Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation
Abstract
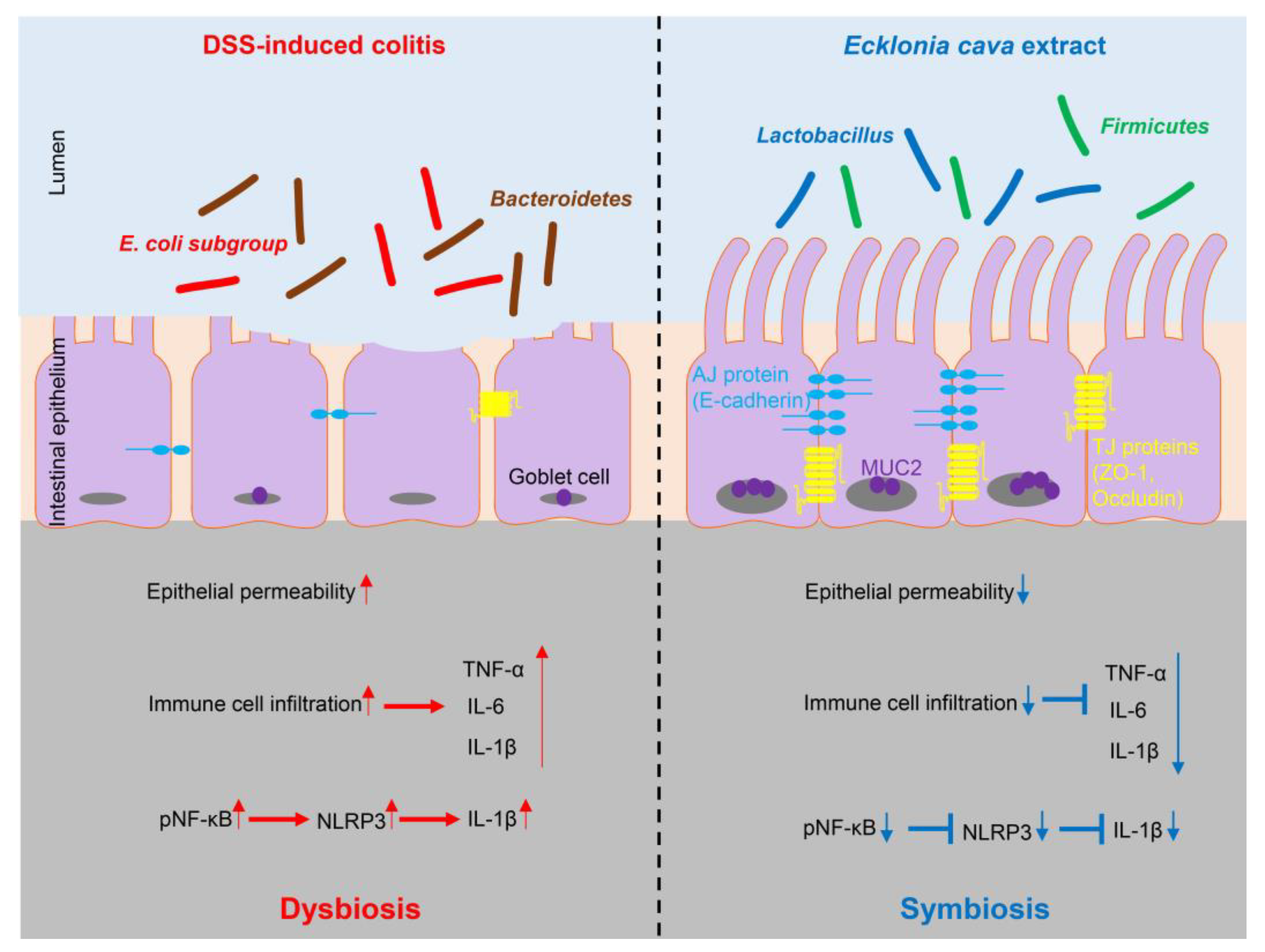
:1. Introduction
2. Results
2.1. Ecklonia cava Extract Protects against DSS-Induced Colitis
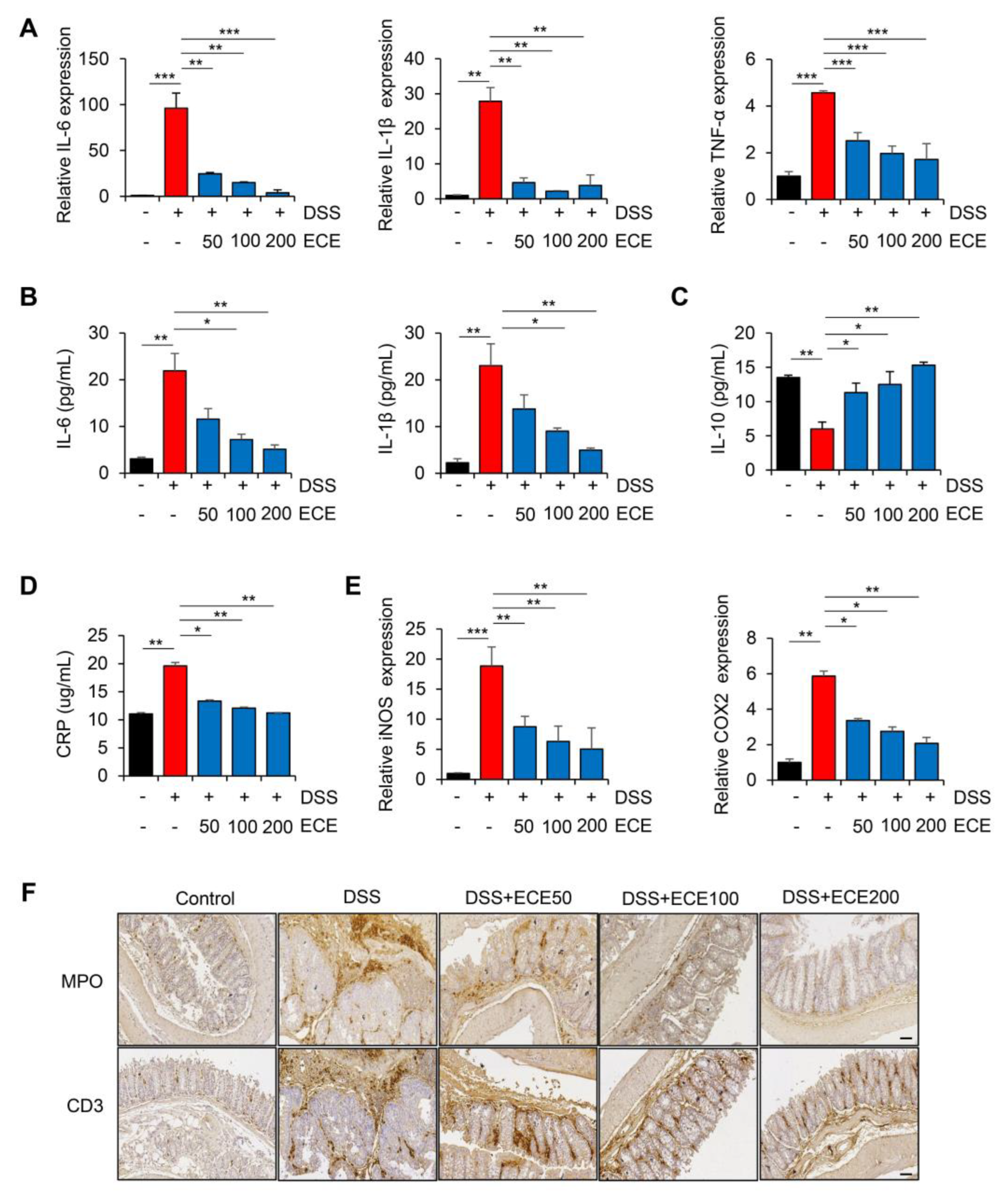
2.2. Ecklonia cava Extract Reduces Inflammatory Response in DSS-Induced Colitis
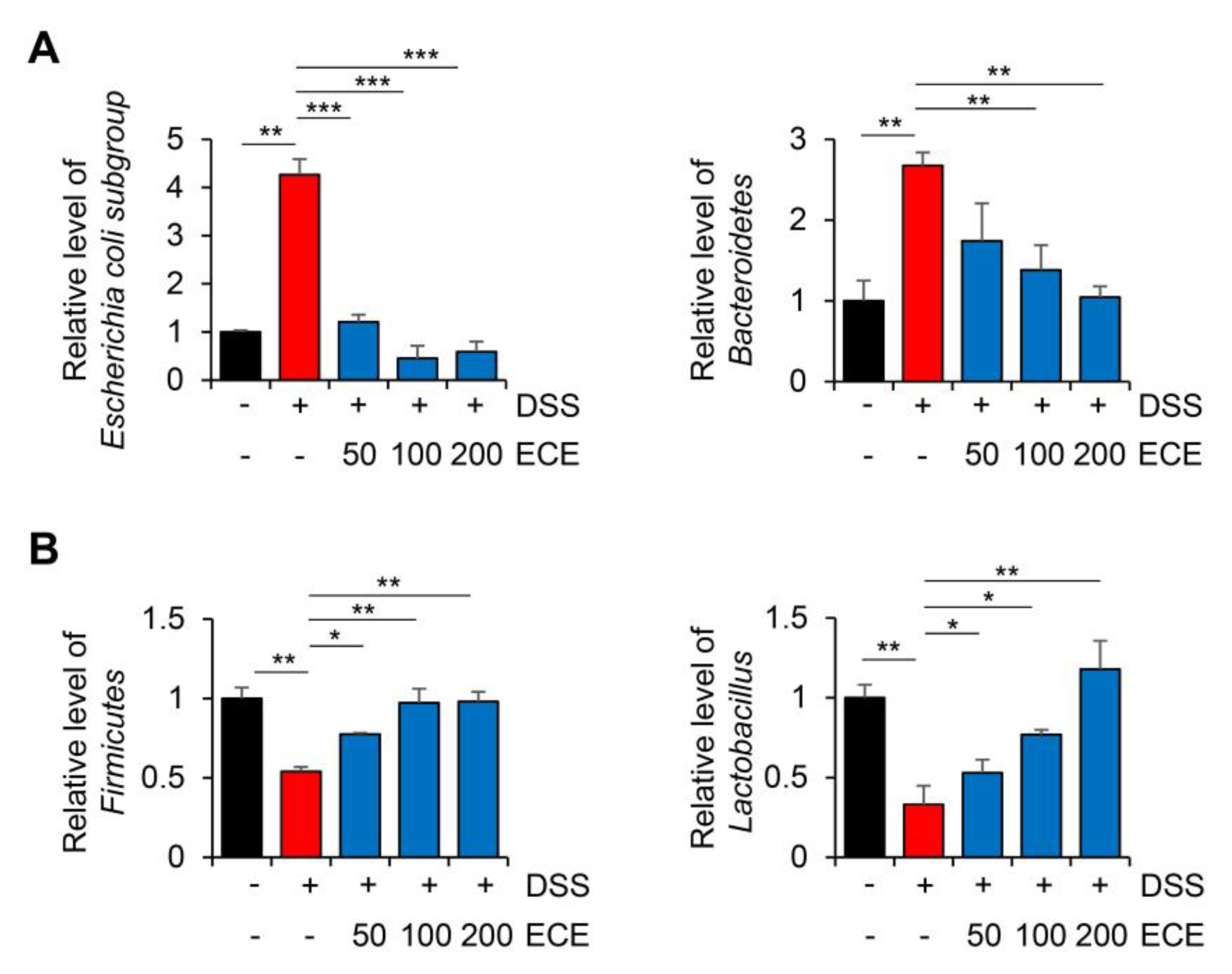
2.3. Ecklonia cava Extract Ameliorates Gut Microbiome Imbalance with DSS-Induced Colitis
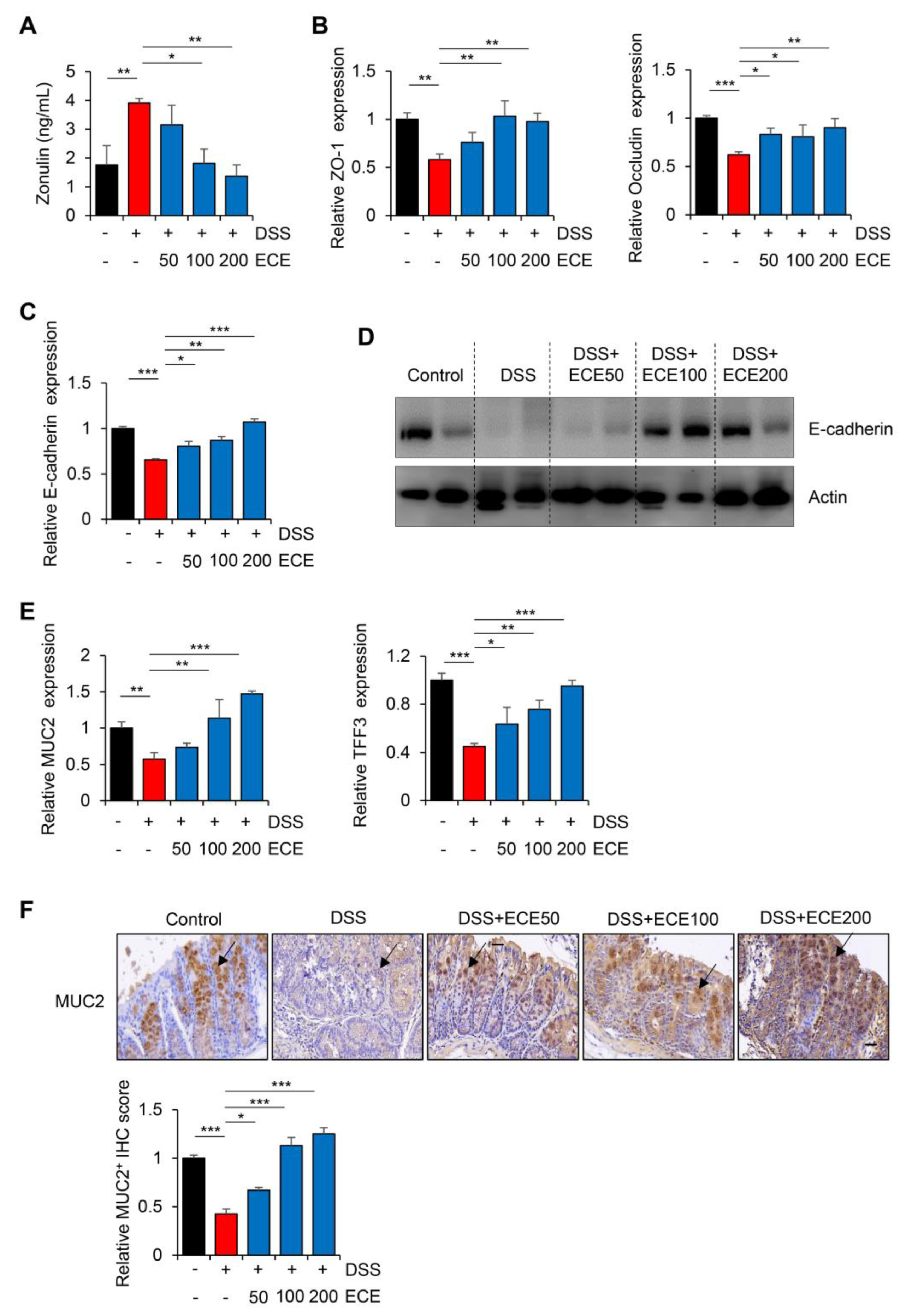
2.4. Ecklonia cava Extract Restores Stability of Intestinal Barrier
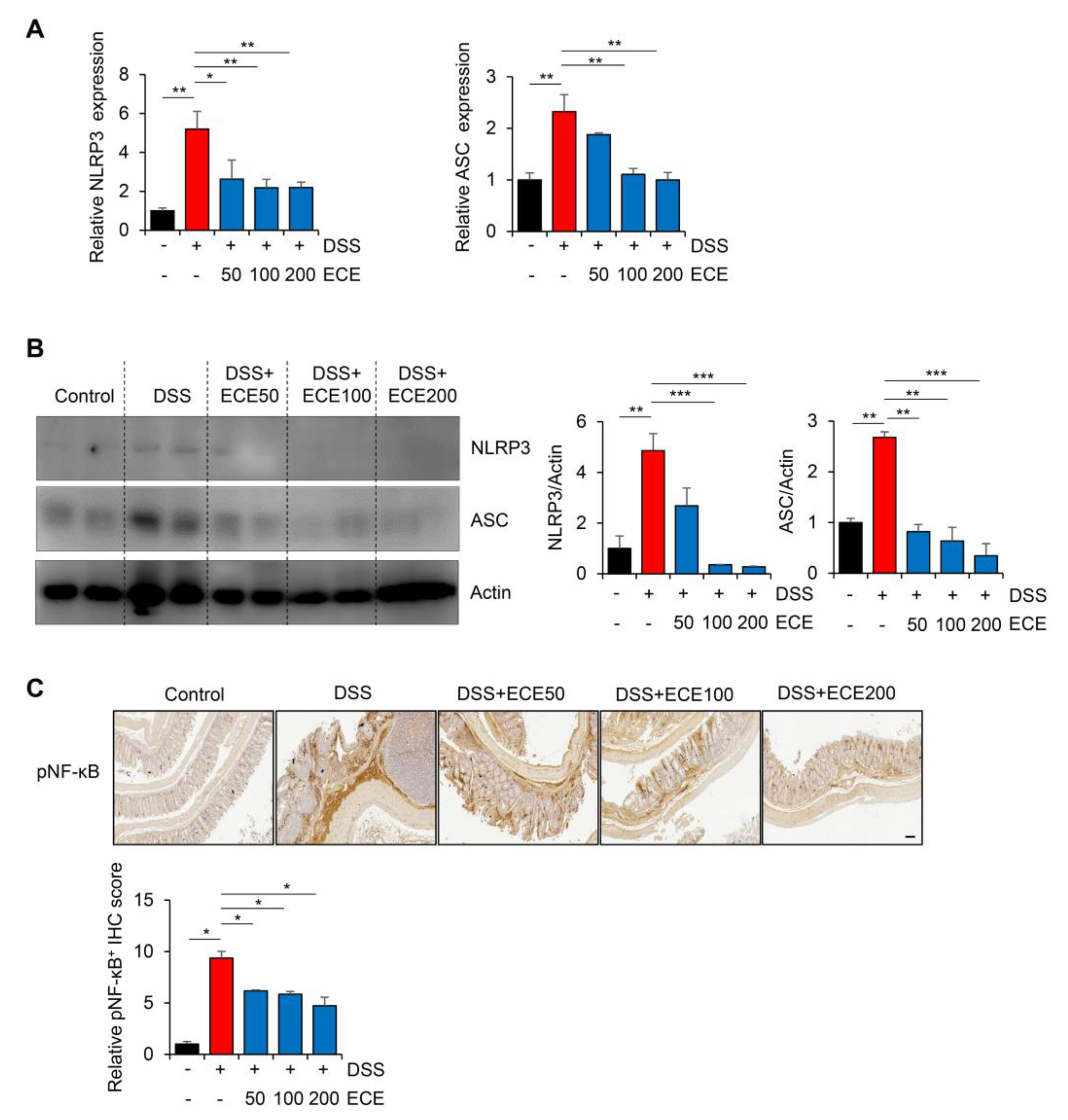
2.5. Ecklonia cava Extract Suppresses the Pathological Inflammatory Signaling
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Characterization of Ecklonia cava Extract Using High-Performance Liquid Chromatography Analysis and Toxicity of Ecklonia cava Extract
4.3. DSS-Induced Colitis Mouse Model
4.4. Western Blot
4.5. Total RNA Isolation and Quantitative Real-Time PCR
4.6. Bacterial DNA Extraction from Mice Ceca and Microbiota Analysis
4.7. ELISA for Serum Markers
4.8. Hematoxylin–Eosin Staining and Immunohistochemistry
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Hodson, R. Inflammatory bowel disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liang, W.; Wang, T.; Sui, J.; Wang, J.; Deng, Z.; Chen, D. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol. Res. 2019, 144, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Arancibia-Cárcamo, C.V.; Fleming, M.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.; Renooij, W.; Murzilli, S.; Klomp, L.W.; Siersema, P.D.; Schipper, M.E.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Engevik, M.A.; Herrmann, B.; Ruan, W.; Engevik, A.C.; Engevik, K.A.; Ihekweazu, F.; Shi, Z.; Luck, B.; Chang-Graham, A.L.; Esparza, M.; et al. Bifidobacterium dentium-derived y-glutamylcysteine suppresses ER-mediated goblet cell stress and reduces TNBS-driven colonic inflammation. Gut Microbes 2021, 13, 1902717. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kitani, A.; Strober, W.; Fuss, I.J. The Role of NLRP3 and IL-1β in the Pathogenesis of Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 2566. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 210–220. [Google Scholar] [PubMed]
