Oxidation of Mesalamine under Phenoloxidase- or Peroxidase-like Enzyme Catalysis
Abstract
:1. Introduction
2. Results and Discussion
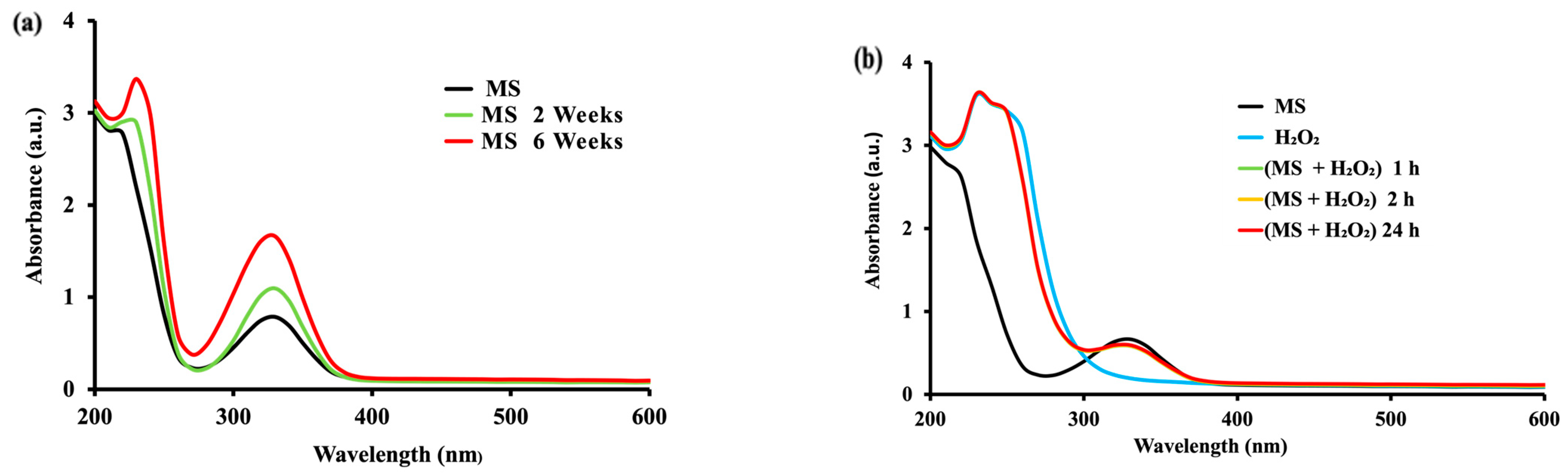
2.1. Effect of Time in the Mesalamine Oxidation
2.2. Oxidation of Mesalamine Using Vegetal and Animal Enzymes
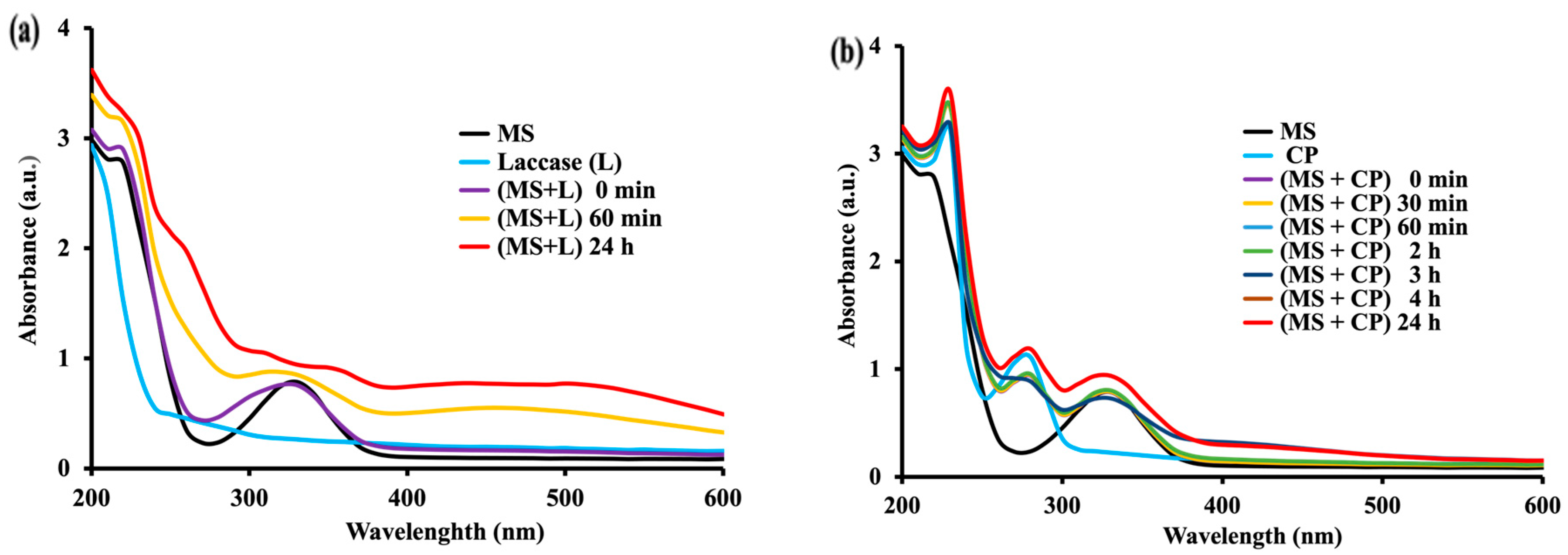
2.2.1. Oxidation of MS with Molecular Oxygen Catalyzed by Laccase and by Ceruloplasmin
SH2 + ½ O2 ---------> Q + H2O
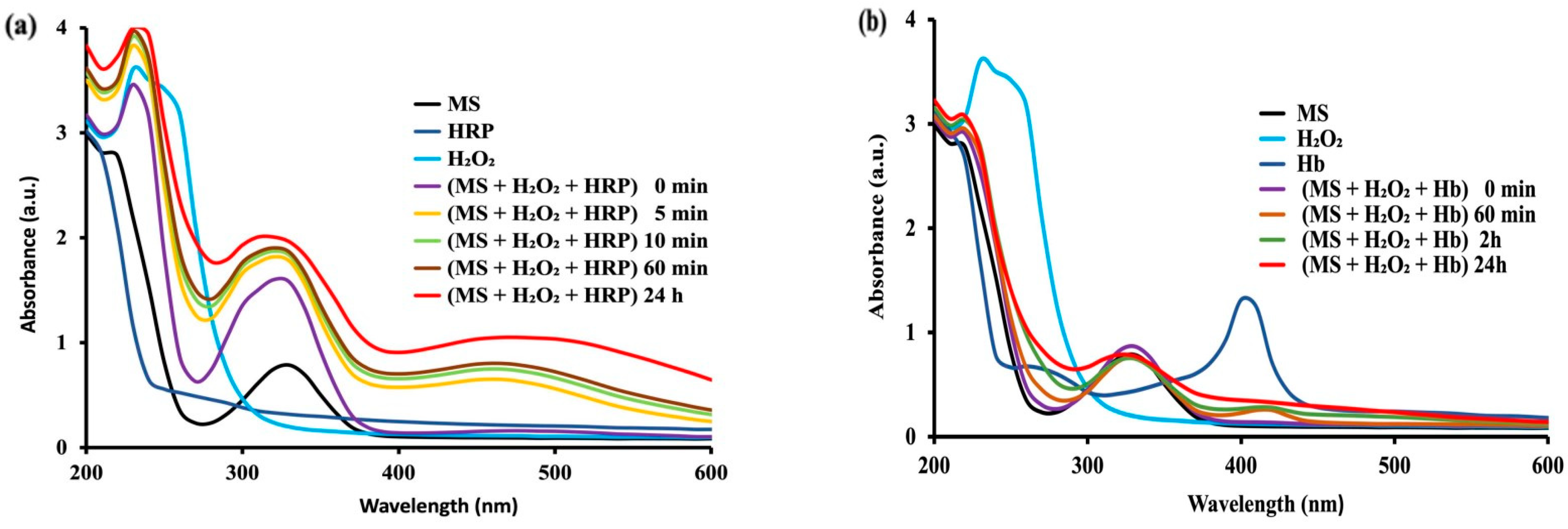
2.2.2. Oxidation of MS with Hydrogen Peroxide Catalyzed by Peroxidase and Hemoglobin
SH2 + H2O2 ----> Q + 2H2O
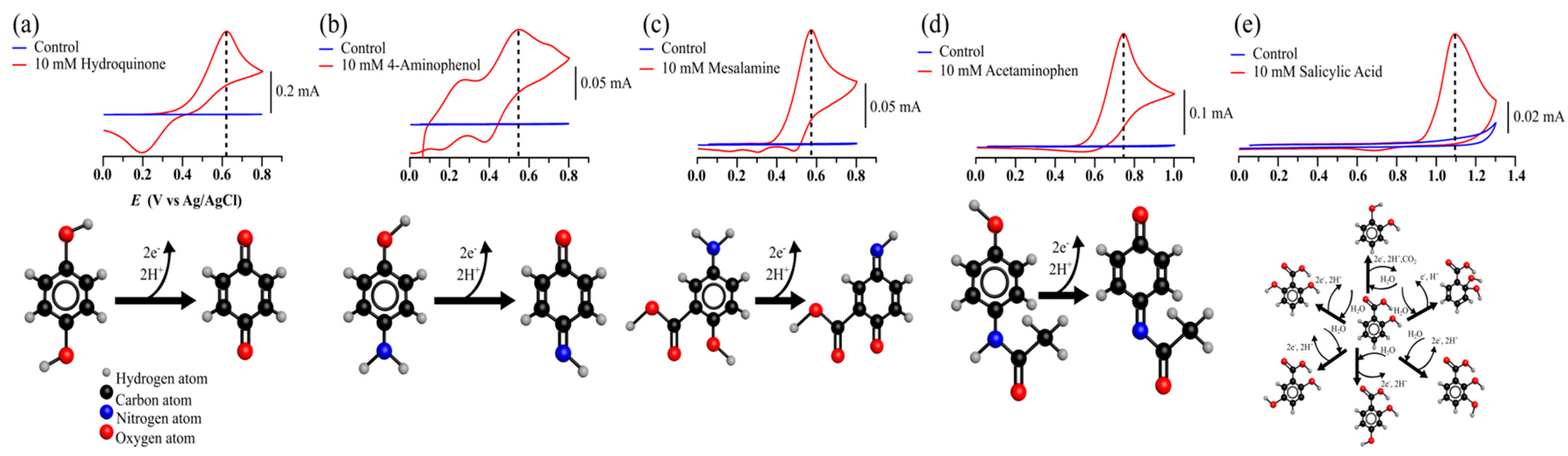
2.3. Evaluation of Susceptibility to Oxidation of Mesalamine by Cyclic Voltametry
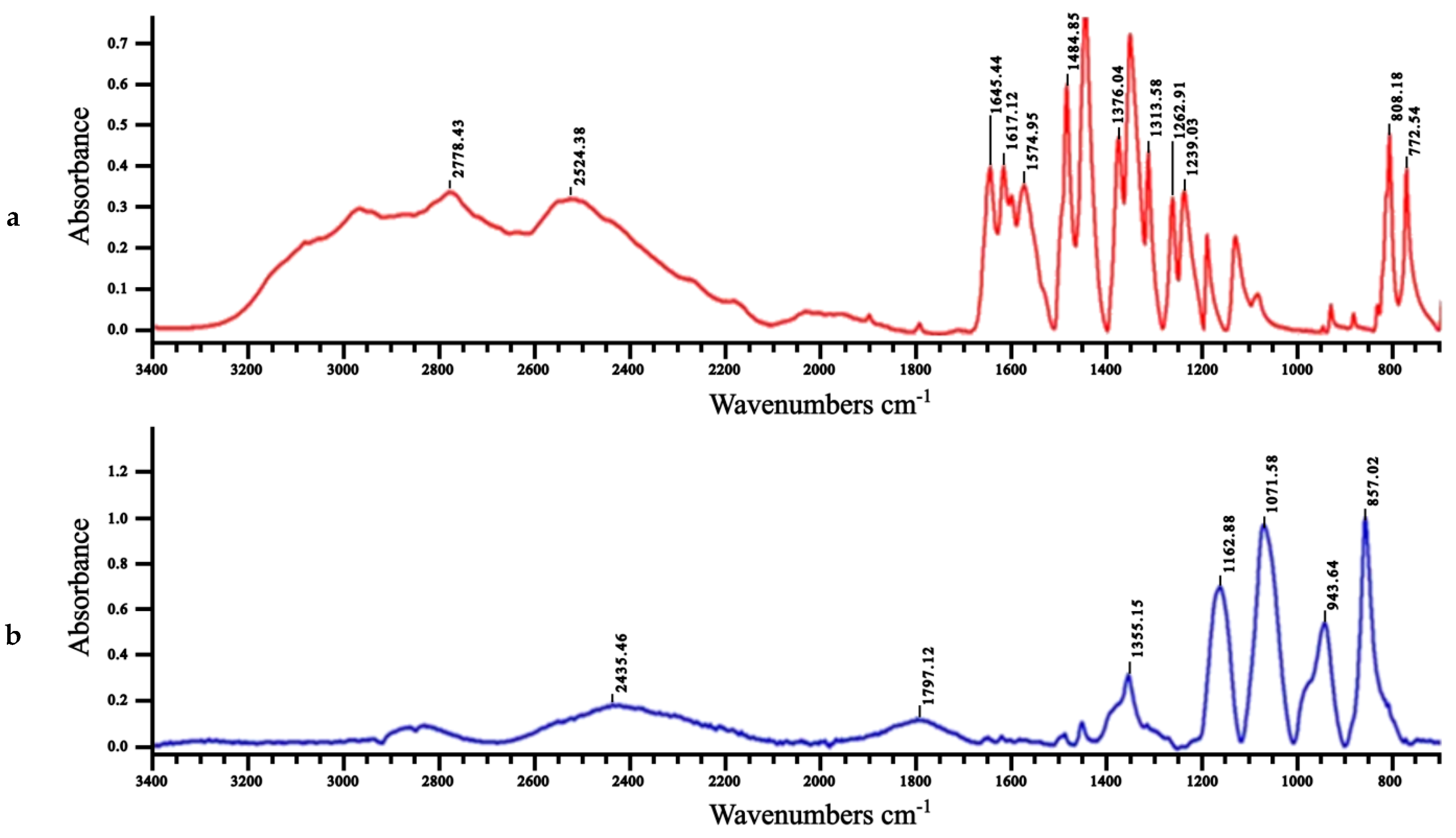
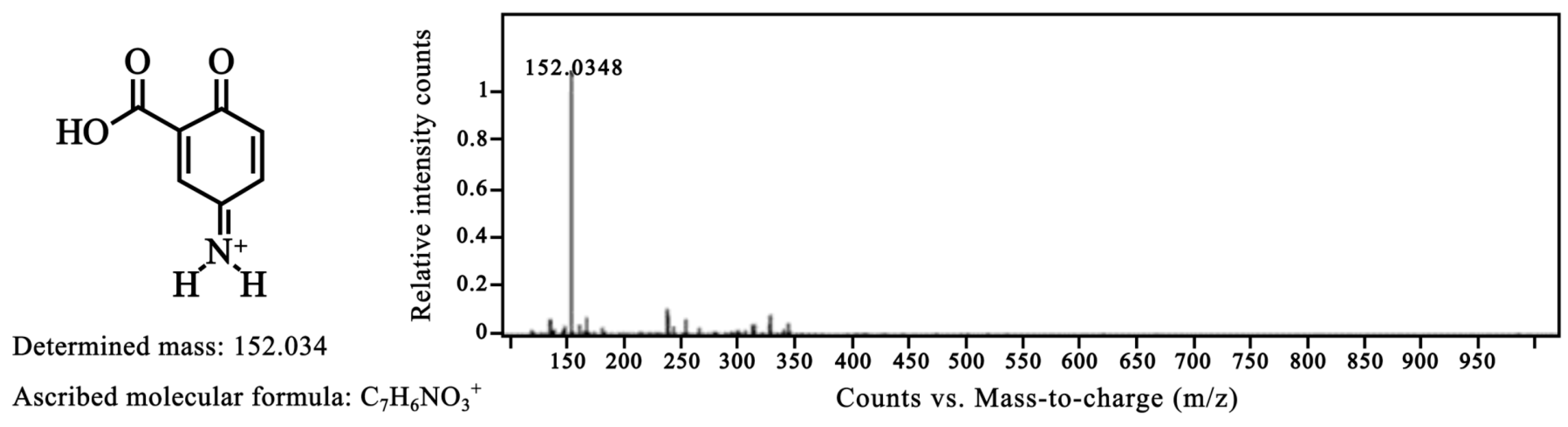
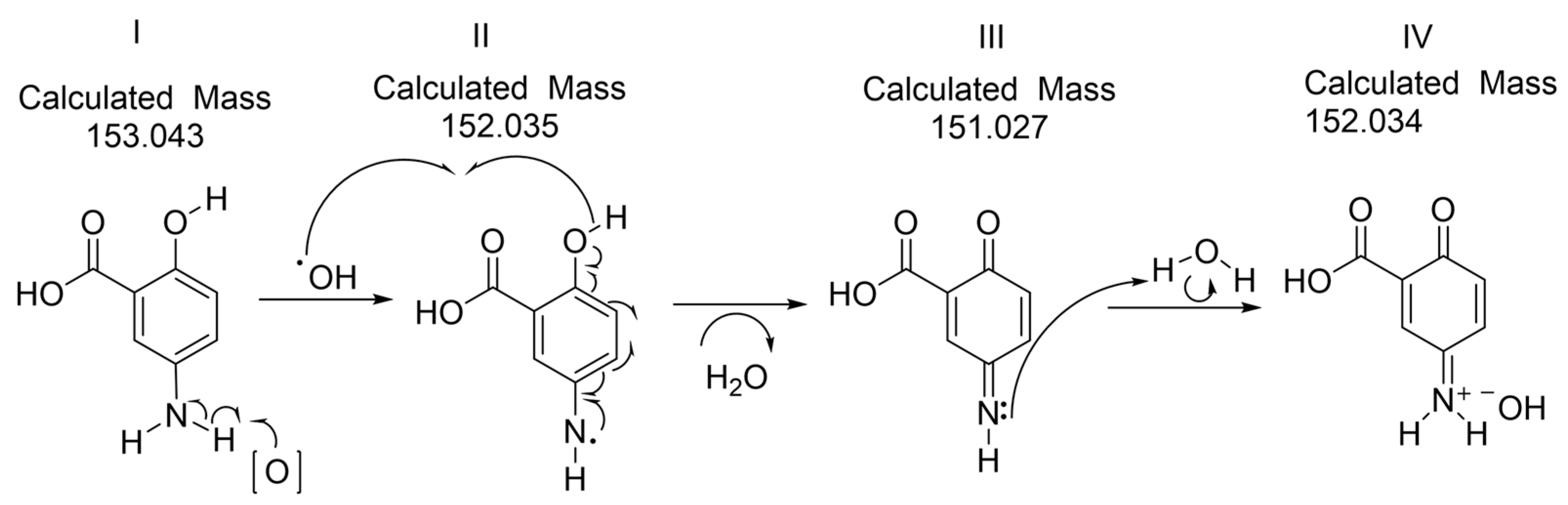
2.4. FTIR, 1H NMR and HRMS of the Oxidized Mesalamine
3. Materials and Methods
3.1. Materials
3.2. Mesalamine Spontaneous Oxidation—Spectrophotometric Measurements
3.3. Assays of Enzyme Activities
3.4. Mesalamine Oxidation under Enzyme Catalysis
3.4.1. The Oxidation of Mesalamine with O2-Dependent Copper-Containing Oxidases: Laccase and CP
3.4.2. The Oxidation of Mesalamine with H2O2-Dependent Peroxidase and Peroxidase-like Hb
3.5. Electrochemical Measurements
3.6. Fourier-Transform Infrared (FTIR)
3.7. 1H NMR Spectroscopy
