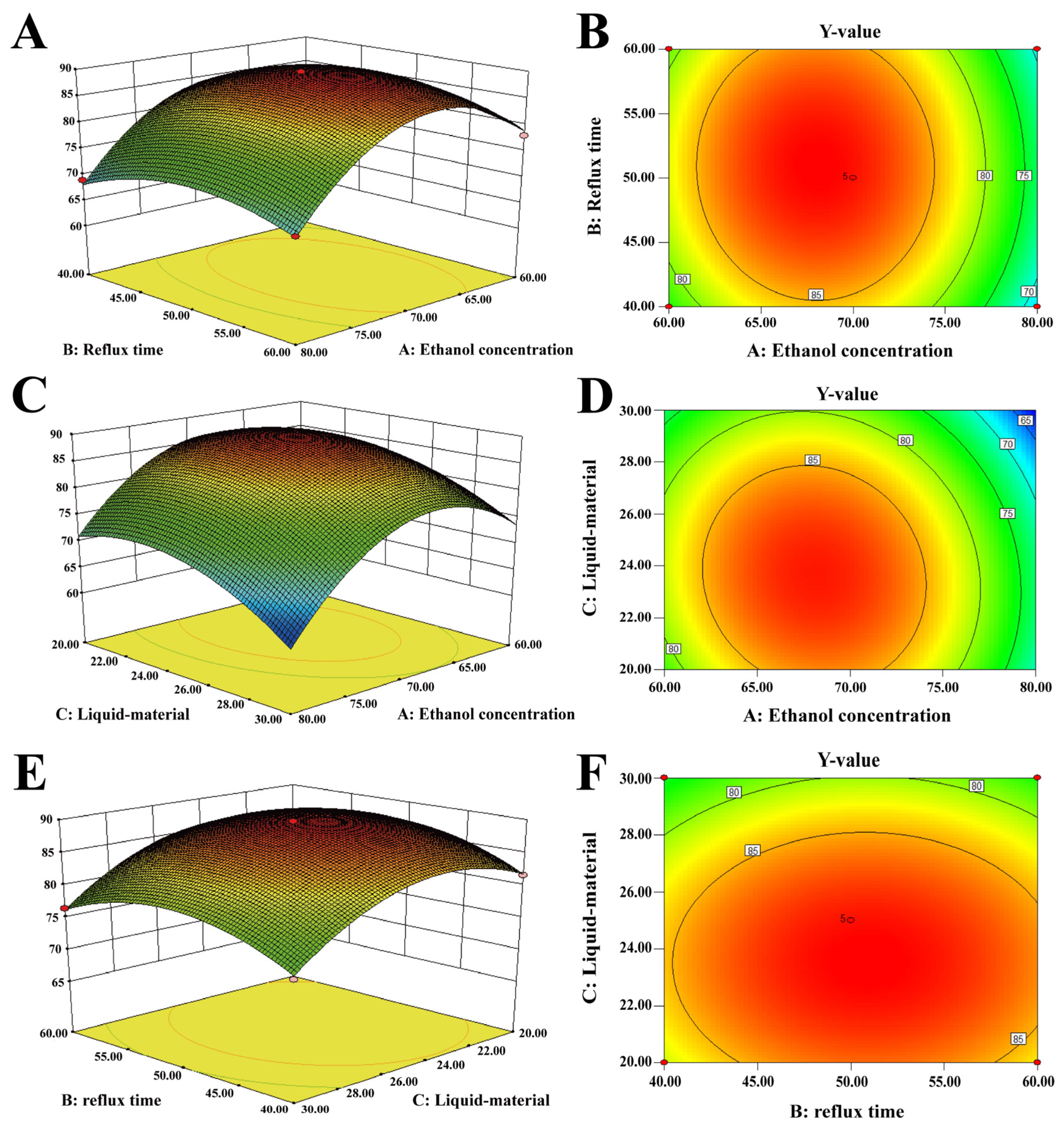
2.6. Response Surface Experiment
2.6.1. Model Establishment and Significance Test
2.6.2. Validation of Response Surface Experiment
2.6.3. Interaction of Various Factors
2.7. Acute Toxicity Test Results
2.7.1. Pre-Experiment Results
2.7.2. Maximum Administration Dosage Test Results
2.8. Subchronic Toxicity Test Results
2.8.1. Observation of Clinical Symptoms and Signs
2.8.2. Analysis of Organ Indices
2.8.3. Detection Results of the Blood Routine and Blood Biochemistry
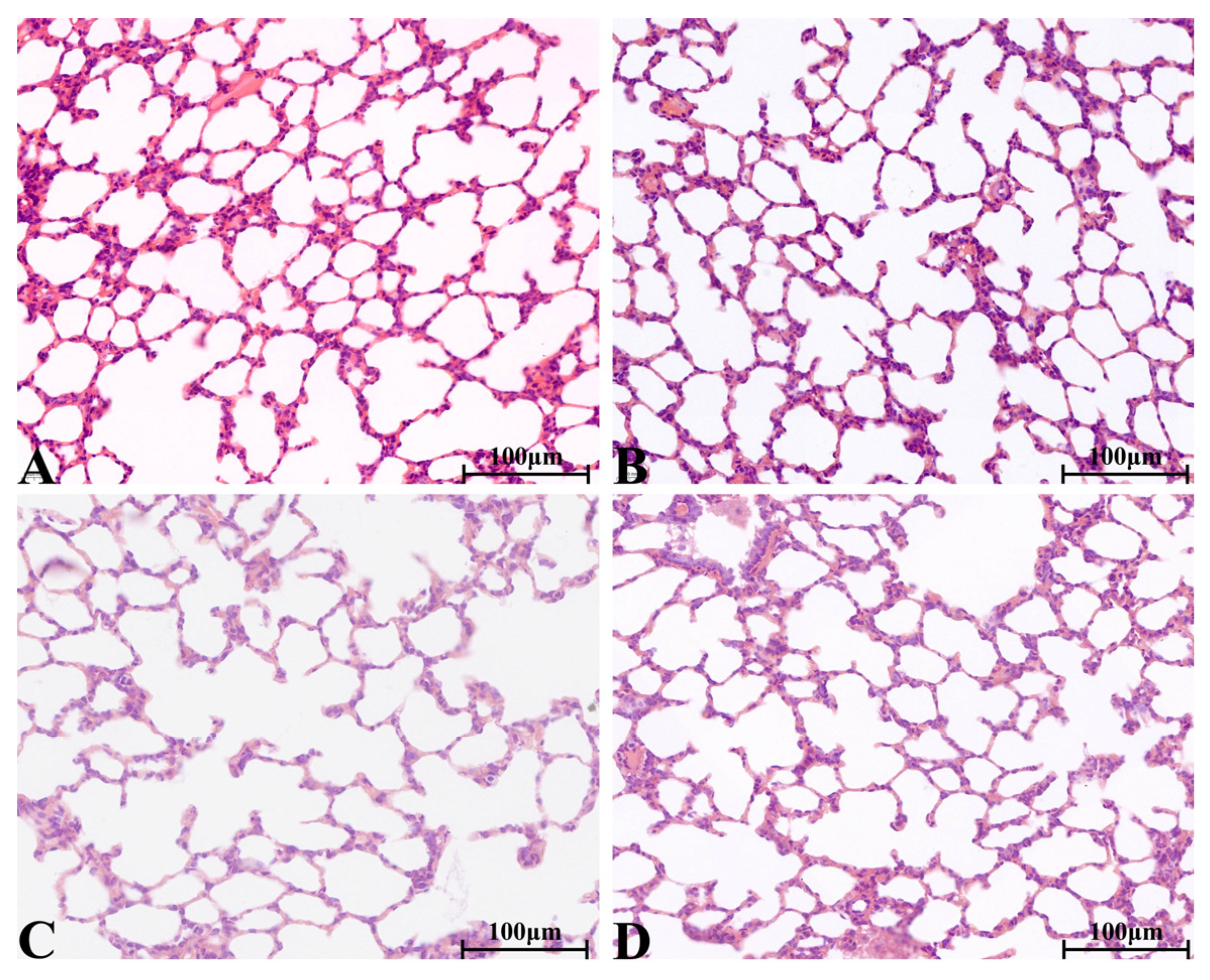
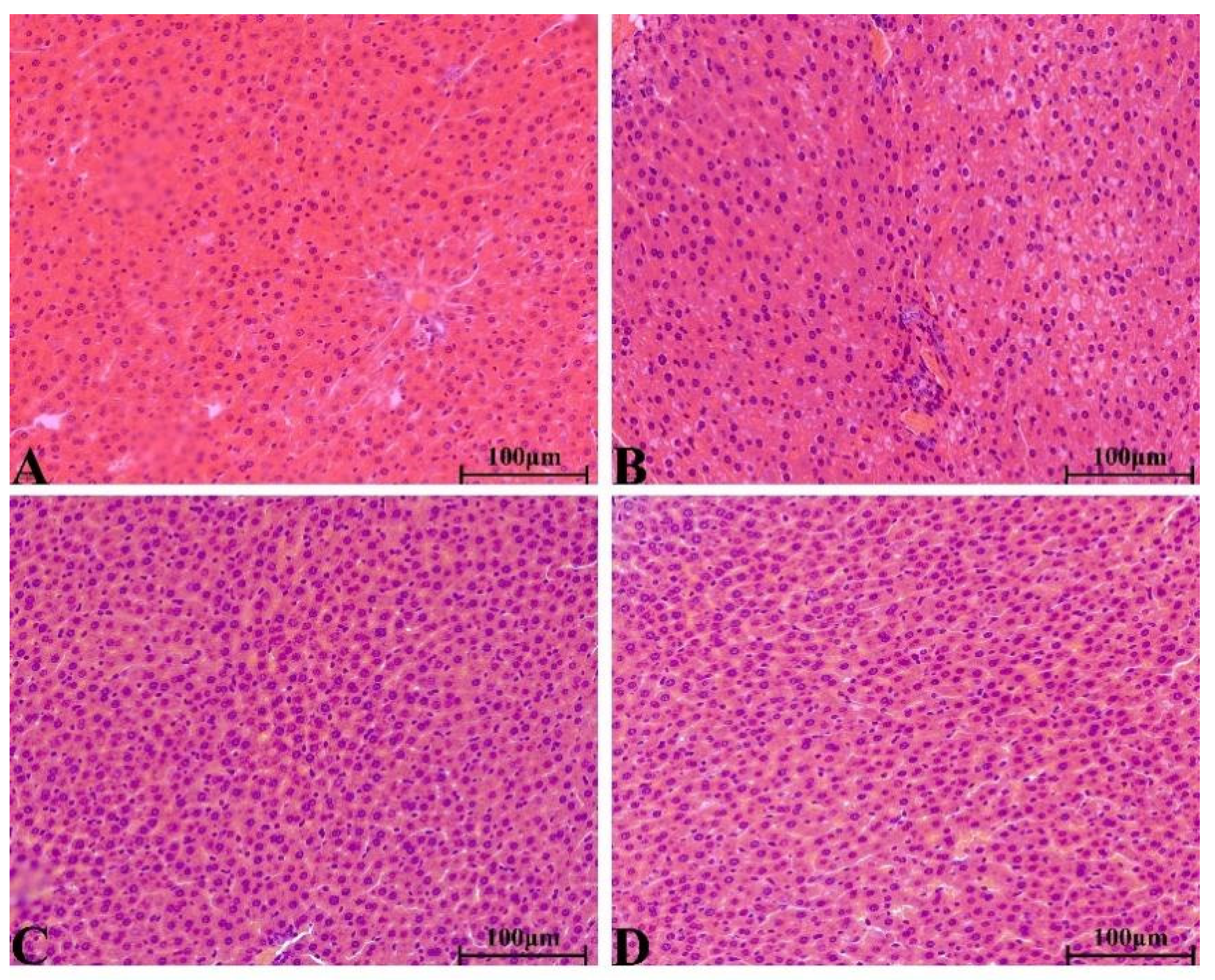
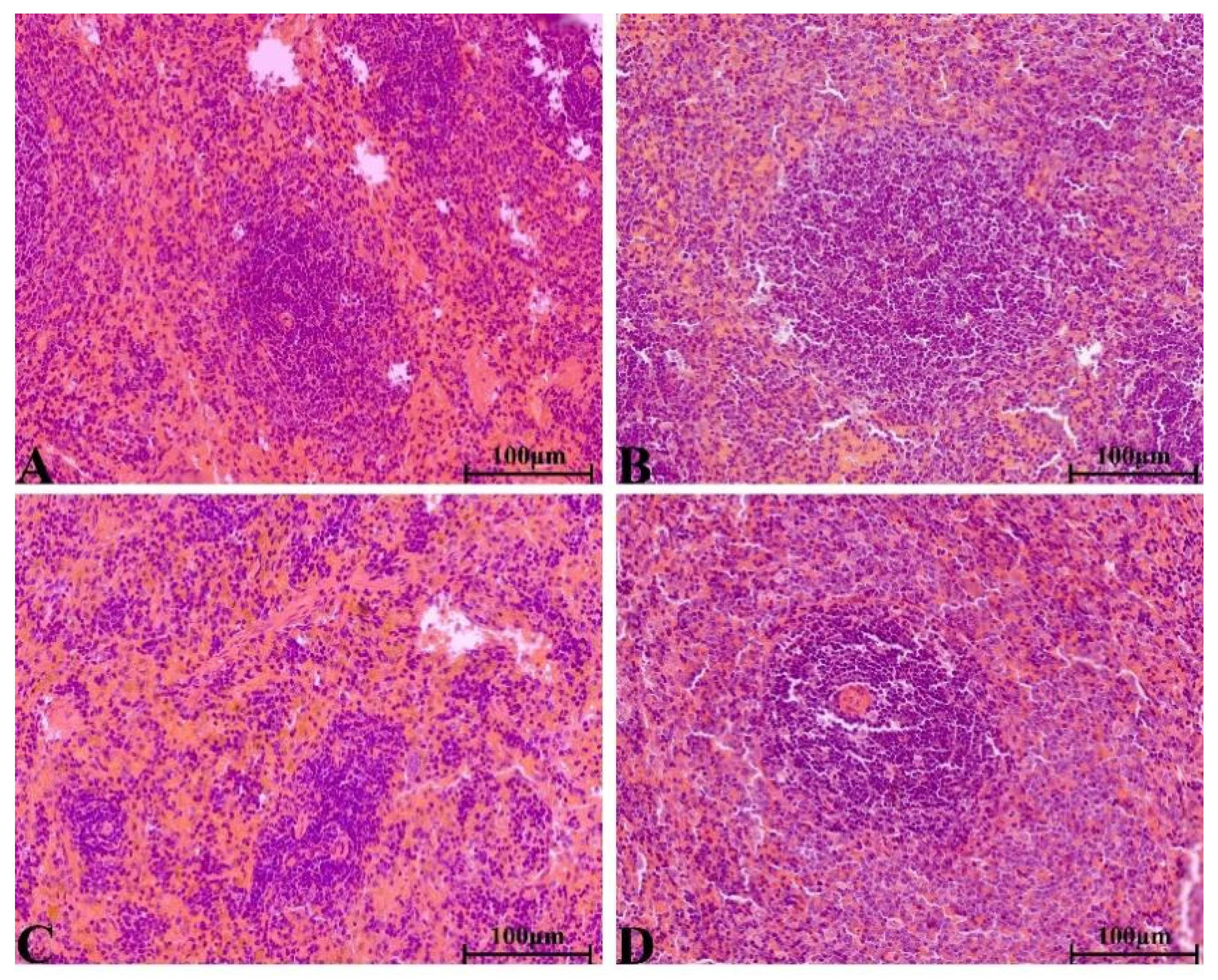
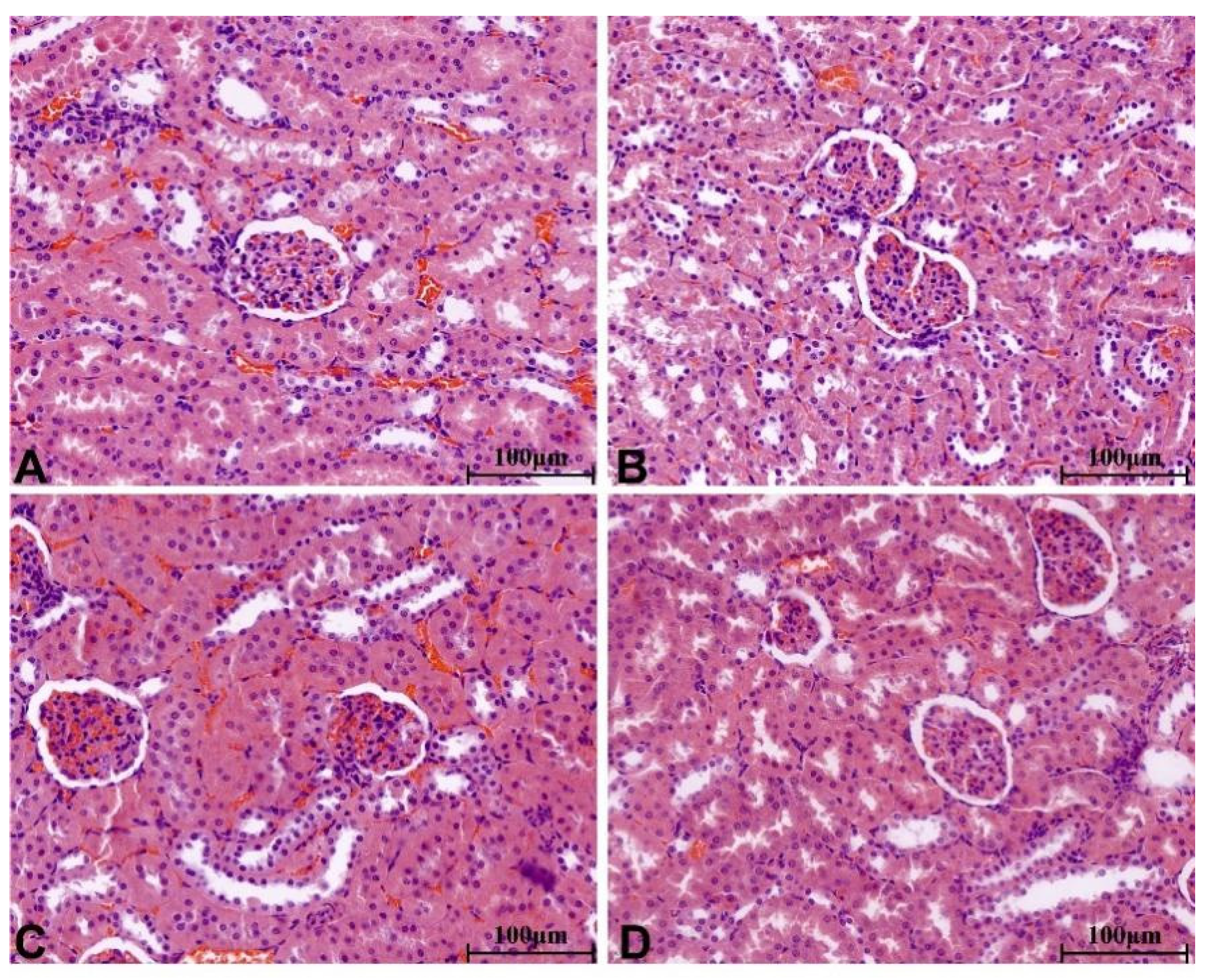
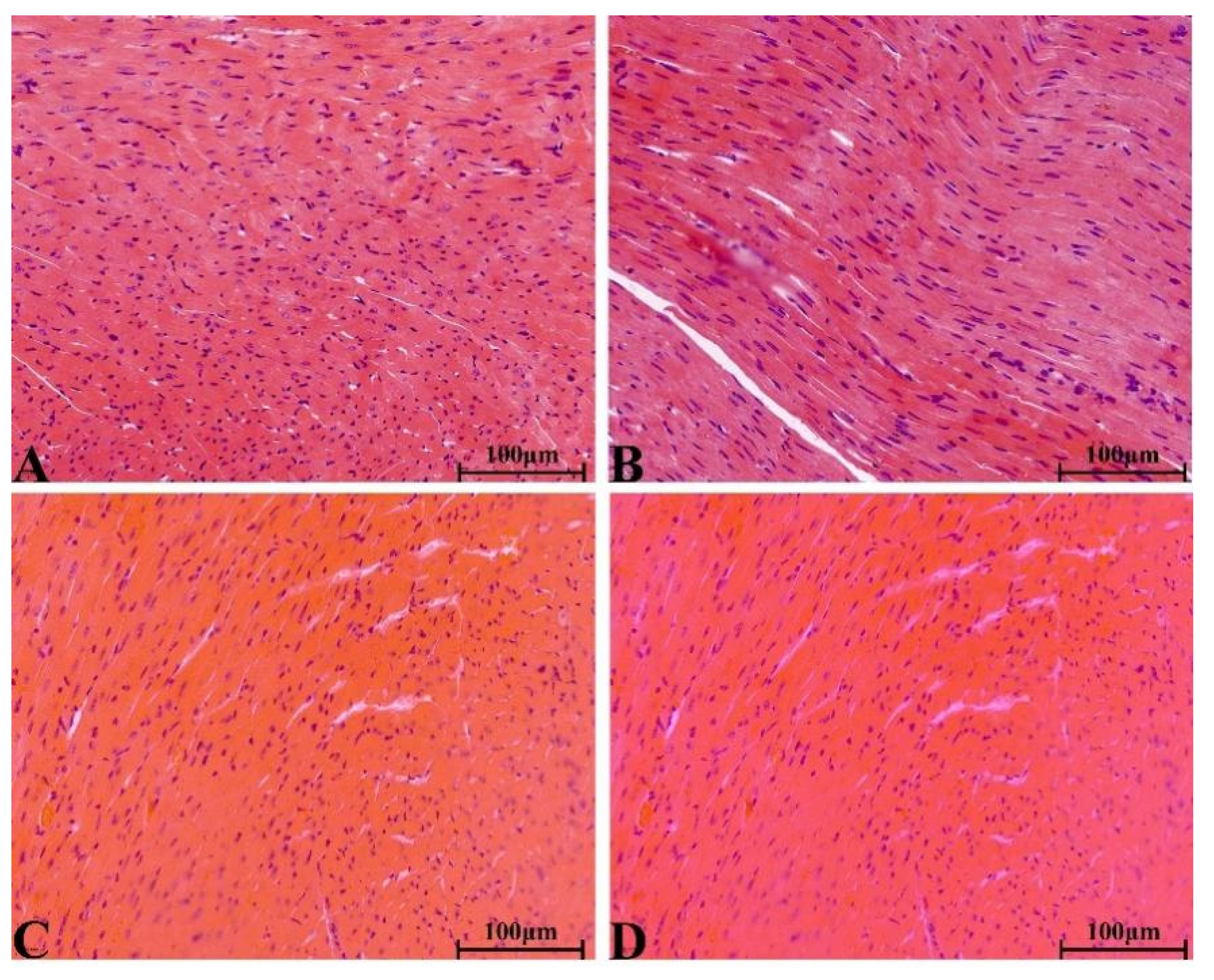
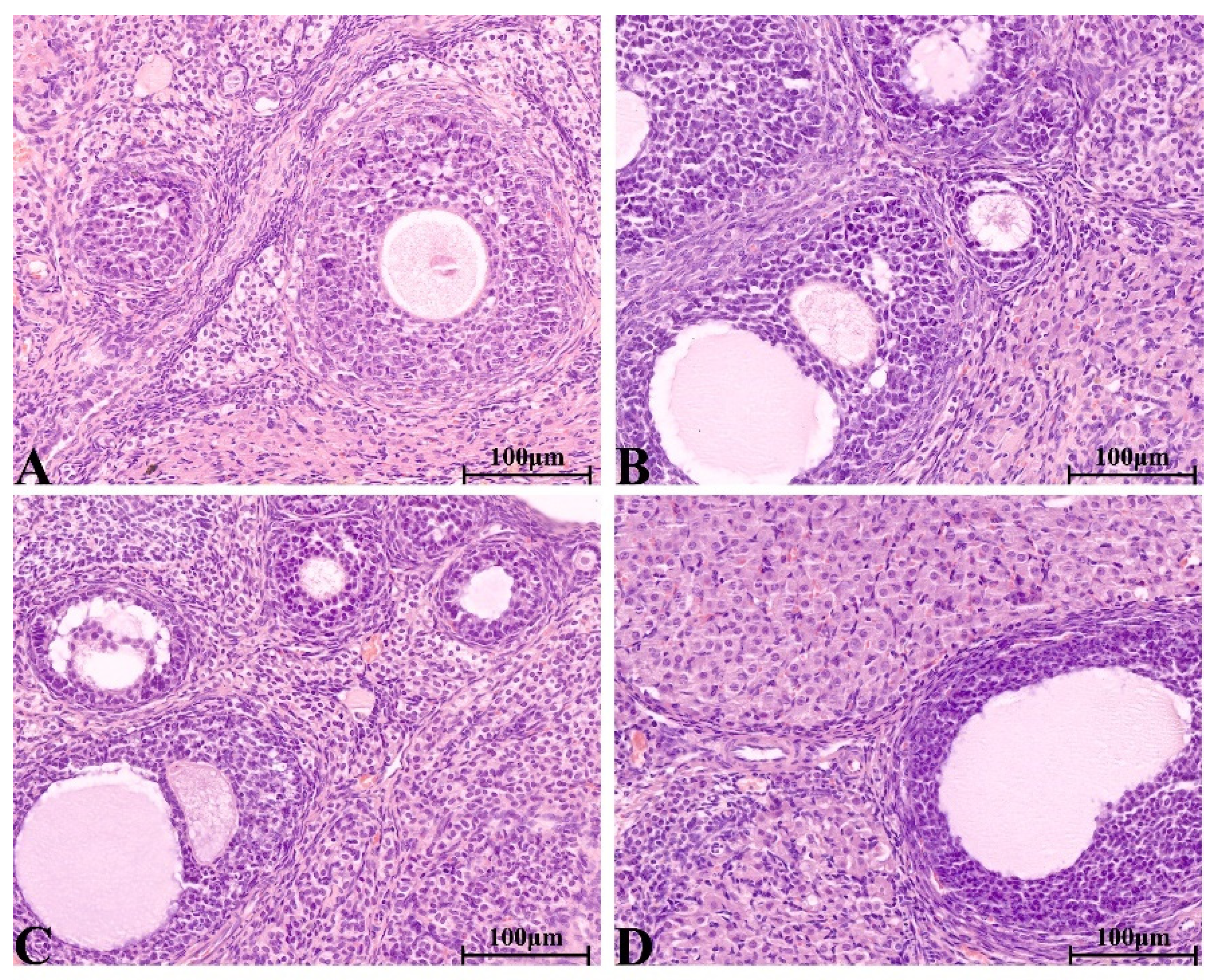
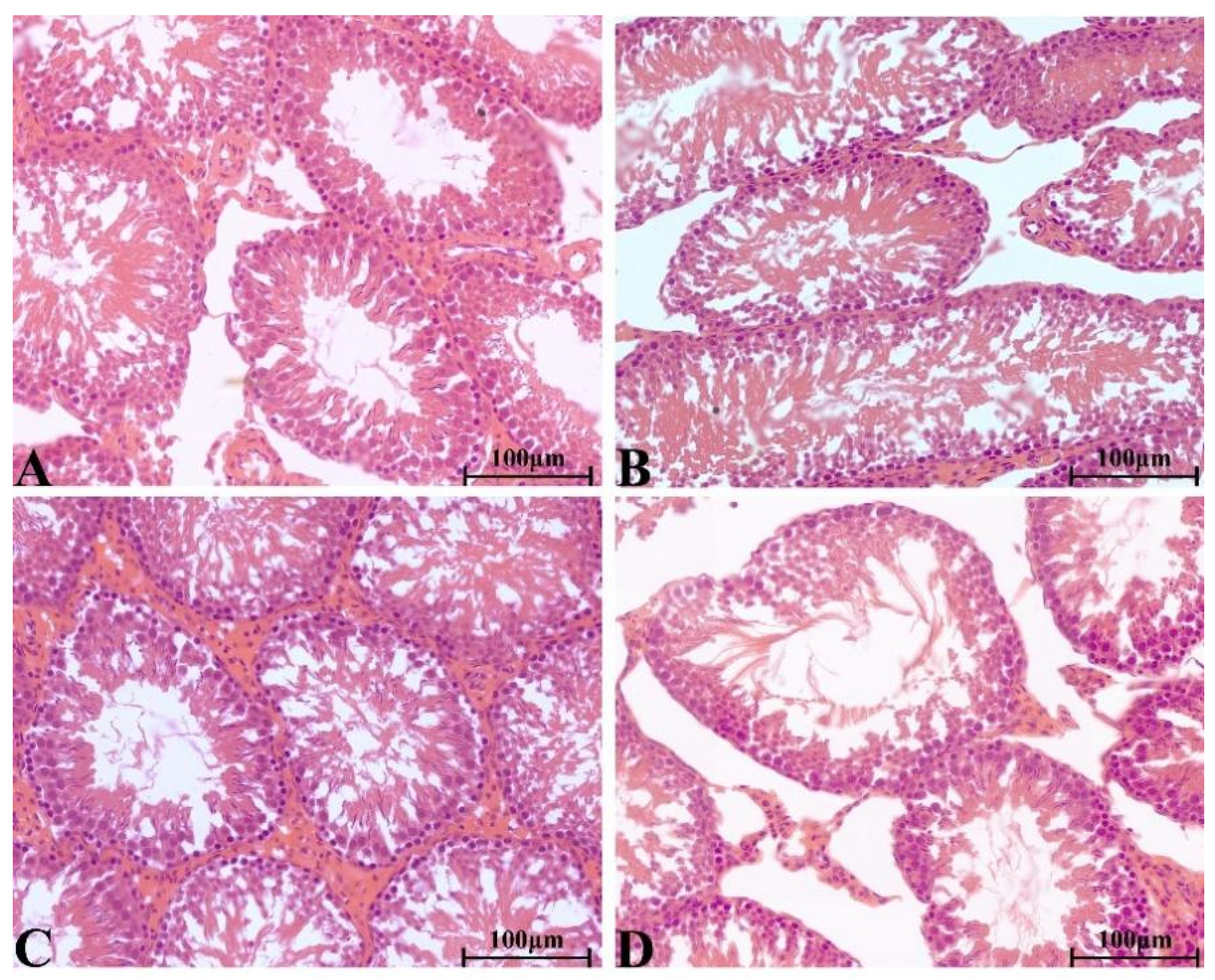
2.8.4. Histopathological Change in Main Organs
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.1.1. Experimental Drugs
4.1.2. Experimental Reagents
4.1.3. Animals
4.2. Drug Preparation
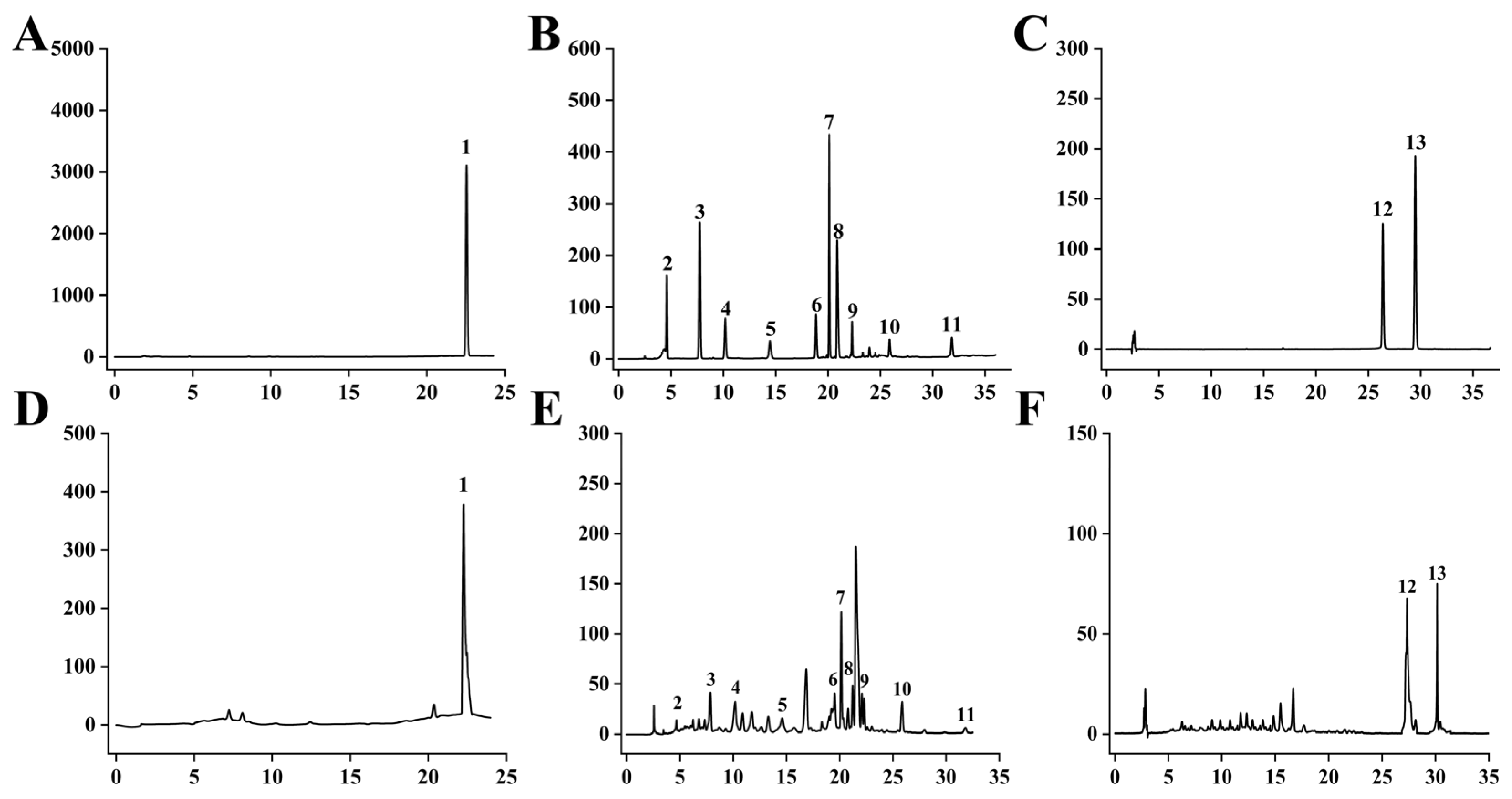
4.3. HPLC Detection
4.3.1. HPLC Chromatographic Condition
4.3.2. Preparation of Standard Solutions
4.3.3. Method Validation with HPLC
4.4. Weight and Calculation of Comprehensive Y Score
4.4.1. Index for Selection
4.4.2. Weight Calculation by AHP
4.4.3. Weigh calculation by CRITIC
4.4.4. Weight Determination by AHP-CRITIC Mixed Weighting Method
4.4.5. Calculation of Comprehensive Y Score
4.5. Single-Factor Experiment
4.6. Optimization of Ethanol Extraction Conditions of YJP by RSM
4.6.1. Response Surface Experimental Design
4.6.2. Verification Experiments
4.7. Acute Toxicity Test
4.7.1. Pre-Experiment
4.7.2. Maximum Administration Dosage Test
4.8. Subchronic Toxicity Test
4.8.1. Animal Experiment
4.8.2. Blood Routine Detection
4.8.3. Blood Biochemistry Detection
4.8.4. Histopathological Observation
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.L. Traditional Chinese Veterinary Medicine; Science Press: Beijing, China, 2013. [Google Scholar]
- Zhang, Y.; Wen, W. Treatment of equine damp-heat diarrhea with modified YuJin powder and acupoint application of traditional Chinese medicine. Chin. J. Vet. Med. 2012, 48, 89. [Google Scholar]
- Ma, H.R. Modified Yujin Powder for Calf Diarrhea. J. Tradit. Chin. Vet. Med. 2007, 26, 1. [Google Scholar]
- Fu, N.X. Study on Jiawei Yujin Powder in the treatment of Bovine Acute Enteritis. Agric. Tech. Serv. 2015, 32, 1. [Google Scholar]
- Yao, W.L. Essence of Large Intestine Dampness-Heat Syndrome and Treatment Mechanisms of Yujin Powder on It. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2017. [Google Scholar]
- Yao, W.L.; Yang, C.X.; Wen, Y.Q.; Zhang, W.D.; Zhang, X.S.; Ma, Q.; Ji, P.; Hua, Y.L.; Wei, Y.M. Treatment effects and mechanisms of Yujin Powder on rat model of large intestine dampness-heat syndrome. J. Ethnopharmacol. 2017, 202, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Q. Effects of Yujin Powder on Gastrointestinal Hormone and Hypothalamus-pituitary-adrenal Axis of Large Intestine Dampness-heat Syndrome Rat Model. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2018. [Google Scholar]