- Catalan-Serra, I.; Brenna, Ø. Immunotherapy in inflammatory bowel disease: Novel and emerging treatments. Hum. Vaccines Immunother. 2018, 14, 2597–2611. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Jeon, Y.J. The Role of Seaweed Polysaccharides in Gastrointestinal Health: Protective Effect against Inflammatory Bowel Disease. Life 2023, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.; Lee, H.G.; Jayawardena, T.U.; Jeon, Y.J. Anti-Fine Dust Effect of Fucoidan Extracted from Ecklonia maxima Laves in Macrophages via Inhibiting Inflammatory Signaling Pathways. Mar. Drugs 2022, 20, 413. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.M.; Lee, H.G.; Nagahawatta, D.P.; Jayawardhana, H.; Ryu, B.; Jeon, Y.J. Characterization and therapeutic effect of Sargassum coreanum fucoidan that inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophages by blocking NF-κB signaling. Int. J. Biol. Macromol. 2022, 223 Pt A, 500–510. [Google Scholar] [CrossRef]
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2019, 126, 326–336. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, H.J.; Heo, H.J. Ecklonia cava Attenuates PM2.5-Induced Cognitive Decline through Mitochondrial Activation and Anti-Inflammatory Effect. Mar. Drugs 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.A.; Oh, S.; Yang, J.Y.; Lee, S.Y.; Son, K.H.; Byun, K. Ecklonia cava extracts decrease hypertension-related vascular calcification by modulating PGC-1α and SOD2. Biomed. Pharmacother. 2022, 153, 113283. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Choi, J.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Phlorotannins from Ecklonia cava Attenuates Palmitate-Induced Endoplasmic Reticulum Stress and Leptin Resistance in Hypothalamic Neurons. Mar. Drugs 2019, 17, 570. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Lee, H.S.; Ryu, B.; Jiang, Y.; Jang, J.T.; Jeon, Y.J.; Byun, K. Pyrogallol-Phloroglucinol-6,6’-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models. Mar. Drugs 2019, 17, 272. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Jung, M.H.; Avunje, S.; Nikapitiya, C.; Kang, S.Y.; Ryu, Y.B.; Lee, W.S.; Jung, S.J. Efficacy of algal Ecklonia cava extract against viral hemorrhagic septicemia virus (VHSV). Fish. Shellfish Immunol. 