3.8. Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nikias, G.; Eisner, T.; Katz, S.; Levin, L.; Eskries, D.; Urmacher, C.; McKinley, M. Crohn’s disease and colorectal carcinoma: Rectal cancer complicating longstanding active perianal disease. Am. J. Gastroenterol. 1995, 90, 216–219. [Google Scholar]
- Abdu-Allah, H.H.; El-Shorbagi, A.; Abdel-Moty, S.G.; El-Awady, R.; Abdel-Alim, A. 5-Aminosalyclic acid (5-ASA): A unique anti-inflammatory salicylate. Med. Chem. 2016, 6, 306–315. [Google Scholar] [CrossRef]
- Mesalazine European Pharmacopoeia (EP) Reference Standard. Y0000297. Available online: http://www.uspbpep.com/ep50/Mesalazine.pdf (accessed on 19 October 2023).
- Frolkis, A.; Dieleman, L.A.; Barkema, H.W.; Panaccione, R.; Ghosh, S.; Fedorak, R.N.; Madsen, K.; Kaplan, G.G.; Consortium, A.I. Environment and the inflammatory bowel diseases. Can. J. Gastroenterol. 2013, 27, e18–e24. [Google Scholar] [CrossRef]
- Oostlander, A.E.; Everts, V.; Schoenmaker, T.; Bravenboer, N.; van Vliet, S.J.; van Bodegraven, A.A.; Lips, P.; de Vries, T.J. T cell-mediated increased osteoclast formation from peripheral blood as a mechanism for crohn’s disease-associated bone loss. J. Cell. Biochem. 2012, 113, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Actis, G.C.; Pazienza, P.; Rosina, F. Mesalamine for inflammatory bowel disease: Recent reappraisals. Inflamm. Allergy-Drug Targets 2008, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, O.