- Yao, Y.; Luo, R.Y.; Shu, X.; Zhang, C.; Zhang, Y.K. Protective effects of curcumin against rat intestinal inflammation-related motility disorders. Mol. Med. Rep. 2021, 23, 319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Ishfaq, M.; Yu, H.X.; Yang, Y.Q.; Li, S.H.; Li, X.T.; Fazlani, S.A.; Guo, W.X.; Zhang, X.Y. Curcumin ameliorates duodenal toxicity of AFB1 in chicken through inducing P-glycoprotein and downregulating cytochrome P450 enzymes. Poult. Sci. 2020, 99, 7035–7045. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, Y.J.; Choi, H.K.; Yoon, Y.G. Fractionated Coptis chinensis Extract and Its Bioactive Component Suppress Propionibacterium acnes-Stimulated Inflammation in Human Keratinocytes. J. Microbiol. Biotechnol. 2018, 28, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Fujii, A.; Okuyama, T.; Wakame, K.; Okumura, T.; Ikeya, Y.; Nishizawa, M. Identification of anti-inflammatory constituents in Phellodendri Cortex and Coptidis Rhizoma by monitoring the suppression of nitric oxide production. J. Nat. Med. 2017, 71, 745–756. [Google Scholar] [CrossRef]
- Georgieva, Y.; Katsarova, M.; Stoyanov, P.; Mladenov, R.; Dimitrova, S. Metabolite Profile and Antioxidant Activity of Some Species of Genus Scutellaria Growing in Bulgaria. Plants 2020, 10, 45. [Google Scholar] [CrossRef]
- Liao, H.F.; Ye, J.; Gao, L.L.; Liu, Y.L. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.C.; Liu, Y.H.; Li, C.; Xu, M.; Xie, H.T.; Zhao, J. Using Network Pharmacology for Systematic Understanding of Geniposide in Ameliorating Inflammatory Responses in Colitis Through Suppression of NLRP3 Inflammasome in Macrophage by AMPK/Sirt1 Dependent Signaling. Am. J. Chin. Med. 2020, 48, 1693–1713. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Liang, K.; An, R.; Wang, X. Baicalin protects against ethanol-induced chronic gastritis in rats by inhibiting Akt/NF-κB pathway. Life Sci. 2019, 239, 117064. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Guan, X.; Hou, Y.; Liu, Y.L.; Wei, W.; Cai, X.Y.; Zhang, Y.Y.; Wang, G.J.; Zheng, X.; Hao, H.P. Paeoniflorin modulates gut microbial production of indole-3-lactate and epithelial autophagy to alleviate colitis in mice. Phytomedicine Int. J. Phytother. Phytopharm. 2020, 79, 153345. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Liu, L.G.; Hu, J.Y.; Deng, X.T.; Tang, Y.H.; Chen, Y.T.; Bai, X.L. On the Antibacterial Effects of Anthraquinone Compounds in Rhubarb. J. Hangzhou Norm. Univ. 2021, 20, 54–58. [Google Scholar]
- Lee, H.S.; Jeong, G.S. Chrysophanol Attenuates Manifestations of Immune Bowel Diseases by Regulation of Colorectal Cells and T Cells Activation In Vivo. Molecules 2021, 26, 1682. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Xia, P.F.; Ma, X.; Yang, R.J.; Hu, J.R.; Fan, Q.; Jin, X.J.; Wu, X.Y.; Peng, X.J.; et al. Research progress on chemical composition and pharmacological effects of Rhei Radix et Rhizoma and predictive analysis on quality markers. Chin. Tradit. Herb. Drugs 2019, 50, 4821–4837. [Google Scholar]
- Qiao, X.; Ye, M.; Xiang, C.; Wang, Q.; Liu, C.F.; Miao, W.J.; Guo, D.A. Analytical strategy to reveal the in vivo process of multicomponent herbal medicine: A pharmacokinetic study of licorice using liquid chromatography coupled with triple quadrupole mass spectrometry. J. Chromatogr. A 2012, 1258, 84–93. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C.; Huang, T.C.; Lee, S.Z.; Lin, S.S. Comparison of modified ultrasound-assisted and traditional extraction methods for the extraction of baicalin and baicalein from Radix Scutellariae. Ind. Crop. Prod. 2013, 45, 182–190. [Google Scholar] [CrossRef]
- Yang, B.Y.; Zhang, M.Y.; Weng, H.Y.; Xu, Y.; Zeng, L.H. Optimization of ultrasound assisted extraction (UAE) of kinsenoside compound from Anoectochilus roxburghii (Wall.) Lindl by response surface methodology (RSM). Molecules 2020, 25, 193. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, Y.B.; Liang, Z.S.; Wang, W.L. Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason. Sonochem. 2010, 17, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Elhag, H.E.E.A.; Naila, A.; Ajit, A.; Aziz, B.A.; Sulaiman, A.Z. Sequential extraction of saponins from Eurycoma longifolia roots by water extraction and ultrasound-assisted extraction. Mater. Today Proc. 2018, 5, 21672–21681. [Google Scholar] [CrossRef]