2018, 72, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Kim, S.; Baek, S.M.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Ecklonia cava Extract Exerts Anti-Inflammatory Effect in Human Gingival Fibroblasts and Chronic Periodontitis Animal Model by Suppression of Pro-Inflammatory Cytokines and Chemokines. Foods 2021, 10, 1656. [Google Scholar] [CrossRef]
- Jo, S.L.; Yang, H.; Jeong, K.J.; Lee, H.W.; Hong, E.J. Neuroprotective Effects of Ecklonia cava in a Chronic Neuroinflammatory Disease Model. Nutrients 2023, 15, 2007. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Anti-Inflammatory Effect of Ecklonia cava Extract on Porphyromonas gingivalis Lipopolysaccharide-Stimulated Macrophages and a Periodontitis Rat Model. Nutrients 2019, 11, 1143. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Lee, H.S.; Chung, D.M.; Jang, J.T.; Jeon, Y.J.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Ecklonia Cava Extract Attenuates Endothelial Cell Dysfunction by Modulation of Inflammation and Brown Adipocyte Function in Perivascular Fat Tissue. Nutrients 2019, 11, 2795. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Choi, J.; Jang, J.T.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. The Phlorotannin-Rich Fraction of Ecklonia cava Extract Attenuated the Expressions of the Markers Related with Inflammation and Leptin Resistance in Adipose Tissue. Int. J. Endocrinol. 2020, 2020, 9142134. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Nepal, N.; Li, G.; Yang, N.; Chen, H.; Lin, Q.; Ji, X.; Zhang, S.; Jin, S. Dental pulp stem cells overexpressing hepatocyte growth factor facilitate the repair of DSS-induced ulcerative colitis. Stem Cell Res. Ther. 2021, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Jialing, L.; Yangyang, G.; Jing, Z.; Xiaoyi, T.; Ping, W.; Liwei, S.; Simin, C. Changes in serum inflammatory cytokine levels and intestinal flora in a self-healing dextran sodium sulfate-induced ulcerative colitis murine model. Life Sci. 2020, 263, 118587. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.G.; Elazab, S.T.; Abdelfattah-Hassan, A.; Mahfouz, H.; Salem, G.A.; Sheraiba, N.I.; Mohamed, E.A.A.; Attia, M.S.; El-Shetry, E.S.; Saleh, A.A.; et al. Multi-Strain-Probiotic-Loaded Nanoparticles Reduced Colon Inflammation and Orchestrated the Expressions of Tight Junction, NLRP3 Inflammasome and Caspase-1 Genes in DSS-Induced Colitis Model. Pharmaceutics 2022, 14, 1183. [Google Scholar] [CrossRef] [PubMed]
