Recent Progress in Photocatalytic Degradation of Water Pollution by Bismuth Tungstate
Abstract
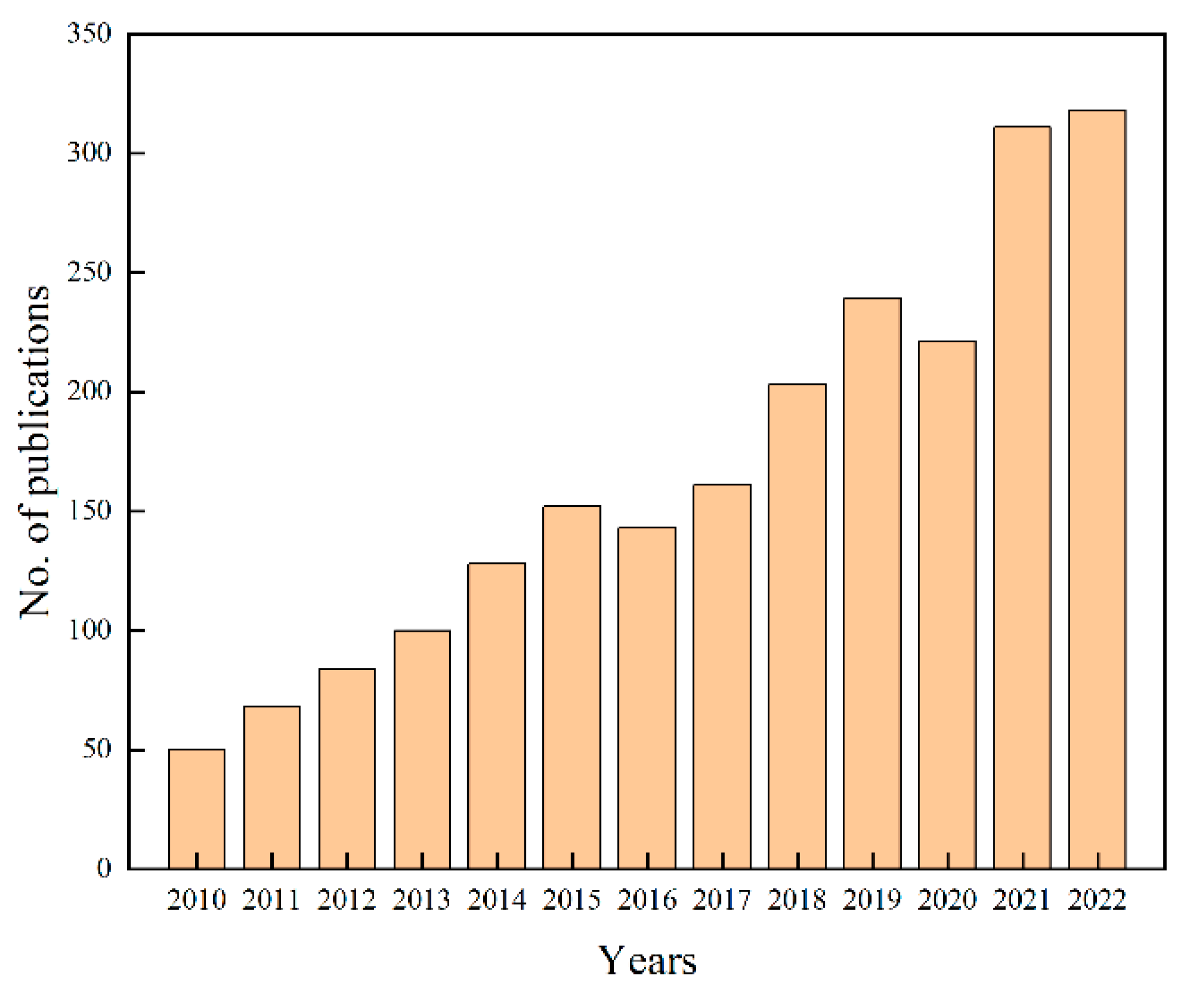
1. Introduction
2. Structure, Property Characteristics, and Photocatalytic Fundamentals of Bi2WO6
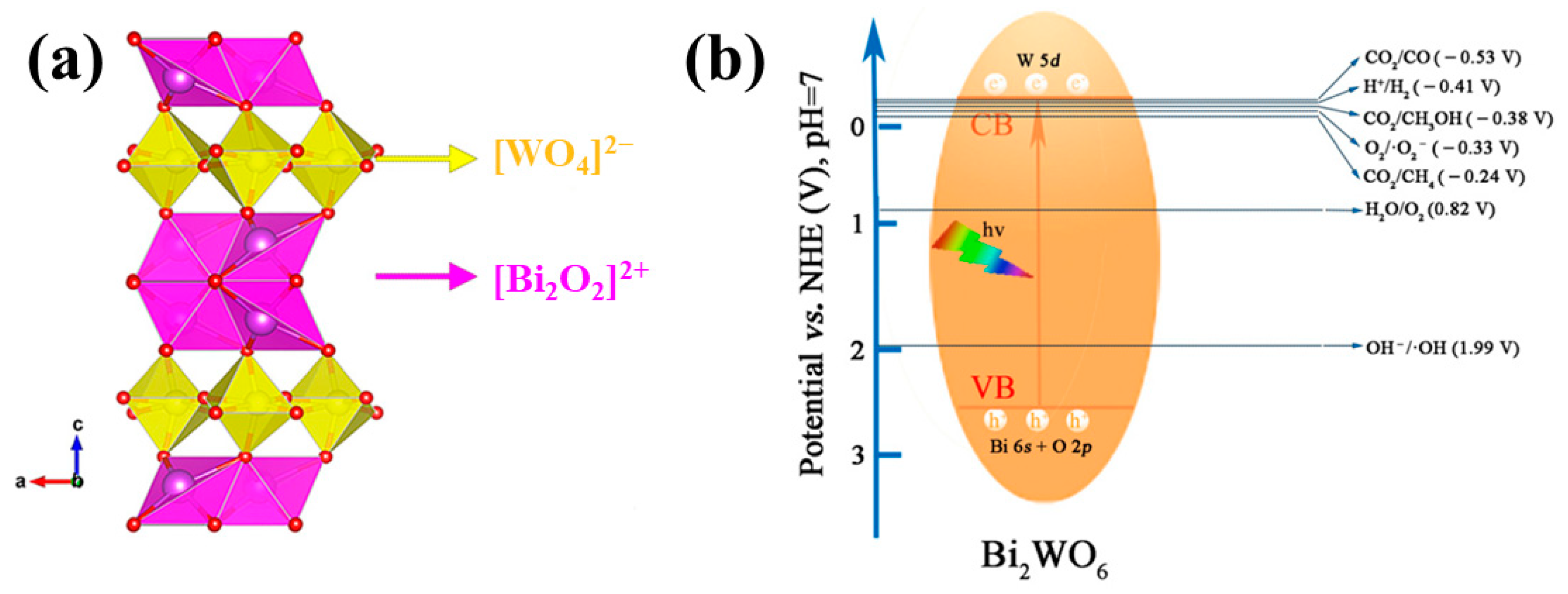
2.1. Structure and Property Characteristics
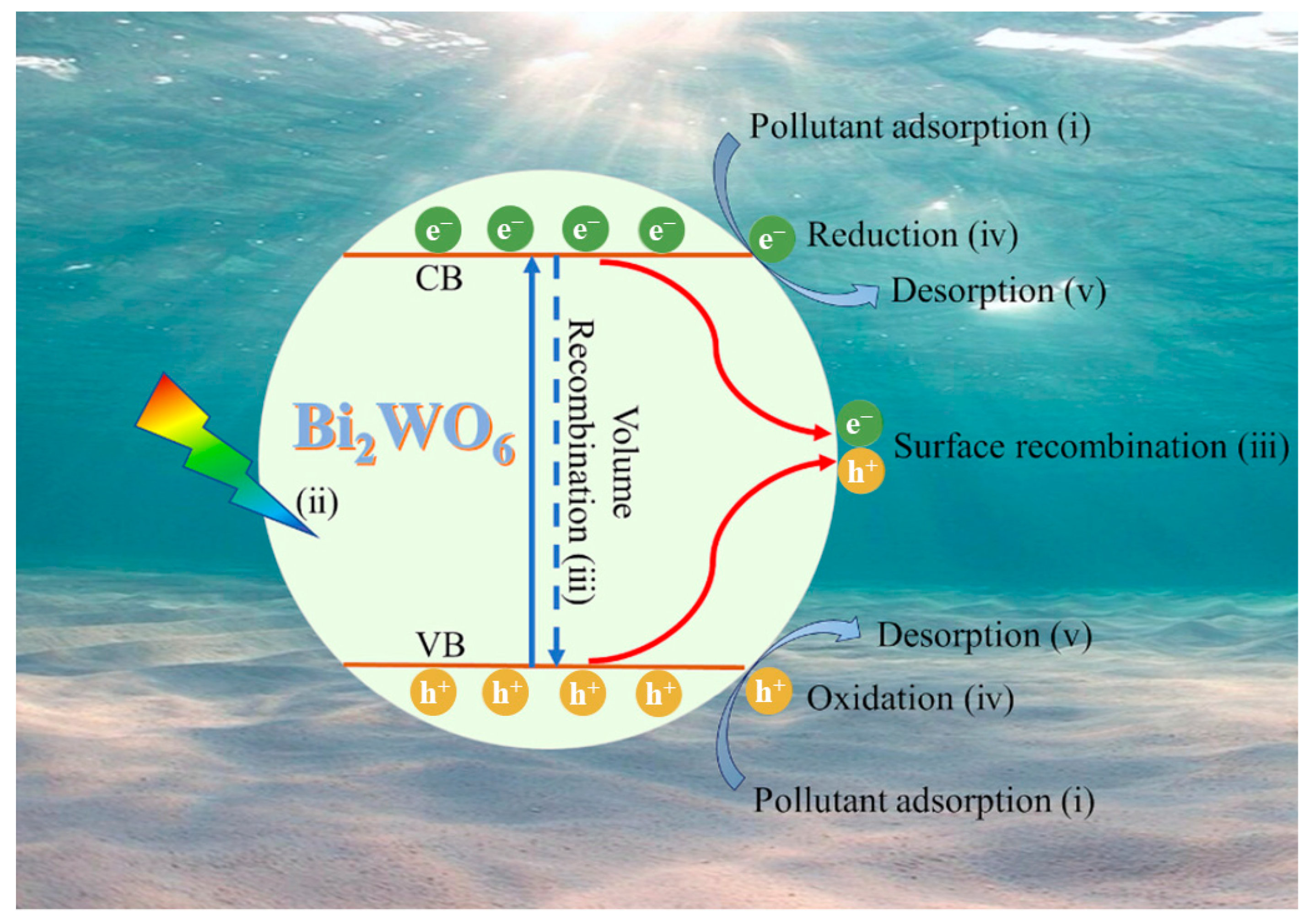
2.2. Fundamentals of Photocatalysis
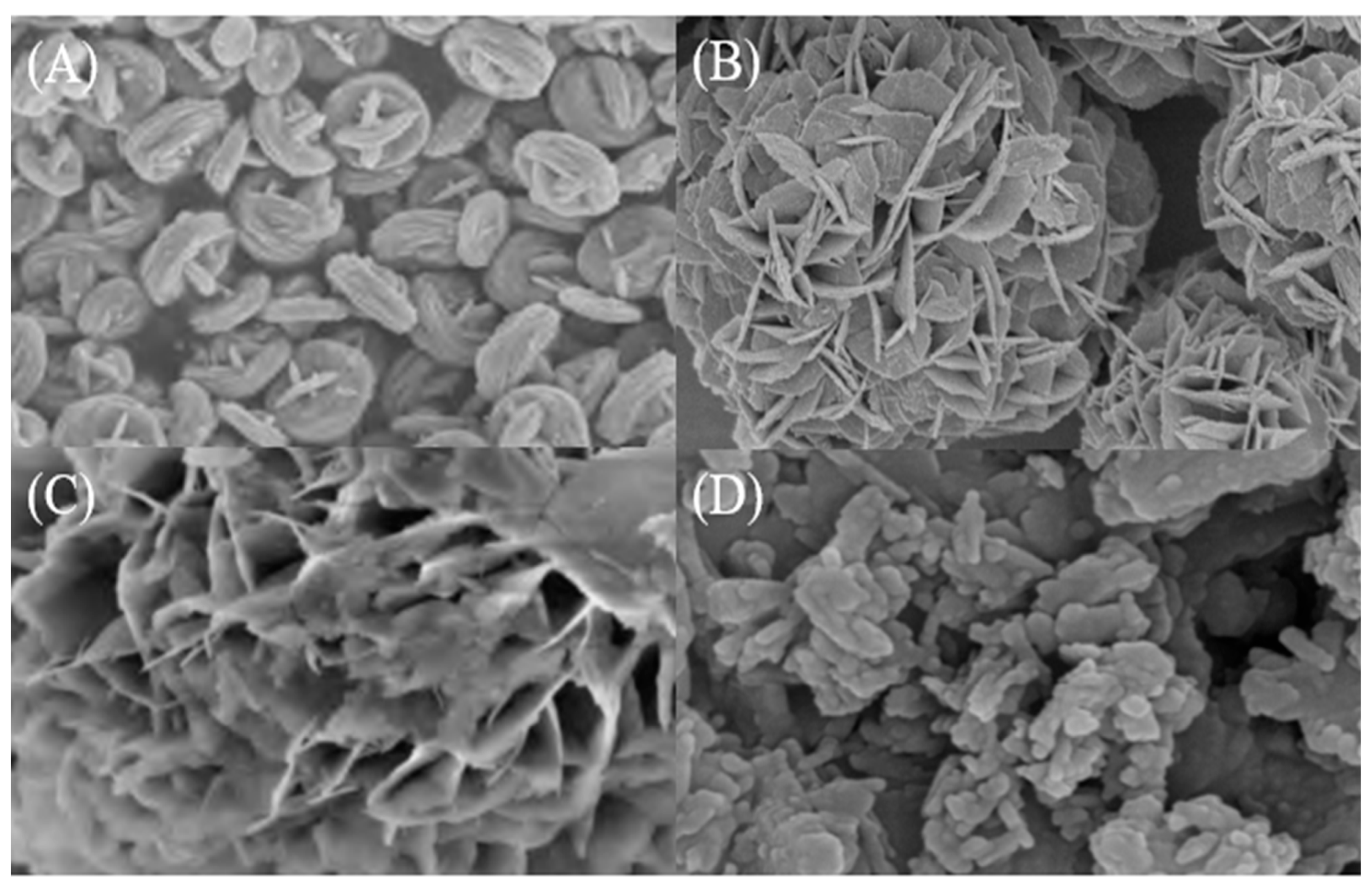
3. Morphology Control
4. Metal Doping
5. Non-Metal Doping
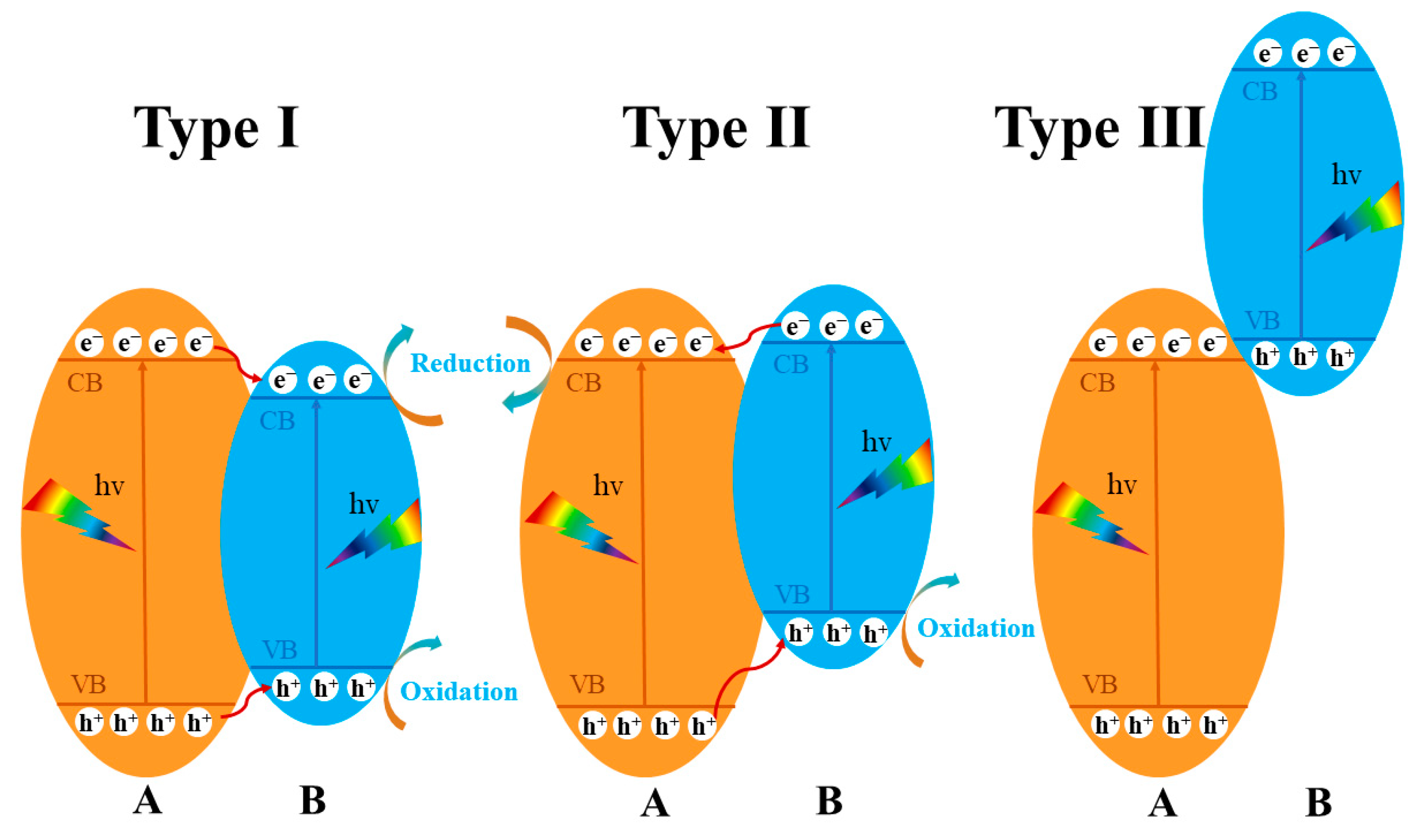
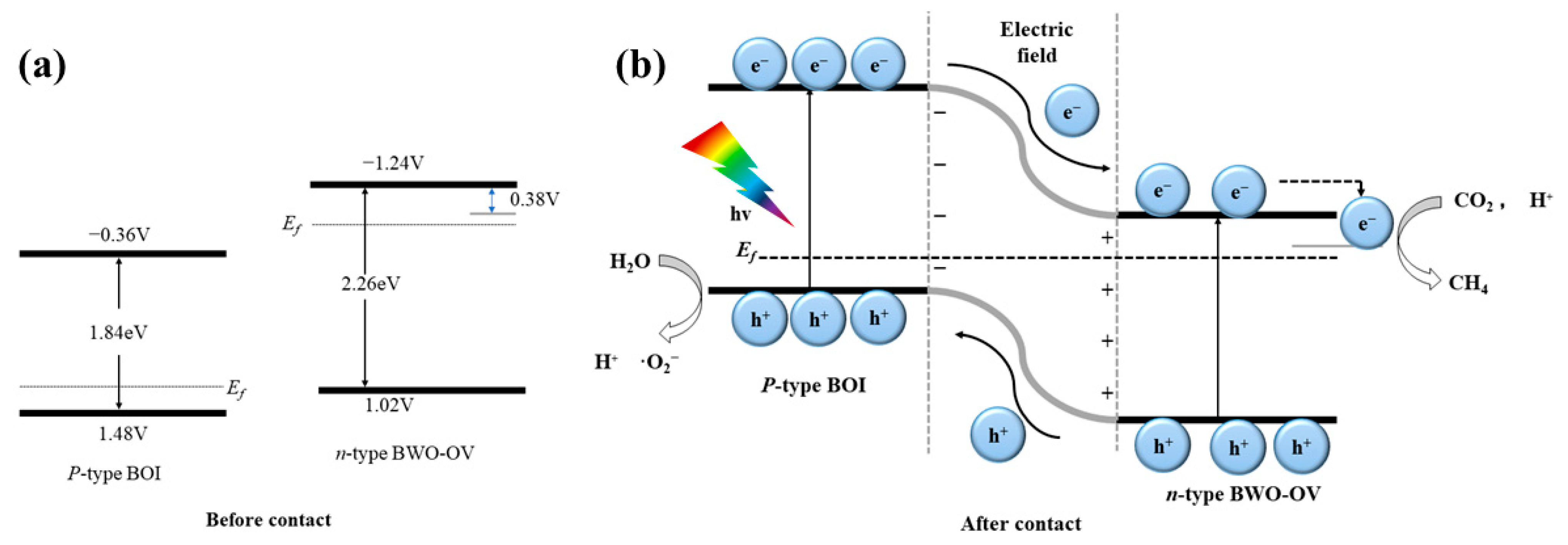
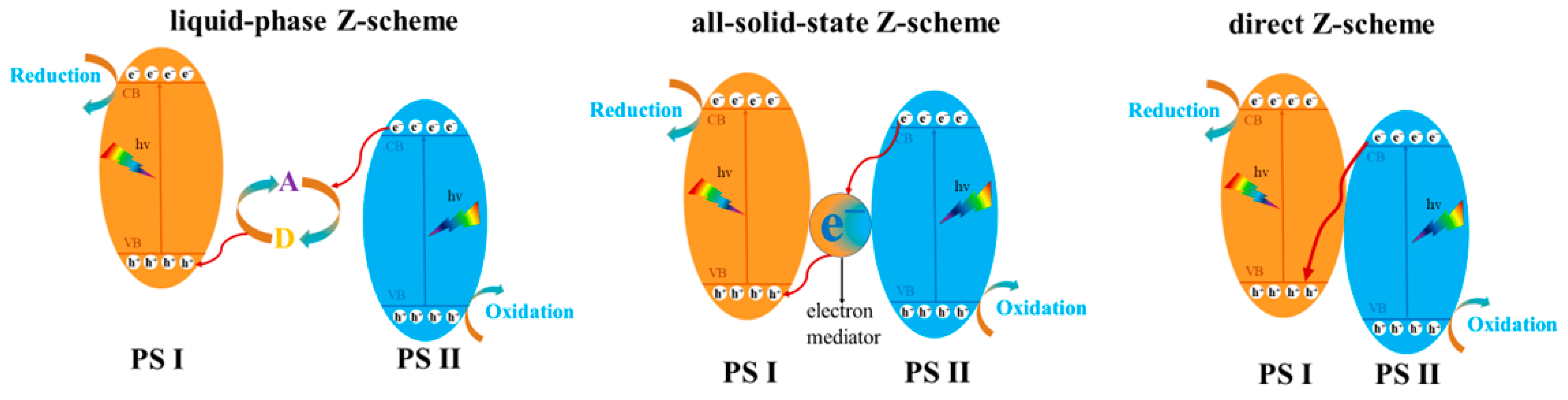
6. Semiconductor Heterojunction
7. Defect Engineering
8. Bi2WO6 Photoelectrochemistry
9. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, K.; Wang, L.; Xu, X.; Dou, S.X.; Hao, W.; Du, Y. Two Dimensionl Bismuth-Based Layered Materials for Energy-Related Applications. Energy Storage Mater. 2019, 19, 446–463. [Google Scholar] [CrossRef]
- Tu, S.; Guo, Y.; Zhang, Y.; Hu, C.; Zhang, T.; Ma, T.; Huang, H. Piezocatalysis and Piezo-Photocatalysis: Catalysts Classification and Modification Strategy, Reaction Mechanism, and Practical Application. Adv. Funct. Mater. 2020, 30, 2005158. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, B.; Zhang, L.; Yu, J.; Li, Y.; Wageh, S.; Al-Ghamdi, A.A. S-Scheme 2D/2D Bi2MoO6/BiOI van der Waals Heterojunction for CO2 Photoreduction. Chin. J. Catal. 2022, 43, 1657–1666. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Chen, Z.; Zhang, Z. Cobalt-Ferrite Functionalized Graphitic Carbon Nitride (CoFe2O4@g-C3N4) Nanoconfined Catalytic Membranes for Efficient Water Purification: Performance and Mechanism. J. Mater. Chem. A 2023, 11, 18933–18944. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, W.; Guo, S.; Zhou, J.; Lu, T.; Xing, P.; Cai, Q.; Sun, R. Hazard of heavy metal pollution in water and its treatment technology. Mod. Agric. Sci. Technol. 2022, 6, 129–132. [Google Scholar]
- Li, J. Analysis of pretreatment methods and detection techniques of organic pollutants in water. Leather Manuf. Environ. Technol. 2022, 3, 18–20. [Google Scholar]
- Dai, X.; Feng, S.; Zheng, W.; Wu, W.; Zhou, Y.; Ye, Z.; Cao, X.; Wang, Y. Ag–AgBr/g-C3N4/ZIF-8 Prepared with Ionic Liquid as Template for Highly Efficient Photocatalytic Hydrogen Evolution under Visible Light. Int. J. Hydrogen Energy 2022, 47, 10603–10615. [Google Scholar] [CrossRef]
- Wang, X.; Ng, D.; Du, H.; Hornung, C.H.; Polyzos, A.; Seeber, A.; Li, H.; Huo, Y.; Xie, Z. Copper Decorated Indium Oxide Rods for Photocatalytic CO2 Conversion under Simulated Sun Light. J. CO2 Util. 2022, 58, 101909. [Google Scholar] [CrossRef]
- Adamou, P.; Harkou, E.; Hafeez, S.; Manos, G.; Villa, A.; Al-Salem, S.M.; Constantinou, A.; Dimitratos, N. Recent Progress on Sonochemical Production for the Synthesis of Efficient Photocatalysts and the Impact of Reactor Design. Ultrason. Sonochem. 2023, 100, 106610. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, C.; Liu, Y. Ta3N5/CdS Core-Shell S-Scheme Heterojunction Nanofibers for Efficient Photocatalytic Removal of Antibiotic Tetracycline and Cr (VI): Performance and Mechanism Insights. Adv. Fiber Mater. 2023, 5, 994–1007. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled Adsorption and Photocatalysis of G-C3N4 Based Composites: Material Synthesis, Mechanism, and Environmental Applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Yan, R.; Chen, X. Constructing Cd0.5Zn0.5S/Bi2WO6 S-Scheme Heterojunction for Boosted Photocatalytic Antibiotic Oxidation and Cr (VI) Reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, L.; Yu, J. Research Progress on S-Scheme Heterojunction Photocatalyst. J. Chin. Ceram. Soc. 2023, 51, 73–81. [Google Scholar]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-Based Materials for Photocatalytic Water Splitting and Environmental Application: A Critical Review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, J.; Jiang, C.; Yu, J.; Zhang, P. Review on design and evaluation of environmental photocatalysts. Front. Environ. Sci. Eng. 2018, 12, 1–32. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-Scheme MIL-101(Fe) Octahedrons Modified Bi2WO6 Microspheres for Photocatalytic Decontamination of Cr (VI) and Tetracycline Hydrochloride: Synergistic Insights, Reaction Pathways, and Toxicity Analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent Advances in Floating TiO2-Based Photocatalysts for Environmental Application. Appl. Catal. B-Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Ong, C.; Ng, L.; Mohammad, A. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sust. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Wang, Z.; Zhou, Y.; Qin, Y.; Wang, Z.L. Piezotronic Effect Enhanced Photocatalysis in Strained Anisotropic ZnO/TiO2 Nanoplatelets via Thermal Stress. ACS Nano 2016, 10, 2636–2643. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Li, W.; Li, J.; Li, Y.; Chen, Q. A Novel Bi2S3 Nanowire @ TiO2 Nanorod Heterogeneous Nanostructure for Photoelectrochemical Hydrogen Generation. Chem. Eng. J. 2016, 302, 717–724. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Leong, S.; Lin, X.; Wei, J.; Kong, B.; Xu, Y.; Low, Z.-X.; Yao, J.; Wang, H. Rapid Construction of ZnO@ZIF-8 Heterostructures with Size-Selective Photocatalysis Properties. ACS Appl. Mater. Interfaces 2016, 8, 9080–9087. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zuo, F.; Liu, J.; Ma, Q.; Wang, C.; Sun, D.; Bartels, L.; Feng, P. Synthesis and Efficient Visible Light Photocatalytic Hydrogen Evolution of Polymeric G-C3N4 Coupled with CdS Quantum Dots. J. Phys. Chem. C 2012, 116, 13708–13714. [Google Scholar] [CrossRef]
- Wu, S.; Sun, J.; Li, Q.; Hood, Z.D.; Yang, S.; Su, T.; Peng, R.; Wu, Z.; Sun, W.; Kent, P.R.C.; et al. Effects of Surface Terminations of 2D Bi2WO6 on Photocatalytic Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 20067–20074. [Google Scholar] [CrossRef]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-Scheme g-C3N4/Bi/BiVO4 Hybrid Photocatalysts toward Artificial Carbon Cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- Li, Y.; Liao, D.; Li, T.; Zhong, W.; Wang, X.; Hong, X.; Yu, H. Plasmonic Z-Scheme Pt-Au/BiVO4 Photocatalyst: Synergistic Effect of Crystal-Facet Engineering and Selective Loading of Pt-Au Cocatalyst for Improved Photocatalytic Performance. J. Colloid Interface Sci. 2020, 570, 232–241. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, K.; Guo, T.; Wu, X.; Zhang, G. Fabrication of Z-Scheme MoO3/Bi2O4 Heterojunction Photocatalyst with Enhanced Photocatalytic Performance under Visible Light Irradiation. Chin. J. Catal. 2020, 41, 161–169. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Z.; Sun, M.; Liu, Y.; Liu, L.; Liu, H.; Huang, C.; Qu, J.; Li, J. Formation of Bi2WO6 Bipyramids with Vacancy Pairs for Enhanced Solar-Driven Photoactivity. Adv. Funct. Mater. 2015, 25, 3726–3734. [Google Scholar] [CrossRef]
- Lian, X.; Xue, W.; Dong, S.; Liu, E.; Li, H.; Xu, K. Construction of S-Scheme Bi2WO6/g-C3N4 Heterostructure Nanosheets with Enhanced Visible-Light Photocatalytic Degradation for Ammonium Dinitramide. J. Hazard. Mater. 2021, 412, 125217. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, J.; Wu, Z.; Liu, M.; Zhang, H.; Han, W. Preparation of Tin Dioxide/Bismuth Tungstate Composite Photocatalytic Materials by Hydrothermal Method and Its Catalytic Activity. J. Synth. Cryst. 2022, 51, 502–507. [Google Scholar]
- Li, C.; Chen, G.; Sun, J.; Feng, Y.; Liu, J.; Dong, H. Ultrathin Nanoflakes Constructed Erythrocyte-like Bi2WO6 Hierarchical Architecture via Anionic Self-Regulation Strategy for Improving Photocatalytic Activity and Gas-Sensing Property. Appl. Catal. B Environ. 2015, 163, 415–423. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Rao, J.; Han, Z.; Hu, Y.; Zhou, Y. A Novel Mesoporous Single-Crystal-Like Bi2WO6 with Enhanced Photocatalytic Activity for Pollutants Degradation and Oxygen Production. ACS Appl. Mater. Interfaces 2015, 7, 25716–25724. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Du, X.; Wang, R.; Liu, E.; Jia, J.; Bai, X.; Hu, X.; Fan, J. Mesoporous Nanoplate Multi-Directional Assembled Bi2WO6 for High Efficient Photocatalytic Oxidation of NO. Chemosphere 2018, 193, 737–744. [Google Scholar] [CrossRef]