- Xue, J.; Wen, Y.Q.; Dong, J.Q.; Zhang, W.D.; Zhang, Y.H.; Xiao, T.; Cao, Y.X.; Yao, W.L.; Wei, Y.M. Extraction Efficiency Comparison of 3 Extraction Methods for Effective Components of Yujin Powder. Prog. Vet. Med. 2021, 42, 81–87. [Google Scholar]
- Shi, G.; Shen, J.; Wei, T.; Ren, F.; Guo, F.; Zhou, Y. UPLC-ESI-MS/MS Analysis and Evaluation of Antioxidant Activity of Total Flavonoid Extract from Paeonia lactiflora Seed Peel and Optimization by Response Surface Methodology (RSM). BioMed Res. Int. 2021, 2021, 7304107. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Choi, Y.H. Optimization of ultrasonic-assisted extraction of bioactive alkaloid compounds from rhizoma coptidis (Coptis chinensis Franch.) using response surface methodology. Food Chem. 2014, 142, 299–305. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Zhou, X.; Xie, F.R.; Li, Z.C. Comparison of three weighing methods for evaluation of the HPLC fingerprint of cortex fraxini. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2008–2019. [Google Scholar] [CrossRef]
- Xie, R.F.; Shi, Z.N.; Li, Z.C.; Chen, P.P.; Li, Y.M.; Zhou, X. Optimization of high pressure machine decocting process for Dachengqi Tang using HPLC fingerprints combined with the Box–Behnken experimental design. J. Pharm. Anal. 2015, 5, 110–119. [Google Scholar] [CrossRef]
- Ouyang, L.M.; Wang, X.H.; Zhu, S.M.; You, L.S.; An, R.; Cui, B. Based on the AHPCRITIC method to explore the optimal harvest period of Atractylodes sinensis. Chin. Pat. Med. 2020, 42, 708–713. [Google Scholar]
- Yang, Z.C.; Yang, Y.J.; He, Y.; Qi, J.; E, X.H. Application of multi-attribute decision making in the research and development and production of traditional Chinese medicine. Chin. Pat. Med. 2021, 43, 988–993. [Google Scholar]
- Liu, H.H.; Zhang, Q.; Xu, T.T.; Wang, Y.Z.; Wang, J.; Zhao, L.A.; Yao, Y.; Zhang, X.L.; Liu, S.J. Optimization of Water Extracting Procession of Qingwei San through Multi-Targets Integrated Weighted Method. J. Nanjing Univ. Tradit. Chin. Med. 2021, 37, 450–456. [Google Scholar]
- Ji, P.; Li, C.C.; Wei, Y.M.; Wu, F.L.; Liu, S.L.; Hua, Y.L.; Yao, W.L.; Zhang, X.S.; Yuan, Z.W.; Wen, Y.Q. Screening study of blood-supplementing active components in water decoction of angelica sinensis processed with yellow rice wine based on response surface methodology. Pharm. Biol. 2020, 58, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, J.; Jiang, M.Y.; Yan, D.; Yang, R.P.; Zhang, C.N. Study on Extraction technology of Sanqi Traumatic Ointment based on Multi-index weight analysis and Orthogonal design method. J. Chin. Med. Mater. 2017, 40, 412–415. [Google Scholar]
- Liu, X.M.; Wang, S.S.; Shi, C.J.; Cheng, Z.Q.; Yuan, Q.H.; Song, Y. Optimization of Extraction Technique for Shenxi Oral Liquid by Box-Behnken Response Surface Methodology Based on AHP-CRITIC Weighted Analysis. J. Chin. Med. Mater. 2019, 42, 150–155. [Google Scholar]
- Shi, J.M.; Zhou, C.; Yu, X.M.; Wu, Z.Y.; Wang, J.; Lei, L.; Li, Z.Z. Optimization of the Decoction Process for Shengma Gegen Decoction with Boiling Machine by Orthogonal Design and AHP-CRITIC Weight Analysis. China Pharm. 2020, 23, 49–52. [Google Scholar]
- Wu, D.; Lei, C. Simultaneous Determination of Four Flavonoids in Huaihua Powder by HPLC. Chin. J. Inf. Tradit. Chin. Med. 2020, 27, 69–72. [Google Scholar]
- Xia, W.X.; Liu, Q.S.; Zhou, H.; Hua, S.Y.; Fu, X.y. Study on the Spectrum-Effect Relationship of the Traditional Effect of Saponins in Glycyrrhiza uralensis Fisch. Int. J. Anal. Chem. 2021, 2021, 6617033. [Google Scholar] [CrossRef]