- Bodammer, P.; Zirzow, E.; Klammt, S.; Maletzki, C.; Kerkhoff, C. Alteration of DSS-mediated immune cell redistribution in murine colitis by oral colostral immunoglobulin. BMC Immunol. 2013, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Sousa, T.; Ladeirinha, A.F.; Santiago, A.R.; Carvalheiro, H.; Raposo, B.; Alarcão, A.; Cabrita, A.; Holmdahl, R.; Carvalho, L.; Souto-Carneiro, M.M. Deficient production of reactive oxygen species leads to severe chronic DSS-induced colitis in Ncf1/p47phox-mutant mice. PLoS ONE 2014, 9, e97532. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Vester-Andersen, M.K.; Mirsepasi-Lauridsen, H.C.; Prosberg, M.V.; Mortensen, C.O.; Träger, C.; Skovsen, K.; Thorkilgaard, T.; Nøjgaard, C.; Vind, I.; Krogfelt, K.A.; et al. Increased abundance of proteobacteria in aggressive Crohn’s disease seven years after diagnosis. Sci. Rep. 2019, 9, 13473. [Google Scholar] [CrossRef]
- Zegarra Ruiz, D.F.; Kim, D.V.; Norwood, K.; Saldana-Morales, F.B.; Kim, M.; Ng, C.; Callaghan, R.; Uddin, M.; Chang, L.C.; Longman, R.S.; et al. Microbiota manipulation to increase macrophage IL-10 improves colitis and limits colitis-associated colorectal cancer. Gut Microbes 2022, 14, 2119054. [Google Scholar] [CrossRef]
- Zuo, T.; Lu, X.J.; Zhang, Y.; Cheung, C.P.; Lam, S.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Zhao, R.; Chan, P.K.S.; et al. Gut mucosal virome alterations in ulcerative colitis. Gut 2019, 68, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xie, J.; Wang, Y.; Jiang, H.; Chen, K.; Li, D.; Wang, J.; Liu, Y.; He, J.; Zhou, J.; et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat. Commun. 2022, 13, 4804. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113 Pt 24, 4435–4440. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Gilboa, T.; Ogata, A.F.; Senussi, Y.; Lazarovits, R.; Boribong, B.P.; Bartsch, Y.C.; Loiselle, M.; Rivas, M.N.; Porritt, R.A.; et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Investig. 2021, 131, e149633. [Google Scholar] [CrossRef] [PubMed]