- Wang, S.; Yang, H.; Yi, Z.; Wang, X. Enhanced Photocatalytic Performance by Hybridization of Bi2WO6 Nanoparticles with Honeycomb-like Porous Carbon Skeleton. J. Environ. Manag. 2019, 248, 109341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, Y.; Li, W.; Wang, F.; Li, H.; Liu, X.; Zhang, W.; Ren, C. Double Z-Scheme System of Silver Bromide@bismuth Tungstate/Tungsten Trioxide Ternary Heterojunction with Enhanced Visible-Light Photocatalytic Activity. J. Colloid Interface Sci. 2018, 509, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ren, J.; Hua, J.; Xia, L.; He, J.; Huo, D.; Hu, Y. Multifunctional Bi2WO6 Nanoparticles for CT-Guided Photothermal and Oxygen-free Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1132–1146. [Google Scholar] [CrossRef]
- Chang, C.; Chen, J.; Lin, K.; Wei, Y.; Chao, P.; Huang, C. Enhanced Visible-Light-Driven Photocatalytic Degradation by Metal Wire-Mesh Supported Ag/Flower-like Bi2WO6 Photocatalysts. J. Alloys Compd. 2020, 813, 152186. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Yang, J.; Yu, Q.; Zhu, X.; Chu, J.; Du, Y.; Wang, C.; Hua, Y.; Li, H.; et al. Plasma Treated Bi2WO6 Ultrathin Nanosheets with Oxygen Vacancies for Improved Photocatalytic CO2 Reduction. Inorg. Chem. Front. 2020, 7, 597–602. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Du, Y.; Zhou, C.; Zhang, M.; Wang, Z.; Weng, Y.; Long, J.; Hofkens, J.; Steele, J.A.; et al. Direct Z-Scheme Heterojunction of Semicoherent FAPbBr3/Bi2WO6 Interface for Photoredox Reaction with Large Driving Force. ACS Nano 2020, 14, 16689–16697. [Google Scholar] [CrossRef]
- Guo, W.; Jian, L.; Wang, X.; Zeng, W. Hydrothermal Synthesis of Ni-Doped Hydrangea-like Bi2WO6 and the Enhanced Gas Sensing Property to n-Butanol. Sens. Actuators B Chem. 2022, 357, 131396. [Google Scholar] [CrossRef]
- Sun, D.; Le, Y.; Jiang, C.; Cheng, B. Ultrathin Bi2WO6 Nanosheet Decorated with Pt Nanoparticles for Efficient Formaldehyde Removal at Room Temperature. Appl. Surf. Sci. 2018, 441, 429–437. [Google Scholar] [CrossRef]
- Peng, R.; Kang, Y.; Deng, X.; Zhang, X.; Xie, F.; Wang, H.; Liu, W. Topotactic Transformed Face-to-Face Heterojunction of BiOCl/Bi2WO6 for Improved Tetracycline Photodegradation. J. Environ. Chem. Eng. 2021, 9, 106750. [Google Scholar] [CrossRef]
- Ren, X.; Wu, K.; Qin, Z.; Zhao, X.; Yang, H. The Construction of Type II Heterojunction of Bi2WO6/BiOBr Photocatalyst with Improved Photocatalytic Performance. J. Alloys Compd. 2019, 788, 102–109. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Liu, E.; Fan, J.; Hu, X. Bi2WO6 Nanoflowers: An Efficient Visible Light Photocatalytic Activity for Ceftriaxone Sodium Degradation. Appl. Surf. Sci. 2018, 436, 854–864. [Google Scholar] [CrossRef]
- Karbasi, M.; Hashemifar, S.J.; Karimzadeh, F.; Giannakis, S.; Pulgarin, C.; Raeissi, K.; Sienkiewicz, A. Decrypting the Photocatalytic Bacterial Inactivation of Hierarchical Flower-like Bi2WO6 Microspheres Induced by Surface Properties: Experimental Studies and Ab Initio Calculations. Chem. Eng. J. 2022, 427, 131768. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Chen, Z.; Wong, P.; Liu, J. Bi2WO6 Micro/Nano-Structures: Synthesis, Modifications and Visible-Light-Driven Photocatalytic Applications. Appl. Catal. B 2011, 106, 1–13. [Google Scholar] [CrossRef]
- Yi, H.; Qin, L.; Huang, D.; Zeng, G.; Lai, C.; Liu, X.; Li, B.; Wang, H.; Zhou, C.; Huang, F.; et al. Nano-Structured Bismuth Tungstate with Controlled Morphology: Fabrication, Modification, Environmental Application and Mechanism Insight. Chem. Eng. J. 2019, 358, 480–496. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lin, M.; Long, J.; Zhang, Z.; Lin, H.; Wu, J.C.-S.; Wang, X. Monolayered Bi2WO6 Nanosheets Mimicking Heterojunction Interface with Open Surfaces for Photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef]
- Lin, S.; Liu, L.; Hu, J.; Liang, Y.; Cui, W. Nano Ag@AgBr Surface-Sensitized Bi2WO6 Photocatalyst: Oil-in-Water Synthesis and Enhanced Photocatalytic Degradation. Appl. Surf. Sci. 2015, 324, 20–29. [Google Scholar] [CrossRef]
- Kim, M.; Jo, W. Visible-Light-Activated N-Doped CQDs/g-C3N4/Bi2WO6 Nanocomposites with Different Component Arrangements for the Promoted Degradation of Hazardous Vapors. J. Mater. Sci. Technol. 2020, 40, 168–175. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.; Hu, C.; Huang, H. Recent Advances on Bi2WO6-Based Photocatalysts for Environmental and Energy Applications. Chin. J. Catal. 2021, 42, 1413–1438. [Google Scholar] [CrossRef]