- Qu, K.S.; Luo, Y.; Yan, X.N.; Kuai, L.; Ru, Y.; Luo, Y.; Song, J.K.; Ji, W.L.; Li, B.; Xing, M. Qinzhuliangxue mixture alleviates psoriasis-like skin lesions via inhibiting the IL6/STAT3 axis. J. Ethnopharmacol. 2021, 274, 114041. [Google Scholar] [CrossRef]
- Li, Y.N.; Li, J.N.; Qin, O.Y.; Zhou, Y.B.; Lei, C.; Cui, M.J.; Fu, K.C.; Li, J.; Huang, J.M.; Hou, A. Determination of maytansinoids in Trewia nudiflora using QuEChERS extraction combined with HPLC. J. Pharm. Biomed. Anal. 2021, 198, 113993. [Google Scholar] [CrossRef]
- Wu, Q.; Erbu, A.; Liang, X.X.; Luan, S.X.; He, C.L.; Yin, L.Z.; Yin, Z.Q.; Zou, Y.F.; Li, L.X.; Song, X. Determination of the main naphthoquinones in Onosma hookeri Clarke. var. longiforum Duthie and its optimization of the ultrasound-assisted extraction using response surface methodology. J. Food Sci. 2021, 86, 357–365. [Google Scholar] [CrossRef]
- Pan, P.C.; Chen, X.F.; Chen, W.; Guo, Z.Y.; Chai, Y.F. Optimization of the compound herba lysimachiae extraction prosess based on orthogonal experiment and response surface method. J. Pharm. Pract. 2020, 38, 27–34. [Google Scholar]
- Jiang, Y.M.; Wang, S.; Yu, M.; Wu, D.; Lei, J.; Li, W.W.; He, Y.Q.; Gang, W. Ultrasonic-Assisted Ionic Liquid Extraction of Two Biflavonoids from Selaginella tamariscina. ACS Omega 2020, 5, 33113–33124. [Google Scholar] [CrossRef]
- Li, C.Q.; Cui, Y.P.; Lu, J.; Liu, C.Y.; Chen, S.T.; Ma, C.Y.; Liu, Z.H.; Wang, J.M.; Kang, W.Y. Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC and Artificial Neural Network Analysis for Ganoderma lucidum. Molecules 2020, 25, 1309. [Google Scholar] [CrossRef]
- Zhao, J. The Study on Quality Standards od Gastrodiae Rhizoma and Its Extracts. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2019. [Google Scholar]
- Wang, Y.L.; Tao, R.; Xu, K.X.; Li, J.Y.; Wang, Y.; Song, X.S.; Wang, J.; Gao, S.J. Study on Determination of Flavonoids in Chinese Medicine Weight Loss and Lipid-lowering Compound. Contemp. Chem. Ind. 2020, 49, 1114–1117. [Google Scholar]
- Hu, Y.P.; Cui, X.M.; Zhang, Z.J.; Chen, L.J.; Zhang, Y.M.; Wang, C.X.; Yang, X.Y.; Qu, Y.; Xiong, Y. Optimisation of Ethanol-Reflux Extraction of Saponins from Steamed Panax notoginseng by Response Surface Methodology and Evaluation of Hematopoiesis Effect. Molecules 2018, 23, 1206. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.P.; Zhang, M.; Fu, X.B. Three statistical experimental designs for enhancing yield of active compounds from herbal medicines and anti-motion sickness bioactivity. Pharmacogn. Mag. 2015, 11, 435–443. [Google Scholar]
- Chen, G. Study of Extraction and Pirification of Schisandrin b in Schisandra Chinensis (Turcz.) Baill Leaves and Combined with Paclitaxel to Inhibit Cancerstudy and Compound Antioxidant with Total Flavonoids of Citrus Peel. Master’s Thesis, Jinzhou Medical University, Jinzhou, China, 2019. [Google Scholar]
- Wang, L.J.; Gao, X.; Liu, Q.C.; Yang, J.Z.; Sun, G.X.; Yu, J. Optimization of the Ultrasound Extraction Technology of Jinjuan Shengban Capsules by Response Surface Methodology Combined with Multi-index Comprehensive Scoring. China Pharm. 2019, 30, 1781–1785. [Google Scholar]
- Hou, T.H.; Yang, J.Y.; Yang, X.X.; Xu, X.S.; Su, Y.Y.; Qu, X.L. Optimization of polysaccharide extraction from ganoderma spore powder residue by response surface methodology. Lishizhen Med. Mater. Med. Res. 2020, 31, 199–202. [Google Scholar]
- Yi, C.Z.; Wu, Y.N.; Xiao, P.; Cui, L.W.; Zhou, Y.C.; Hu, Y.M. Study on sub-chronic toxicity of Curcuma aromatica Salisb extract. Cap. J. Public Health 2018, 12, 250–252. [Google Scholar]