- Hoebler, C.; Gaudier, E.; De Coppet, P.; Rival, M.; Cherbut, C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig. Dis. Sci. 2006, 51, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Chirlaque, C.; Aranda, C.J.; Ocón, B.; Capitán-Cañadas, F.; Ortega-González, M.; Carrero, J.J.; Suárez, M.D.; Zarzuelo, A.; Sánchez de Medina, F.; Martínez-Augustin, O. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J. Crohns Colitis 2016, 10, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Boltin, D.; Perets, T.T.; Vilkin, A.; Niv, Y. Mucin function in inflammatory bowel disease: An update. J. Clin. Gastroenterol. 2013, 47, 106–111. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Zhang, B.C.; Li, Z.; Xu, W.; Xiang, C.H.; Ma, Y.F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar]
- Zhang, Y.; Tao, M.; Chen, C.; Zhao, X.; Feng, Q.; Chen, G.; Fu, Y. BAFF Blockade Attenuates DSS-Induced Chronic Colitis via Inhibiting NLRP3 Inflammasome and NF-κB Activation. Front. Immunol. 2022, 13, 783254. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Byun, K.A.; Jang, J.T.; Choi, C.H.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease by Decreasing the NLRP3 Inflammasome and Pyroptosis. Mar. Drugs 2021, 19, 318. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Yang, J.; Park, C.; Son, K.; Byun, K. Dieckol Attenuated Glucocorticoid-Induced Muscle Atrophy by Decreasing NLRP3 Inflammasome and Pyroptosis. Int. J. Mol. Sci. 2021, 22, 8057. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Stallhofer, J.; Guse, J.; Kesselmeier, M.; Grunert, P.C.; Lange, K.; Stalmann, R.; Eckardt, V.; Stallmach, A. Immunomodulator comedication promotes the reversal of anti-drug antibody-mediated loss of response to anti-TNF therapy in inflammatory bowel disease. Int. J. Color. Dis. 2023, 38, 54. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Le Berre, C. Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. N. Engl. J. Med. 2021, 385, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Di, B.; Xu, L.L. Recent advances in the treatment of IBD: Targets, mechanisms and related therapies. Cytokine Growth Factor. Rev. 2023, 71–72, 1–12. [Google Scholar] [CrossRef]