- Linghu, X.; Shu, Y.; Liu, L.; Zhao, Y.; Zhang, J.; Chen, Z.; Shan, D.; Wang, B. Hydro/Solvothermally Synthesized Bismuth Tungstate Nanocatalysts for Enhanced Photocatalytic Degradation of Dyes, Antibiotics, and Bacteria in Wastewater: A Review. J. Water Process. Eng. 2023, 54, 103994. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Siddique, M.S.; Yang, H.; Xia, Y.; Su, J.; Tang, B.; Wang, L.; Kang, L.; Huang, Z. Novel Z-Scheme In2S3/Bi2WO6 Core-Shell Heterojunctions with Synergistic Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. J. Clean. Prod. 2022, 339, 130634. [Google Scholar] [CrossRef]
- Chen, T.; Xu, C.; Zou, C.; Fan, L.; Xu, Q. Self-Assembly of PDINH/TiO2/Bi2WO6 Nanocomposites for Improved Photocatalytic Activity Based on a Rapid Electron Transfer Channel. Appl. Surf. Sci. 2022, 584, 152667. [Google Scholar] [CrossRef]
- Lv, Y.; He, R.; Chen, Z.; Li, X.; Xu, Y. Fabrication of Hierarchical Copper Sulfide/Bismuth Tungstate p-n Heterojunction with Two-Dimensional (2D) Interfacial Coupling for Enhanced Visible-Light Photocatalytic Degradation of Glyphosate. J. Colloid Interface Sci. 2020, 560, 293–302. [Google Scholar] [CrossRef]
- Guo, Y.; Chu, D. Controlled synthesis of knob-shaped Bi2WO6 micro-nano materials by hydrothermal method. J. Henan Univ. Eng. (Nat. Sci. Ed.) 2022, 34, 11–15. [Google Scholar]
- Zheng, C.; Yang, H.; Cui, Z. Enhanced Photocatalytic Performance of Au Nanoparticles-Modified Rose Flower-like Bi2WO6 Hierarchical Architectures. J. Ceram. Soc. Jpn. 2017, 125, 887–893. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Wang, R.; Zhang, Y.; Zhao, F.; Su, X. Preparation and Photocatalytic Activity of Bi2O3/Bi2WO6 composite catalyst. Liaoning Chem. Ind. 2022, 51, 1497–1500. [Google Scholar]
- Zhou, L.; Jin, C.; Yu, Y.; Chi, F.; Lv, Y.; Ran, S. Low-temperature Solid-state Synthesis of Bi2WO6 Powders and Its Visible-light Photocatalytic Activity. Chin. J. Process Eng. 2016, 16, 895–899. [Google Scholar]
- Yu, M.; Pu, Q.; Liao, X.; Yu, H.; Lin, S.; Li, Z.; Yu, C.; Wang, H.; Zhong, X. Controllable Synthesis of Bismuth Tungstate Photocatalysts with Different Morphologies for Degradation of Antibiotics under Visible-Light Irradiation. J. Mater. Sci.-Mater. Electron. 2021, 32, 17848–17864. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, Y.; Cao, H.; Sun, J.; Xu, Q.; Wang, L. Insight into the Influence of Morphology of Bi2WO6 for Photocatalytic Degradation of VOCs under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 327–333. [Google Scholar] [CrossRef]
- Gou, Z.; Dai, J.; Bai, J. Synthesis of Mesoporous Bi2WO6 Flower-Like Spheres with Photocatalysis Properties under Visible Light. Int. J. Electrochem. Sci. 2020, 15, 10684–10693. [Google Scholar] [CrossRef]
- Pu, Q.; Li, Z.; Li, D. Preparation of Bismuth Tungstate Nanoscale Photocatalyst and the Study on Degradation Performance of Tetracycline Antibiotics. Synth. Mater. Aging Appl. 2018, 47, 72–75. [Google Scholar]
- Yang, L.; Li, X.; Wang, Z.; Zhao, J.; Dong, D.; Wang, M. Yeast-assisted Synthesis of Bismuth Tungstate Hollow Microsphere and Its Photocatalysis for Tetracycline Hydrochloride. New Chem. Mater. 2016, 44, 98–101. [Google Scholar]
- Liu, S.; Hou, Y.; Zheng, S.; Zhang, Y.; Wang, Y. One-Dimensional Hierarchical Bi2WO6 Hollow Tubes with Porous Walls: Synthesis and Photocatalytic Property. CrystEngComm 2013, 15, 4124–4130. [Google Scholar] [CrossRef]
- Yang, A.; Han, Y.; Li, S.; Xing, H.; Pan, Y.; Liu, W. Synthesis and Comparison of Photocatalytic Properties for Bi2WO6 Nanofibers and Hierarchical Microspheres. J. Alloys Compd. 2017, 695, 915–921. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Wang, H.; Dong, F.; Wu, Z. Fe-Ions Modified Mesoporous Bi2WO6 Nanosheets with High Visible Light Photocatalytic Activity. J. Colloid Interface Sci. 2012, 369, 373–380. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, F.; Zhang, X.; Zhong, Y.; Wang, J.; Jiao, Y.; Luo, Y.; Feng, W. Synthesis of Tin-Doped Three-Dimensional Flower-like Bismuth Tungstate with Enhanced Photocatalytic Activity. Int. J. Mol. Sci. 2022, 23, 8422. [Google Scholar] [CrossRef]