- Deng, N. Study on Liver and Kidney Toxicity of Rhubarb and Its Total Anthraquinones. Master’s Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2018. [Google Scholar]
- Liu, F.; Li, L.L.; Liu, G.J.; Jiang, H.; Zhang, Q.Z. Study on the acute toxicity of water and alcohol extract of radix seutellariae. Hebei J. Tradit. Chin. Med. 2014, 36, 425–427. [Google Scholar]
- Li, H.F.; Zhang, H.H.; Xia, L. Study on chronic hepatic and renal toxicity of gardenia extract in normal and jaundice model rats. Clin. Basic Bridg. Res. 2020, 36, 3755–3759. [Google Scholar]
- Zhang, Z.Y.; Wu, R. Study on the sensitivity of six disinfectants such as Jiududi liquid and Strong antiseptic liquid and three Chinese herbs such as coptis chinensis, glycyrrhiza chinensis and Phellodendri chinensis to laboratory air bacteria. Chin. J. Tradit. Vet. Sci. 2017, 197, 10–12. [Google Scholar]
- Liu, Y.; Ran, C.; You, G.X.; He, J.Z.; Wu, W.; Hou, K. Determination of Berberine Hydrochloride in Crystal of Phellodendron chinensis by HPLC and Evaluation of Bacteriostatic Activity. J. Agric. Sci. Technol. 2020, 22, 179–186. [Google Scholar]
- Li, X.F.; Xiong, H.B.; Zhang, H.F.; Zi, Y.L.; Zhang, Y.; Gao, Y.T.; Zhang, Y.L. Spectrometric investigation of the antioxidant activities of Terminalia polyphenol against DPPH free radicals. Hubei Agric. Sci. 2019, 58, 121–125. [Google Scholar]
- Peng, Z.T.; Li, C.; Hu, S.Q. Effect of total glucosides of paeony plus glycyrrhizic acid on inflammatory response of brain-gut tissue in diarrheal irritable bowel sydrome. Chin. J. Hosp. Pharm. 2021, 41, 1536–1540. [Google Scholar]
- Zhu, Y.; Yu, S.R.; Zhang, X.Y.; Zhou, C.; Gui, L.L.; Yu, H.F.; Wei, J. Optimization of Extraction Process for Compound Roucongrong Mixture by Analytic Hierarchy Process Combined with Box-Behnken Response Surface Methodology. Chin. J. Mod. Appl. Pharm. 2020, 37, 2852–2858. [Google Scholar]
- Lan, T.J.; Chang, M.; Li, Q.F.; Liang, W.P.; Yun, C.X.; Guo, H.W. Optimization of Formation Process for Leigong Antihypertensive Granules by Central Composite Design-Respones Surface Methodology Based on CRITIC Weighted Evaluation. J. Chin. Med. Mater. 2019, 42, 1356–1359. [Google Scholar]
| Name | Regression Equation | Linear Range (μg/mL) | Correlation Coefficient R2 |
|---|---|---|---|
| Germacrone | Y = 57355X + 126.29 | 27.80–222.25 | 0.9995 |
| Gallic acid | Y = 16357X − 25.834 | 6.30–24.32 | 0.9993 |
| Geniposide | Y = 21963X − 4.9886 | 26.24–100.00 | 0.9995 |
| Paeoniflorin | Y = 5646.1X − 9.9892 | 20.82–80.00 | 0.9996 |
| Chebulinic acid | Y = 16412X − 213.1 | 14.87–57.12 | 0.9996 |
| Coptisine hydrochloride | Y = 59330X + 6.3053 | 3.12–12.00 | 0.9995 |
| Baicalin | Y = 101568X − 202.95 | 26.03–100.00 | 0.9991 |
| Berberine | Y = 51677X + 10.098 | 9.48–36.40 | 0.9990 |
| Wogonoside | Y = 28001X − 30.971 | 4.17–16.00 | 0.9997 |
| Baicalein | Y = 23263X + 53.541 | 2.60–100.00 | 0.9996 |
| Wogonin | Y = 18631X − 2.4212 | 4.16–15.96 | 0.9992 |
| Emodin | Y = 7890.3X + 9.258 | 7.50–28.80 | 0.9992 |
| Chrysophanol | Y = 6005.5X − 23.322 | 4.87–18.72 | 0.9993 |
| Name | Average | SD | RSD (%) |
|---|---|---|---|
| Germacrone | 465.9 | 4.28 | 0.92 |
| Gallic acid | 1106.12 | 6.67 | 0.60 |
| Geniposide | 5999 | 38.86 | 0.65 |
| Paeoniflorin | 3964 | 31.37 | 0.73 |
| Chebulinic acid | 4914 | 12.8 | 0.36 |
| Coptisine hydrochloride | 3951 | 33.1 | 0.83 |
| Baicalin | 4459 | 25.5 | 0.57 |
| Berberine | 15,464 | 64.1 | 0.41 |
| Wogonoside | 1654 | 14.53 | 0.87 |
| Baicalein | 2654 | 19.1 | 0.72 |
| Wogonin | 848 | 5.36 | 0.63 |
| Emodin | 1014 | 7.62 | 0.75 |