- Godala, M.; Gaszyńska, E.; Zatorski, H.; Małecka-Wojciesko, E. Dietary Interventions in Inflammatory Bowel Disease. Nutrients 2022, 14, 4261. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Li, Y.Y.; Cui, Y.; Dong, W.R.; Liu, T.T.; Zhou, G.; Chen, Y.X. Terminalia bellirica Fruit Extract Alleviates DSS-Induced Ulcerative Colitis by Regulating Gut Microbiota, Inflammatory Mediators, and Cytokines. Molecules 2023, 28, 5783. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, W.; Lin, Y.; Zhang, S.; Zou, B.; Xiao, D.; Lin, L.; Zhong, Y.; Zheng, H.; Liao, Q.; et al. Compound polysaccharides ameliorate experimental colitis by modulating gut microbiota composition and function. J. Gastroenterol. Hepatol. 2019, 34, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Yang, F.; Fu, Z.; Dong, Y.; Zhang, Z.; Ju, J. The role of enteric dysbacteriosis and modulation of gut microbiota in the treatment of inflammatory bowel disease. Microb. Pathog. 2022, 165, 105381. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, H.; Huang, H.; Xiao, Y.; Wu, X.; Li, M.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; et al. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. 2021, 12, 3142–3158. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Shibata, H.; Kimura-Takagi, I.; Hashimoto, S.; Aiyama, R.; Ueyama, S.; Yokokura, T. Anti-ulcer effects and biological activities of polysaccharides from marine algae. Biofactors 2000, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Liu, L.; Lin, P.; Dong, X.; Ni, L.; Che, H.; Xie, W. Saccharina japonica Ethanol Extract Ameliorates Dextran Sulfate Sodium-Induced Colitis via Reshaping Intestinal Microenvironment and Alleviating Inflammatory Response. Foods 2023, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Filippone, A.; Mannino, D.; Scuderi, S.A.; Casili, G.; Lanza, M.; Cucinotta, L.; Campolo, M.; Esposito, E. Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-κB/Nrf2/SIRT1 Signaling Pathways. J. Clin. Med. 2022, 11, 4301. [Google Scholar] [CrossRef]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Tanaka, R.; Matsushita, T.; Miyata, M. Dieckol isolated from a brown alga, Eisenia nipponica, suppresses ear swelling from allergic inflammation in mouse. J. Food Biochem. 2021, 45, e13659. [Google Scholar] [CrossRef]