- Bunluesak, T.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Visible-Light-Driven Heterostructure Ag/Bi2WO6 Nanocomposites Synthesized by Photodeposition Method and Used for Photodegradation of Rhodamine B Dye. Res. Chem. Intermed. 2021, 47, 3079–3092. [Google Scholar] [CrossRef]
- Gao, X.; Fei, J.; Dai, Y.; Fu, F. Hydrothermal Synthesis of Series Cu-Doped Bi2WO6 and Its Application in Photo-Degradative Removal of Phenol in Wastewater with Enhanced Efficiency. J. Mol. Liq. 2018, 256, 267–276. [Google Scholar] [CrossRef]
- Zhu, F.; Lv, Y.; Li, J.; Ding, J.; Xia, X.; Wei, L.; Jiang, J.; Zhang, G.; Zhao, Q. Enhanced Visible Light Photocatalytic Performance with Metal-Doped Bi2WO6 for Typical Fluoroquinolones Degradation: Efficiencies, Pathways and Mechanisms. Chemosphere 2020, 252, 126577. [Google Scholar] [CrossRef] [PubMed]
- Phuruangrat, A.; Buapoon, S.; Bunluesak, T.; Suebsom, P.; Thongtem, S.; Thongtem, T. Facile Synthesis of Pd-Doped Bi2WO6 Nanoplates Used for Enhanced Visible-Light-Driven Photocatalysis. Inorg. Nano-Met. Chem. 2023, 53, 219–227. [Google Scholar]
- Tu, Y.; Ling, L.; Li, Q.; Long, X.; Liu, N.; Li, Z. Greatly Enhanced Photocatalytic Activity over Bi2WO6 by MIL-53(Fe) Modification. Opt. Mater. 2020, 110, 110500. [Google Scholar] [CrossRef]
- Tahir, N.; Zahid, M.; Bhatti, I.A.; Jamil, Y. Fabrication of Visible Light Active Mn-Doped Bi2WO6-GO/MoS2 Heterostructure for Enhanced Photocatalytic Degradation of Methylene Blue. Environ. Sci. Pollut. Res. 2022, 29, 6552–6567. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, S.; Xu, L.; Liu, C.; Zhang, R.; Tan, W. Hydrothermal Preparation of Flower-like Ni2+ Doped Bi2WO6 for Enhanced Photocatalytic Degradation. J. Phys. Chem. Solids 2022, 170, 110954. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, K.; Wang, B.; Wang, R. Synergistic Effect of Amorphous Ti(IV)-Hole and Ni(II)-Electron Cocatalysts for Enhanced Photocatalytic Performance of Bi2WO6. Catalysts 2022, 12, 1633. [Google Scholar] [CrossRef]
- Pinchujit, S.; Phuruangrat, A.; Wannapop, S.; Sakhon, T.; Kuntalue, B.; Thongtem, T.; Thongtem, S. Synthesis and Characterization of Heterostructure Pt/Bi2WO6 Nanocomposites with Enhanced Photodegradation Efficiency Induced by Visible Radiation. Solid State Sci. 2022, 134, 107064. [Google Scholar] [CrossRef]
- Song, X.C.; Li, W.T.; Huang, W.Z.; Zhou, H.; Yin, H.Y.; Zheng, Y.F. Enhanced Photocatalytic Activity of Cadmium-Doped Bi2WO6 Nanoparticles under Simulated Solar Light. J. Nanopart. Res. 2015, 17, 134. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Buapoon, S.; Bunluesak, T.; Suebsom, P.; Wannapop, S.; Thongtem, T.; Thongtem, S. Hydrothermal Preparation of Au-Doped Bi2WO6 Nanoplates for Enhanced Visible-Light-Driven Photocatalytic Degradation of Rhodamine B. Solid State Sci. 2022, 128, 106881. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Kadirova, Z.C.; Zahedi, E.; Onna, D.; Claudia Marchi, M.; Zhu, G.; Matsushita, N.; Hasegawa, M.; Aldabe Bilmes, S.; Okada, K. Tuning the Morphological Structure, Light Absorption, and Photocatalytic Activity of Bi2WO6 and Bi2WO6-BiOCl through Cerium Doping. Arab. J. Chem. 2020, 13, 2844–2857. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, N.; Yu, H.; Xu, J.; He, Q.; Zheng, S.; Ding, B.; Yan, X. Enhancing oxygen vacancy photocatalytic efficiency of bismuth tungstate using In-doped W site. Acta Phys. Sin. 2019, 68, 217102. [Google Scholar] [CrossRef]
- Kumar, R.; Raizada, P.; Verma, N.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Le, Q.V.; Nguyen, V.-H.; Selvasembian, R.; Singh, P. Recent Advances on Water Disinfection Using Bismuth Based Modified Photocatalysts: Strategies and Challenges. J. Clean. Prod. 2021, 297, 126617. [Google Scholar] [CrossRef]
- Ferreira, V.R.A.; Santos, P.R.M.; Silva, C.I.Q.; Azenha, M.A. Latest Developments on TiO2-Based Photocatalysis: A Special Focus on Selectivity and Hollowness for Enhanced Photonic Efficiency. Appl. Catal. A Gen. 2021, 623, 118243. [Google Scholar] [CrossRef]
- Li, P.; Zhao, X.; Dai, J.; Han, Y.; Jiang, J.; Zhang, Y. Preparation of Large-Faced Flower-like Bi2WO6 Using Carbon as a Template to Enhanced Photocatalytic Activity under Visible Light. J. Phys. Chem. Solids 2022, 171, 110968. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Xiong, Z.; Gao, T.; Gong, B.; Liu, P.; Liu, J.; Zhang, J. Elemental Mercury Removal by I−-Doped Bi2WO6 with Remarkable Visible-Light-Driven Photocatalytic Oxidation. Appl. Catal. B Environ. 2021, 282, 119534. [Google Scholar] [CrossRef]