| Chrysophanol | 119 | 0.77 | 0.65 |
| Name | Average | SD | RSD (%) |
|---|---|---|---|
| Germacrone | 388.46 | 4.76 | 1.23 |
| Gallic acid | 568.2 | 5.4 | 0.95 |
| Geniposide | 2406 | 8.4 | 0.35 |
| Paeoniflorin | 617 | 4.56 | 0.74 |
| Chebulinic acid | 711 | 3.23 | 0.45 |
| Coptisine hydrochloride | 846 | 3.65 | 0.55 |
| Baicalin | 2454 | 21.14 | 0.86 |
| Berberine | 2646 | 21.57 | 0.82 |
| Wogonoside | 560.6 | 3.98 | 0.71 |
| Baicalein | 524.5 | 4.87 | 0.93 |
| Wogonin | 670.5 | 5.14 | 0.77 |
| Emodin | 2008 | 7.85 | 0.39 |
| Chrysophanol | 343 | 3.16 | 0.92 |
| Name | Average | SD | RSD (%) |
|---|---|---|---|
| Germacrone | 309.24 | 3.01 | 0.97 |
| Gallic acid | 518.12 | 6.71 | 1.29 |
| Geniposide | 2245 | 15.74 | 0.70 |
| Paeoniflorin | 594 | 3.67 | 0.62 |
| Chebulinic acid | 664 | 7.84 | 1.18 |
| Coptisine hydrochloride | 762 | 3.19 | 0.42 |
| Baicalin | 2371 | 13.15 | 0.55 |
| Berberine | 2196 | 24.30 | 1.11 |
| Wogonoside | 524.5 | 4.63 | 0.88 |
| Baicalein | 497.31 | 4.11 | 0.82 |
| Wogonin | 641.3 | 4.29 | 0.67 |
| Emodin | 1974.9 | 6.35 | 0.32 |
| Chrysophanol | 290.1 | 1.47 | 0.51 |
| Name | Average | SD | RSD (%) | Rate of Recovery (%) |
|---|---|---|---|---|
| Germacrone | 324.6 | 2.37 | 0.73 | 95.63 |
| Gallic acid | 901.06 | 8.47 | 0.94 | 93.71 |
| Geniposide | 1855.7 | 11.32 | 0.61 | 92.60 |
| Paeoniflorin | 1196.55 | 6.94 | 0.58 | 97.65 |
| Chebulinic acid | 2374.47 | 11.16 | 0.47 | 93.42 |
| Coptisine hydrochloride | 1929 | 9.45 | 0.49 | 94.28 |
| Baicalin | 2385.1 | 6.44 | 0.27 | 99.30 |
| Berberine | 10,112 | 15.45 | 0.15 | 92.88 |
| Wogonoside | 641.9 | 1.99 | 0.31 | 96.85 |
| Baicalein | 1109 | 8.98 | 0.81 | 95.52 |
| Wogonin | 829 | 1.16 | 0.14 | 96.44 |
| Emodin | 982 | 5.99 | 0.61 | 93.79 |
| Chrysophanol | 131.37 | 0.67 | 0.51 | 95.77 |
| Name | Weight Coefficients |
|---|---|
| Germacrone | 0.1541 |
| Gallic acid | 0.0467 |
| Geniposide | 0.0897 |
| Paeoniflorin | 0.0467 |
| Chebulinic acid | 0.0467 |
| Coptisine hydrochloride | 0.0467 |
| Baicalin | 0.0897 |
| Berberine | 0.0897 |
| Wogonoside | 0.0897 |
| Baicalein | 0.0897 |
| Wogonin | 0.0897 |
| Emodin | 0.0467 |
| Chrysophanol | 0.0467 |
| Yield of dry extract | 0.0272 |
| Name | Weight Coefficients |
|---|---|
| Germacrone | 0.0711 |
| Gallic acid | 0.0776 |
| Geniposide | 0.0916 |
| Paeoniflorin | 0.0767 |
| Chebulinic acid | 0.0524 |
| Coptisine hydrochloride | 0.0607 |
| Baicalin | 0.0584 |
| Berberine | 0.0611 |
| Wogonoside | 0.0654 |
| Baicalein | 0.0697 |
| Wogonin | 0.0653 |
| Emodin | 0.0726 |
| Chrysophanol | 0.0751 |
| Yield of dry extract | 0.1024 |
| Name | Comprehensive Weight Coefficients |
|---|---|
| Germacrone | 0.1564 |
| Gallic acid | 0.0518 |
| Geniposide | 0.1173 |
| Paeoniflorin | 0.0512 |
| Chebulinic acid | 0.0350 |
| Coptisine hydrochloride | 0.0405 |
| Baicalin | 0.0748 |
| Berberine | 0.0782 |
| Wogonoside | 0.0837 |
| Baicalein | 0.0893 |
| Wogonin | 0.0836 |
| Emodin | 0.0484 |
| Chrysophanol | 0.0501 |
| Yield of dry extract | 0.0398 |
| No. | (A) Ethanol Concentration% | (B) Reflux Time min | (C) Liquid–Material Ratio mL/g | Y Value% |
|---|---|---|---|---|
| 1 | 80.00 | 50.00 | 30:1 | 62.4835 |
| 2 | 70.00 | 50.00 | 25:1 | 89.7089 |
| 3 | 70.00 | 50.00 | 25:1 | 88.5748 |
| 4 | 60.00 | 50.00 | 30:1 | 75.3015 |
| 5 | 70.00 | 60.00 | 20:1 | 83.8701 |
| 6 | 80.00 | 60.00 | 25:1 | 69.4049 |
| 7 | 70.00 | 40.00 | 20:1 | 81.6292 |
| 8 | 70.00 | 50.00 | 25:1 | 88.7082 |
| 9 | 70.00 | 60.00 | 30:1 | 76.5164 |
| 10 | 60.00 | 60.00 | 25:1 | 78.0018 |
| 11 | 80.00 | 50.00 | 20:1 | 71.4199 |
| 12 | 60.00 | 50.00 | 20:1 | 79.4086 |
| 13 | 70.00 | 50.00 | 25:1 | 89.6892 |