- Pyeon, D.B.; Lee, S.E.; Yoon, J.W.; Park, H.J.; Park, C.O.; Kim, S.H.; Oh, S.H.; Lee, D.G.; Kim, E.Y.; Park, S.P. The antioxidant dieckol reduces damage of oxidative stress-exposed porcine oocytes and enhances subsequent parthenotes embryo development. Mol. Reprod. Dev. 2021, 88, 349–361. [Google Scholar] [CrossRef]
- Yayeh, T.; Im, E.J.; Kwon, T.H.; Roh, S.S.; Kim, S.; Kim, J.H.; Hong, S.B.; Cho, J.Y.; Park, N.H.; Rhee, M.H. Hemeoxygenase 1 partly mediates the anti-inflammatory effect of dieckol in lipopolysaccharide stimulated murine macrophages. Int. Immunopharmacol. 2014, 22, 51–58. [Google Scholar] [CrossRef]
- Dai, W.; Dai, Y.G.; Ren, D.F.; Zhu, D.W. Dieckol, a natural polyphenolic drug, inhibits the proliferation and migration of colon cancer cells by inhibiting PI3K, AKT, and mTOR phosphorylation. J. Biochem. Mol. Toxicol. 2023, 37, e23313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, Y.; Zhang, Y.; Su, X.; Luo, C.; Alarifi, S.; Yang, H. Dieckol alleviates dextran sulfate sodium-induced colitis via inhibition of inflammatory pathway and activation of Nrf2/HO-1 signaling pathway. Environ. Toxicol. 2021, 36, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Park, C.H.; Kang, H. Effect of E. cava and C. indicum Complex Extract on Phorbol 12-Myristate 13-Acetate (PMA) Stimulated Inflammatory Response in Human Pulmonary Epithelial Cells and Particulate Matter (PM)2.5-Induced Pulmonary Inflammation in Mice. Pharmaceutics 2023, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Choi, J.; Jang, J.T.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on Hypertensive Nephropathy in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2021, 22, 4230. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Yang, J.Y.; Park, C.H.; Son, K.H.; Byun, K. Dieckol Reduces Muscle Atrophy by Modulating Angiotensin Type II Type 1Receptor and NADPH Oxidase in Spontaneously Hypertensive Rats. Antioxidants 2021, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shim, M.; Son, M.; Jang, J.T.; Son, K.H.; Byun, K. Attenuating Effects of Dieckol on Endothelial Cell Dysfunction