Ø.; Cortot, A.; Jewell, D.; Wright, J.P.; Winter, T.; Veloso, F.T.; Vatn, M.; Persson, T.; Pettersson, E. A comparison of budesonide and mesalamine for active Crohn’s disease. N. Engl. J. Med. 1998, 339, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Meczker, Á.; Mikó, A.; Hegyi, P. 5-ASA induces mild acute pancreatitis. Case report and review of the literature. J. Gastrointest. Liver Dis. 2018, 27, 189–194. [Google Scholar]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Kang, S.; Kim, W.; Jeong, S.; Lee, Y.; Nam, J.; Lee, S.; Jung, Y. Oxidized 5-aminosalicylic acid activates Nrf2-HO-1 pathway by covalently binding to Keap1: Implication in anti-inflammatory actions of 5-aminosalicylic acid. Free Radic. Biol. Med. 2017, 108, 715–724. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Murakami, K.; Yoshino, M. Prooxidant activity of aminophenol compounds: Copper-dependent generation of reactive oxygen species. BioMetals 2022, 35, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Mazaleuskaya, L.L.; Sangkuhl, K.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenetics Genom. 2015, 25, 416. [Google Scholar] [CrossRef]
- Ba, S.; Arsenault, A.; Hassani, T.; Jones, J.P.; Cabana, H. Laccase immobilization and insolubilization: From fundamentals to applications for the elimination of emerging contaminants in wastewater treatment. Crit. Rev. Biotechnol. 2013, 33, 404–418. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-catalyzed oxidative polymerization of phenolic compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Rich, S.; Horsfall, J.G. Relation of polyphenol oxidases to fungitoxicity. Proc. Natl. Acad. Sci. USA 1954, 40, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.J.; Chiamori, N.; Jacobs, S.; Segalove, M. Determination of ceruloplasmin oxidase in serum. Proc. Soc. Exp. Biol. Med. 1960, 104, 620–624. [Google Scholar] [CrossRef]
- Peisach, J.; Levine, W.G. A comparison of the enzymic activities of pig ceruloplasmin and Rhus vernicifera laccase. J. Biol. Chem. 1965, 240, 2284–2289. [Google Scholar] [CrossRef]
- Mateescu, M.A.; Schell, H.D.; Budu, C.E. Spectrophotometric method for rapid determination of peroxidase activity: Possibilities of simultaneous determination of p-diphenoloxidase (laccase). Rev. Roum. Biochim. 1979, 16, 115–120. [Google Scholar]
- Everse, J. Heme Proteins. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 354–361. [Google Scholar]
- Biggs, K. Phenolic cross-linking in the plant cell wall. In Physiology of Expansion during Plant Growth; American Society of Plant Physiologists: Rockville, PA, USA, 1987; pp. 46–57. [Google Scholar]