| 14 | 60.00 | 40.00 | 25:1 | 76.9670 |
| 15 | 80.00 | 40.00 | 25:1 | 68.9748 |
| 16 | 70.00 | 40.00 | 30:1 | 74.4967 |
| 17 | 70.00 | 50.00 | 25:1 | 89.7066 |
| Project | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Modal | 1123.35 | 9 | 124.82 | 181.33 | <0.0001 ** | Significant |
| A | 174.81 | 1 | 174.81 | 253.96 | <0.0001 ** | |
| B | 4.10 | 1 | 4.10 | 5.95 | 0.0448 * | |
| C | 94.74 | 1 | 94.74 | 137.63 | <0.0001 ** | |
| AB | 0.091 | 1 | 0.091 | 0.13 | 0.7263 | |
| AC | 5.83 | 1 | 5.83 | 8.47 | 0.0226 * | |
| BC | 0.012 | 1 | 0.012 | 0.018 | 0.8977 | |
| A2 | 552.74 | 1 | 552.74 | 803.02 | <0.0001 ** | |
| B2 | 84.61 | 1 | 84.61 | 122.93 | <0.0001 ** | |
| C2 | 135.20 | 1 | 135.20 | 196.42 | <0.0001 ** | |
| Residual error | 4.82 | 7 | 0.69 | |||
| Misfit term | 3.46 | 3 | 1.15 | 3.40 | 0.1340 | Not significant |
| Pure error | 1.36 | 4 | 0.34 | |||
| Total deviation | 1128.17 | 16 |
| Name | Content in YEEs |
|---|---|
| Germacrone | 1.3160 ± 0.0055 |
| Gallic acid | 6.5340 ± 0.01078 |
| Geniposide | 17.7005 ± 0.0070 |
| Paeoniflorin | 89.6883 ± 0.0271 |
| Chebulinic acid | 17.5867 ± 0.0093 |
| Coptisine hydrochloride | 3.9456 ± 0.0023 |
| Baicalin | 14.6616 ± 0.0047 |
| Berberine | 98.2565 ± 0.0100 |
| Wogonoside | 5.0976 ± 0.0066 |
| Baicalein | 1.9298 ± 0.0020 |
| Wogonin | 2.8679 ± 0.0020 |
| Emodin | 1.0792 ± 0.0037 |
| Chrysophanol | 17.2449 ± 0.0096 |
| Group | Initial Weight | First Week | Second Week | Third Week | Fourth Week |
|---|---|---|---|---|---|
| HD-YEEs group | 209.71 ± 4.60 | 220.04 ± 3.86 | 243.87 ± 4.49 | 300.19 ± 10.42 | 315.15 ± 6.90 |
| MD-YEEs group | 205.69 ± 2.04 | 227.17 ± 2.80 | 258.02 ± 2.86 | 291.75 ± 2.39 | 320.42 ± 1.55 |
| LD-YEEs group | 210.97 ± 3.93 | 232.75 ± 4.83 | 264.19 ± 3.86 | 304.72 ± 4.54 | 332.90 ± 4.42 |
| VC group | 210.25 ± 4.15 | 233.61 ± 2.25 | 266.38 ± 2.87 | 302.68 ± 5.82 | 323.11 ± 8.64 |
| Group | Initial Weight | First Week | Second Week | Third Week | Fourth Week |
|---|---|---|---|---|---|
| HD-YEEs group | 193.39 ± 5.23 | 196.33 ± 4.14 | 205.21 ± 3.28 | 219.45 ± 1.81 | 228.22 ± 2.68 |
| MD-YEEs group | 189.05 ± 2.95 | 206.18 ± 2.34 | 220.99 ± 1.78 | 229.96 ± 1.66 | 235.44 ± 2.82 |
| LD-YEEs group | 198.12 ± 1.77 | 202.13 ± 3.02 | 213.73 ± 7.42 | 238.49 ± 7.47 | 237.89 ± 11.26 |
| VC group | 196.74 ± 3.13 | 202.57 ± 3.29 | 211.59 ± 3.52 | 227.90 ± 9.04 | 231.23 ± 8.03 |
| Group | Heart | Liver | Spleen | LUNG | Kidney | Testis |
|---|---|---|---|---|---|---|
| HD-YEEs group | 0.30 ± 0.01 | 2.65 ± 0.03 | 0.21 ± 0.01 | 0.43 ± 0.02 | 0.69 ± 0.03 | 1.02 ± 0.16 |
| MD-YEEs group | 0.30 ± 0.01 | 2.68 ± 0.16 | 0.21 ± 0.02 | 0.39 ± 0.01 | 0.66 ± 0.06 | 0.99 ± 0.10 |
| LD-YEEs group | 0.31 ± 0.01 | 2.66 ± 0.06 | 0.21 ± 0.01 | 0.41 ± 0.02 | 0.69 ± 0.02 | 0.99 ± 0.08 |
| VC group | 0.30 ± 0.01 | 2.56 ± 0.05 | 0.20 ± 0.01 | 0.42 ± 0.02 | 0.65 ± 0.02 | 0.91 ± 0.07 |
| Group | Heart | Liver | Spleen | Lung | Kidney | Ovary |
|---|---|---|---|---|---|---|
| HD-YEEs group | 0.32 ± 0.02 | 2.45 ± 0.04 | 0.28 ± 0.02 | 0.57 ± 0.06 | 0.73 ± 0.02 | 0.32 ± 0.03 |
| MD-YEEs group | 0.32 ± 0.01 | 2.52 ± 0.06 | 0.25 ± 0.02 | 0.59 ± 0.07 | 0.73 ± 0.02 | 0.37 ± 0.01 |
| LD-YEEs group | 0.32 ± 0.02 | 2.79 ± 0.20 | 0.26 ± 0.01 | 0.50 ± 0.03 | 0.70 ± 0.10 | 0.33 ± 0.02 |
| VC group | 0.33 ± 0.01 | 2.67 ± 0.11 | 0.26 ± 0.02 | 0.54 ± 0.02 | 0.67 ± 0.07 | 0.34 ± 0.01 |
| Group | WBC (109/L) | LYM (109/L) | HGB (109/L) | RBC (1012/L) | PLT (109/L) |
|---|---|---|---|---|---|
| HD-YEEs group | 7.01 ± 0.11 | 5.41 ± 0.12 | 15.57 ± 0.16 | 7.20 ± 0.15 | 1126.80 ± 7.95 |
| MD-YEEs group | 7.44 ± 0.30 | 6.12 ± 0.27 | 15.60 ± 0.13 | 7.30 ± 0.08 | 1144.55 ± 16.75 |