- Zheng, X.; Tang, Q.; Zhang, H.; Lu, S.; Yang, F. Bi2WO6/SiC Composite Photocatalysts with Enhanced Photocatalytic Performance for Dyes Degradation. Inorg. Chem. Commun. 2022, 140, 109434. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Y.; Wu, W.; Deng, Y.; Xiang, Y. A Novel Rose-Like CuS/Bi2WO6 Composite for Rhodamine B Degradation. ChemistrySelect 2019, 4, 11853–11861. [Google Scholar] [CrossRef]
- Liu, W.; Qi, K.; Wang, Y.; Wen, F.; Wang, J. Halogen-Induced Polymorphic Bi2WO6-xHalogen2x with Highly Photocatalytic Performance and Mechanism Investigation. Appl. Surf. Sci. 2022, 600, 154160. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, X.; Guo, S.; Huang, Y.; Zhu, G.; Zhang, J. The Effects of Br Dopant on the Photo-Catalytic Properties of Bi2WO6. Mater. Sci. Eng. B 2016, 206, 79–84. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Hu, X.; Fan, J. RGO/Bi2WO6 Composite as a Highly Efficient and Stable Visible-Light Photocatalyst for Norfloxacin Degradation in Aqueous Environment. J. Colloid Interface Sci. 2021, 589, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.H.; Phu, N.D.; Peng, H.; Chen, X. High Photocatalytic Activity N-Doped Bi2WO6 Nanoparticles Using a Two-Step Microwave-Assisted and Hydrothermal Synthesis. J. Alloys Compd. 2018, 744, 228–233. [Google Scholar] [CrossRef]
- Sun, H.; Zou, C.; Tang, W. Designing Double Z-Scheme Heterojunction of g-C3N4/Bi2MoO6/Bi2WO6 for Efficient Visible-Light Photocatalysis of Organic Pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130105. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, Y.; Liu, M.; Peng, Y.; Liu, J. 2D-2D WO3-Bi2WO6 Photocatalyst with an S-Scheme Heterojunction for Highly Efficient Cr (VI) Reduction. CrystEngComm 2022, 24, 6902–6909. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; He, Y.; Zhang, T.; Dong, F.; Du, X.; Zhang, Y. In Situ Assembly of BiOI@Bi12O17Cl2 P-n Junction: Charge Induced Unique Front-Lateral Surfaces Coupling Heterostructure with High Exposure of BiOI {001} Active Facets for Robust and Nonselective Photocatalysis. Appl. Catal. B Environ. 2016, 199, 75–86. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, C.; Niu, C.; Zhang, L.; Zeng, G.; Zhang, X. Highly Enhanced Visible Light Photocatalytic Activity of CeO2 through Fabricating a Novel p-n Junction BiOBr/CeO2. Catal. Commun. 2017, 90, 51–55. [Google Scholar] [CrossRef]
- Kong, X.; Lee, W.; Mohamed, A.; Chai, S. Effective Steering of Charge Flow through Synergistic Inducing Oxygen Vacancy Defects and P-n Heterojunctions in 2D/2D Surface-Engineered Bi2WO6/BiOI Cascade: Towards Superior Photocatalytic CO2 Reduction Activity. Chem. Eng. J. 2019, 372, 1183–1193. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Li, J.; Xu, Y.; Han, Q.; Wei, L.; Sun, J.; Guo, J. Preparation of a Novel P-n BiOI/ Bi2WO6 Photocatalyst and Its Application in the Treatment of Tetracycline. J. Mater. Sci. Mater. Electron. 2022, 33, 23212–23223. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, Q. EPR, DFT, and Mott–Schottky Study on Visible Light Photocatalytic Activity of Au and Co3O4 Mediated Bi2WO6: Role of SPR and Multi-Valence States. J. Mater. Sci. Mater. Electron. 2022, 33, 21363–21383. [Google Scholar] [CrossRef]
- Lu, C.; Yang, D.; Wang, L.; Wen, S.; Cao, D.; Tu, C.; Gao, L.; Li, Y.; Zhou, Y.; Huang, W. Facile Construction of CoO/Bi2WO6 p-n Heterojunction with Following Z-Scheme Pathways for Simultaneous Elimination of Tetracycline and Cr (VI) under Visible Light Irradiation. J. Alloys Compd. 2022, 904, 164046. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, L.; Wang, T.; Bai, Y.; Guan, Y. Fabrication of Highly Efficient Bi2WO6/CuS Composite for Visible-Light Photocatalytic Removal of Organic Pollutants and Cr (VI) from Wastewater. Front. Environ. Sci. Eng. 2021, 15, 52. [Google Scholar] [CrossRef]
- Hu, W.; Liu, W. CuAlO2 /Bi2WO6: A Novel p–n Type Composite with Significantly Enhanced Visible-Light Photocatalytic Reduction of Cr (VI). Mater. Res. Express 2021, 8, 065901. [Google Scholar] [CrossRef]