- Saleh Ahammad, A. Hydrogen peroxide biosensors based on horseradish peroxidase and hemoglobin. Biosens. Bioelectron. 2013, 9, 1–11. [Google Scholar]
- Widmer, C.C.; Pereira, C.P.; Gehrig, P.; Vallelian, F.; Schoedon, G.; Buehler, P.W.; Schaer, D.J. Hemoglobin can attenuate hydrogen peroxide–induced oxidative stress by acting as an antioxidative peroxidase. Antioxid. Redox Signal. 2010, 12, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Cortot, A.; Maetz, D.; Degoutte, E.; Delette, O.; Meunier, P.; Tan, G.; Cazals, J.-B.; Dewit, O.; Hebuterne, X.; Beorchia, S. Mesalamine foam enema versus mesalamine liquid enema in active left-sided ulcerative colitis. Am. J. Gastroenterol. 2008, 103, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Cohen, R.D. Mesalamine delivery systems: Do they really make much difference? Adv. Drug Deliv. Rev. 2005, 57, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Prenant, M.; Bessis, M. On the proper use of the Soret band for hemoglobin detection in erythrocytic cells. Blood Cells 1978, 4, 361–367. [Google Scholar] [PubMed]
- Mai, N.L.; Ambauen, N.; Halle, C.; Meyn, T.; Trinh, T.T. Initial degradation mechanism of salicylic acid via electrochemical process. Chem. Phys. 2021, 543, 111071. [Google Scholar] [CrossRef]