| LD-YEEs group | 6.79 ± 0.35 | 6.24 ± 0.46 | 15.90 ± 0.17 | 7.46 ± 0.14 | 1163.63 ± 12.97 |
| VC group | 7.11 ± 0.59 | 5.21 ± 0.55 | 15.54 ± 0.28 | 7.50 ± 0.22 | 1158.75 ± 24.17 |
| Group | WBC (109/L) | LYM (109/L) | HGB (109/L) | RBC (1012/L) | PLT (109/L) |
|---|---|---|---|---|---|
| HD-YEEs group | 3.76 ± 0.27 | 3.48 ± 0.29 | 15.41 ± 0.18 | 7.18 ± 0.11 | 806.75 ± 29.72 |
| MD-YEEs group | 4.00 ± 0.36 | 3.60 ± 0.32 | 15.13 ± 0.15 | 7.43 ± 0.11 | 842.14 ± 42.28 |
| LD-YEEs group | 4.27 ± 0.08 | 3.53 ± 0.29 | 15.37 ± 0.25 | 7.09 ± 0.25 | 794.14 ± 18.29 |
| VC group | 3.72 ± 0.25 | 3.00 ± 0.21 | 15.33 ± 0.21 | 7.07 ± 0.12 | 780.71 ± 80.67 |
| Group | ALT (U/L) | AST (U/L) | ALP (g/L) | Cr (umol/L) | BUN (mg/dL) |
|---|---|---|---|---|---|
| HD-YEEs group | 120.84 ± 30.99 | 119.73 ± 38.95 | 161.86 ± 41.12 | 53.94 ± 7.89 | 21.09 ± 2.62 |
| MD-YEEs group | 115.20 ± 27.58 | 115.54 ± 35.91 | 160.86 ± 38.72 | 51.62 ± 7.94 | 19.81 ± 2.72 |
| LD-YEEs group | 116.09 ± 29.42 | 120.73 ± 35.86 | 162.29 ± 37.37 | 50.04 ± 8.08 | 22.40 ± 2.54 |
| VC group | 114.96 ± 33.85 | 119.49 ± 37.17 | 156.75 ± 39.80 | 50.89 ± 8.17 | 20.44 ± 3.25 |
| Group | ALT (U/L) | AST (U/L) | ALP (g/L) | Cr (umol/L) | BUN (mg/dL) |
|---|---|---|---|---|---|
| HD-YEEs group | 102.38 ± 19.11 | 129.56 ± 23.82 | 58.29 ± 13.56 | 68.30 ± 17.92 | 27.78 ± 3.43 |
| MD-YEEs group | 97.50 ± 22.03 | 126.12 ± 27.63 | 51.27 ± 9.12 | 66.69 ± 16.10 | 25.35 ± 3.65 |
| LD-YEEs group | 97.63 ± 27.86 | 124.91 ± 27.06 | 62.83 ± 14.59 | 67.24 ± 12.03 | 27.20 ± 3.30 |
| VC group | 111.55 ± 19.95 | 130.34 ± 38.44 | 60.72 ± 11.48 | 71.82 ± 11.91 | 29.05 ± 3.72 |
| Level | (A) Ethanol Concentration (%) | (B) Reflux Time (min) | (C) Liquid–Material Ratio (mL/g) |
|---|---|---|---|
| −1 | 60 | 40 | 20:1 |
| 0 | 70 | 50 | 25:1 |
| 1 | 80 | 60 | 30:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zhang, W.; Zhao, W.; Cai, Y.; Qin, X.; Wang, B.; Xue, J.; Wen, Y.; Wei, Y.; Hua, Y.; et al. Optimization of Ethanol Extraction Technology for Yujin Powder Using Response Surface Methodology with a Box–Behnken Design Based on Analytic Hierarchy Process–Criteria Importance through Intercriteria Correlation Weight Analysis and Its Safety Evaluation. Molecules 2023, 28, 8124. https://doi.org/10.3390/molecules28248124
Jiang L, Zhang W, Zhao W, Cai Y, Qin X, Wang B, Xue J, Wen Y, Wei Y, Hua Y, et al. Optimization of Ethanol Extraction Technology for Yujin Powder Using Response Surface Methodology with a Box–Behnken Design Based on Analytic Hierarchy Process–Criteria Importance through Intercriteria Correlation Weight Analysis and Its Safety Evaluation. Molecules. 2023; 28(24):8124. https://doi.org/10.3390/molecules28248124
Chicago/Turabian StyleJiang, Lidong, Wangdong Zhang, Wenbo Zhao, Yanzi Cai, Xue Qin, Baoshan Wang, Jiao Xue, Yanqiao Wen, Yanming Wei, Yongli Hua, and et al. 2023. "Optimization of Ethanol Extraction Technology for Yujin Powder Using Response Surface Methodology with a Box–Behnken Design Based on Analytic Hierarchy Process–Criteria Importance through Intercriteria Correlation Weight Analysis and Its Safety Evaluation" Molecules 28, no. 24: 8124. https://doi.org/10.3390/molecules28248124
APA StyleJiang, L., Zhang, W., Zhao, W., Cai, Y., Qin, X., Wang, B., Xue, J., Wen, Y., Wei, Y., Hua, Y., & Yao, W. (2023). Optimization of Ethanol Extraction Technology for Yujin Powder Using Response Surface Methodology with a Box–Behnken Design Based on Analytic Hierarchy Process–Criteria Importance through Intercriteria Correlation Weight Analysis and Its Safety Evaluation. Molecules, 28(24), 8124. https://doi.org/10.3390/molecules28248124