- Xie, T.; Liu, Y.; Wang, H.; Wu, Z. Layered MoSe2/Bi2WO6 Composite with P-N Heterojunctions as a Promising Visible-Light Induced Photocatalyst. Appl. Surf. Sci. 2018, 444, 320–329. [Google Scholar] [CrossRef]
- Ng, B.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.; Li, Z.; Sun, J.; Yang, J.; Wei, J.; Wang, S.; Song, H.; Hou, Y. Visible Light Driven Antibiotics Degradation Using S-Scheme Bi2WO6/CoIn2S4 Heterojunction: Mechanism, Degradation Pathways and Toxicity Assessment. Chemosphere 2022, 303, 135113. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, F.; Liu, W. Construction of S-Scheme Heterojunction by Doping Bi2WO6 into Bi2O3 for Efficiently Enhanced Visible-Light Photocatalytic Performance. J. Mater. Sci. 2022, 57, 4265–4282. [Google Scholar] [CrossRef]
- Shandilya, P.; Guleria, A.; Fang, B. A Magnetically Recyclable Dual Step-Scheme Bi2WO6/Fe2O3/WO3 Heterojunction for Photodegradation of Bisphenol-A from Aqueous Solution. J. Environ. Chem. Eng. 2021, 9, 106461. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Liu, F. Preparation and Photocatalytic Performance of CdS@Bi2WO6 Hybrid Nanocrystals. J. Alloys Compd. 2021, 889, 161668. [Google Scholar] [CrossRef]
- Wu, X.; Xu, J.; Zhu, P.; Liu, M.; Duan, M.; Zhang, S. High Performance Visible Light Response of a Z-Type Bi2WO6/BiOBr/RGO Heterojunction Photocatalyst for the Degradation of Norfloxacin. Dalton Trans. 2022, 51, 17994–18009. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Sun, L.; Li, Y.; Liao, L.; Guan, Y.; Lao, J.; Yang, Y.; Zhou, T.; Wang, Y.; et al. A Highly Active Z-Scheme SnS/Zn2SnO4 Photocatalyst Fabricated for Methylene Blue Degradation. RSC Adv. 2022, 12, 31985–31995. [Google Scholar] [CrossRef]
- Jin, K.; Qin, M.; Li, X.; Wang, R.; Zhao, Y.; Wang, H. Z-Scheme Au@TiO2/Bi2WO6 Heterojunction as Efficient Visible-Light Photocatalyst for Degradation of Antibiotics. J. Mol. Liq. 2022, 364, 120017. [Google Scholar] [CrossRef]
- Sattari, M.; Farhadian, M.; Reza Solaimany Nazar, A.; Moghadam, M. Enhancement of Phenol Degradation, Using of Novel Z-Scheme Bi2WO6/C3N4/TiO2 Composite: Catalyst and Operational Parameters Optimization. J. Photochem. Photobiol. A Chem. 2022, 431, 114065. [Google Scholar] [CrossRef]
- Ma, C.; Ding, Y.; Ding, X.; Zhao, L.; Xu, Z.; Gao, X.; Chen, D. Synthesis of Z-Scheme g-C3N4/Gd-Doped Bi2WO6 Heterojunction with Enhanced Visible-Light Photodegradation of Organic Dyes. J. Mater. Sci. Mater. Electron. 2022, 33, 14545–14555. [Google Scholar] [CrossRef]
- Jin, J.; Sun, J.; Lv, K.; Guo, X.; Hou, Q.; Liu, J.; Wang, J.; Bai, Y.; Huang, X. Oxygen Vacancy BiO2-x/Bi2WO6 Synchronous Coupling with Bi Metal for Phenol Removal via Visible and near-Infrared Light Irradiation. J. Colloid Interface Sci. 2022, 605, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, S.; Zhong, S.; Su, X.; Ma, C. A Direct Z-Scheme Polypyrrole/Bi2WO6 Nanoparticles with Boosted Photogenerated Charge Separation for Photocatalytic Reduction of Cr (VI): Characteristics, Performance, and Mechanisms. J. Clean. Prod. 2022, 337, 130577. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Ao, M.; Luo, Y.; Zhang, A.; Zhao, L.; Yan, L.; Deng, F.; Luo, X. Solvothermal Synthesis of Z-Scheme AgIn5S8/Bi2WO6 Nano-Heterojunction with Excellent Performance for Photocatalytic Degradation and Cr (VI) Reduction. J. Alloys Compd. 2019, 805, 41–49. [Google Scholar] [CrossRef]
- Maarisetty, D.; Baral, S.S. Defect Engineering in Photocatalysis: Formation, Chemistry, Optoelectronics, and Interface Studies. J. Mater. Chem. A 2020, 8, 18560–18604. [Google Scholar] [CrossRef]
- Gao, W.; Li, G.; Wang, Q.; Zhang, L.; Wang, K.; Pang, S.; Zhang, G.; Lv, L.; Liu, X.; Gao, W.; et al. Ultrathin Porous Bi2WO6 with Rich Oxygen Vacancies for Promoted Adsorption-Photocatalytic Tetracycline Degradation. Chem. Eng. J. 2023, 464, 142694. [Google Scholar] [CrossRef]
- Hang, X.; Zhang, Y.; Li, H.; Wang, Y.; Xiang, M.; Yu, W.; Huang, H.; Ou, H. Surface Cationic and Anionic Dual Vacancies Enhancing Photocatalytic Activity of Bi2WO6. Appl. Surf. Sci. 2022, 602, 154311. [Google Scholar]