- Umesh, N.M.; Amalraj, A.J.J.; Wang, S.-F. Trace level electrochemical detection of mesalazine in human urine sample using poly (N-Vinyl)-2-Pyrrolidone capped Bi-EDTA complex sheets incorporated with ultrasonically exfoliated graphene oxide. J. Taiwan Inst. Chem. Eng. 2021, 122, 67–77. [Google Scholar] [CrossRef]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 6, 1483–1497. [Google Scholar] [CrossRef]
- Vinoth Kumar, J.; Kokulnathan, T.; Chen, S.-M.; Chen, T.-W.; Elgorban, A.M.; Elshikh, M.S.; Marraiki, N.; Nagarajan, E.R. Two-Dimensional Copper Tungstate Nanosheets: Application toward the Electrochemical Detection of Mesalazine. ACS Sustain. Chem. Eng. 2019, 7, 18279–18287. [Google Scholar] [CrossRef]
- Chandrashekar, B.N.; Swamy, B.E.K.; Pandurangachar, M.; Sathisha, T.V.; Sherigara, B.S. Electrochemical Investigation of 4-Aminophenol at CTAB Modified Carbon Paste Electrode: A Cyclic Voltammetric. Anal. Bioanal. Electrochem. 2011, 3, 227–232. [Google Scholar]
- Dou, N.; Zhang, S.; Qu, J. Simultaneous detection of acetaminophen and 4-aminophenol with an electrochemical sensor based on silver–palladium bimetal nanoparticles and reduced graphene oxide. RSC Adv. 2019, 9, 31440–31446. [Google Scholar] [CrossRef] [PubMed]
- Madhu, R.; Palanisamy, S.; Chen, S.-M.; Piraman, S. A low temperature synthesis of activated carbon from the bio waste for simultaneous electrochemical determination of hydroquinone and catechol. J. Electroanal. Chem. 2014, 727, 84–90. [Google Scholar] [CrossRef]