via Modulation of Th17/Treg Balance in the Intestine and Aorta of Spontaneously Hypertensive Rats. Antioxidants 2021, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Ryu, B.; Oh, S.; Chung, D.M.; Seo, M.; Park, S.J.; Byun, K.; Jeon, Y.J. Reversibility of sarcopenia by Ishige okamurae and its active derivative diphloroethohydroxycarmalol in female aging mice. Biomed. Pharmacother. 2022, 152, 113210. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, A.R.; Lee, H.Y.; Moon, Y.A.; Lee, H.J.; Cha, J.Y. Deletion of KLF10 Leads to Stress-Induced Liver Fibrosis upon High Sucrose Feeding. Int. J. Mol. Sci. 2020, 22, 331. [Google Scholar] [CrossRef]
- Jang, J.; Hwang, S.; Oh, A.R.; Park, S.; Yaseen, U.; Kim, J.G.; Park, S.; Jung, Y.; Cha, J.Y. Fructose malabsorption in ChREBP-deficient mice disrupts the small intestine immune microenvironment and leads to diarrhea-dominant bowel habit changes. Inflamm. Res. 2023, 72, 769–782. [Google Scholar] [CrossRef]
- Baek, M.O.; Cho, H.J.; Min, D.S.; Choi, C.S.; Yoon, M.S. Self-transducible LRS-UNE-L peptide enhances muscle regeneration. J. Cachexia Sarcopenia Muscle 2022, 13, 1277–1288. [Google Scholar] [CrossRef]
- Oh, S.; Rho, N.K.; Byun, K.A.; Yang, J.Y.; Sun, H.J.; Jang, M.; Kang, D.; Son, K.H.; Byun, K. Combined Treatment of Monopolar and Bipolar Radiofrequency Increases Skin Elasticity by Decreasing the Accumulation of Advanced Glycated End Products in Aged Animal Skin. Int. J. Mol. Sci. 2022, 23, 2993. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Kim, H.-Y.; Jang, J.-T.; Hong, S. Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules 2023, 28, 8099. https://doi.org/10.3390/molecules28248099
Kim Y-M, Kim H-Y, Jang J-T, Hong S. Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules. 2023; 28(24):8099. https://doi.org/10.3390/molecules28248099
Chicago/Turabian StyleKim, Young-Mi, Hye-Youn Kim, Ji-Tae Jang, and Suntaek Hong. 2023. "Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation" Molecules 28, no. 24: 8099. https://doi.org/10.3390/molecules28248099
APA StyleKim, Y.-M., Kim, H.-Y., Jang, J.-T., & Hong, S. (2023). Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules, 28(24), 8099. https://doi.org/10.3390/molecules28248099