- Okada, Y. Synthetic Semiconductor Photoelectrochemistry. Chem. Rec. 2021, 21, 2223–2238. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Zhang, Y.; Wei, W.D. Plasmonic Photoelectrochemistry: In View of Hot Carriers. Adv. Mater. 2021, 33, 2006654. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Reduced Graphene Oxide Wrapped Bi2WO6 Hybrid with Ultrafast Charge Separation and Improved Photoelectrocatalytic Performance. Appl. Surf. Sci. 2017, 392, 51–60. [Google Scholar] [CrossRef]
- Pedanekar, R.S.; Madake, S.B.; Narewadikar, N.A.; Mohite, S.V.; Patil, A.R.; Kumbhar, S.M.; Rajpure, K.Y. Photoelectrocatalytic Degradation of Rhodamine B by Spray Deposited Bi2WO6 Photoelectrode under Solar Radiation. Mater. Res. Bull. 2022, 147, 11163. [Google Scholar] [CrossRef]


| Pollutants | Morphology | Precursor | Method | Efficiency | Reference |
|---|---|---|---|---|---|
| methylbenzene | Nest-like | Bi(NO3)3·5H2O Na2WO4 | hydrothermal | 100% | [62] |
| tetracycline | Flower-like | Na2WO4·2H2O Bi(NO3)3·5H2O | hydrothermal | 88% | [63] |
| tetracycline | Nanosheet | (NH4)10H2(W2O7)6 Bi(NO3)3·5H2O | hydrothermal | 70% | [64] |
| tetracycline hydrochloride | Hollow microspheres | Na2WO4·2H2O Bi(NO3)3·5H2O | precipitation | 95% | [65] |
| RhB | Tube-like | Bi2O3 Na2WO4·2H2O | solvothermal | 99% | [66] |
| RhB | Nanofiber | Na2WO4·2H2O Bi(NO3)3·5H2O | electrospinning | 71% | [67] |
| Photocatalyst | Method | Modified Band Gap Value | Pollutants | Efficiency | Reference |
|---|---|---|---|---|---|
| Pd/Bi2WO6 | hydrothermal | / | RhB | 99.33% | [73] |
| MIL-53(Fe)/Bi2WO6 | hydrothermal | 2.61 eV | phenol | 98% | [74] |
| Mn-doped Bi2WO6/GO/MoS2 | hydrothermal ultrasonic | 2.2 eV | methylene blue | 99% | [75] |
| Ni/Bi2WO6 | hydrothermal | 2.74 eV | RhB | 93% | [76] |
| Ni/Ti-Bi2WO6 | hydrothermal | 2.89 eV | tetracycline | 92.9% | [77] |
| Pt/Bi2WO6 | precipitation | 3.08 eV | RhB | 98.09% | [78] |
| Cd-Bi2WO6 | hydrothermal | 2.58 eV | RhB | 100% | [79] |
| Au-Bi2WO6 | hydrothermal | 2.96 eV | RhB | 96.25% | [80] |
| Ce-Bi2WO6 | hydrothermal | 2.23~2.26 eV | salicylic acid | 91.6% | [81] |
| In-Bi2WO6 | sol-gel | 2.74 eV | RhB | 90% | [82] |
| Doping Element | Method | Modified Band Gap Value | Pollutants | Efficiency | Reference |
|---|---|---|---|---|---|
| I | solvothermal microwave | 2.29 eV | RhB | 99% | [89] |
| Br | hydrothermal | 0.916 eV | RhB | 95% | [90] |
| rGO | hydrothermal | / | norfloxacin | 87.49% | [91] |
| N | hydrothermal microwave | / | RhB | 81% | [92] |
| Heterostructure | Catalyst | Method | Pollutants | Efficiency | Reference |
|---|---|---|---|---|---|
| S-scheme | Bi2WO6/CoIn2S4 | hydrothermal | tetracycline | 90% | [105] |
| Bi2WO6/Bi2O3 | trituration | nitrobenzene | 95.7% | [106] | |
| Bi2WO6/Fe2O3/WO3 | ultrasonic immersing | bisphenol A | 99% | [107] | |
| CdS@Bi2WO6 | solvothermal | RhB | 96.1% | [108] | |
| Z-scheme | Bi2WO6/BiOBr/rGO | hydrothermal | norfloxacin | 95.12% | [109] |
| SnS/Zn2SnO4 | hydrothermal | methylene blue | 94.5% | [110] | |
| Au@TiO2/Bi2WO6 | sol-gel | tetracycline | 95% | [111] | |
| Bi2WO6/C3N4/TiO2 | sol-gel | phenol | 84.7% | [112] | |
| g-C3N4/Gd/Bi2WO6 | hydrothermal | methylene blue | 92% | [113] | |
| BiO2−x/Bi2WO6 | solvothermal | phenol | 90% | [114] | |
| polypyrrole/Bi2WO6 | precipitation | Cr (VI) | 99.7% | [115] | |
| AgIn5S8/Bi2WO6 | solvothermal | Cr (VI) | 92% | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, H.; Zhai, R.; Zhang, J.; Gao, C.; Qi, K.; Yang, L.; Ma, Q. Recent Progress in Photocatalytic Degradation of Water Pollution by Bismuth Tungstate. Molecules 2023, 28, 8011. https://doi.org/10.3390/molecules28248011
Zhang Y, Yu H, Zhai R, Zhang J, Gao C, Qi K, Yang L, Ma Q. Recent Progress in Photocatalytic Degradation of Water Pollution by Bismuth Tungstate. Molecules. 2023; 28(24):8011. https://doi.org/10.3390/molecules28248011
Chicago/Turabian StyleZhang, Yingjie, Huijuan Yu, Ruiqi Zhai, Jing Zhang, Cuiping Gao, Kezhen Qi, Li Yang, and Qiang Ma. 2023. "Recent Progress in Photocatalytic Degradation of Water Pollution by Bismuth Tungstate" Molecules 28, no. 24: 8011. https://doi.org/10.3390/molecules28248011
APA StyleZhang, Y., Yu, H., Zhai, R., Zhang, J., Gao, C., Qi, K., Yang, L., & Ma, Q. (2023). Recent Progress in Photocatalytic Degradation of Water Pollution by Bismuth Tungstate. Molecules, 28(24), 8011. https://doi.org/10.3390/molecules28248011