- Amiraslanova, M.; Alieva, A.; Akhmedbekova, S.; Ibragimova, M.D.; Mamedzade, F.; Rustamov, R.; Alieva, S.R.; Dadasheva, N.; Isaeva, P. Using IR Spectroscopy to Study Structures of Benzylamine Phenol-Formaldehyde Oligomers. J. Struct. Chem. 2019, 60, 1037–1042. [Google Scholar] [CrossRef]
- Mecke, B.; Noack, K. Untersuchungen über die Beeinflussung der Frequenz und Intensität der νC-O-und νC-C-Banden im IR-Spektrum ungesättigter Ketone durch Konjugation und sterische Hinderung. Spectrochim. Acta 1958, 12, 391–393. [Google Scholar] [CrossRef]
- Hrycay, E.G.; Bandiera, S.M. Monooxygenase, Peroxidase and Peroxygenase Properties and Reaction Mechanisms of Cytochrome P450 Enzymes; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Nakayama, T.; Honda, R. Electrochemical and mechanistic study of superoxide elimination by mesalazine through proton-coupled electron transfer. Pharmaceuticals 2021, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- MacFie, T.S.; Poulsom, R.; Parker, A.; Warnes, G.; Boitsova, T.; Nijhuis, A.; Suraweera, N.; Poehlmann, A.; Szary, J.; Feakins, R. DUOX2 and DUOXA2 form the predominant enzyme system capable of producing the reactive oxygen species H2O2 in active ulcerative colitis and are modulated by 5-aminosalicylic acid. Inflamm. Bowel Dis. 2014, 20, 514–524. [Google Scholar] [CrossRef]
- Alothman, M.; Ispas-Szabo, P.; Mateescu, M.A. Design of catalase monolithic tablets for intestinal targeted delivery. Pharmaceutics 2021, 13, 69. [Google Scholar] [CrossRef]
- Wang, X.; Dumoulin, M.; Befani, O.; Mondovi, B.; Mateescu, M. Joint chromatographic purification of bovine serum ceruloplasmin and amineoxidase. Prep. Biochem. Biotechnol. 1994, 24, 237–250. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Zein, R.; Ispas-Szabo, P.; Jafari, M.; Siaj, M.; Mateescu, M.A. Oxidation of Mesalamine under Phenoloxidase- or Peroxidase-like Enzyme Catalysis. Molecules 2023, 28, 8105. https://doi.org/10.3390/molecules28248105
El Zein R, Ispas-Szabo P, Jafari M, Siaj M, Mateescu MA. Oxidation of Mesalamine under Phenoloxidase- or Peroxidase-like Enzyme Catalysis. Molecules. 2023; 28(24):8105. https://doi.org/10.3390/molecules28248105
Chicago/Turabian StyleEl Zein, Rimaz, Pompilia Ispas-Szabo, Maziar Jafari, Mohamed Siaj, and Mircea Alexandru Mateescu. 2023. "Oxidation of Mesalamine under Phenoloxidase- or Peroxidase-like Enzyme Catalysis" Molecules 28, no. 24: 8105. https://doi.org/10.3390/molecules28248105
APA StyleEl Zein, R., Ispas-Szabo, P., Jafari, M., Siaj, M., & Mateescu, M. A. (2023). Oxidation of Mesalamine under Phenoloxidase- or Peroxidase-like Enzyme Catalysis. Molecules, 28(24), 8105. https://doi.org/10.3390/molecules28248105








