In Situ Synthesis of Bi2S3/BiFeO3 Nanoflower Hybrid Photocatalyst for Enhanced Photocatalytic Degradation of Organic Pollutants
Abstract
:1. Introduction
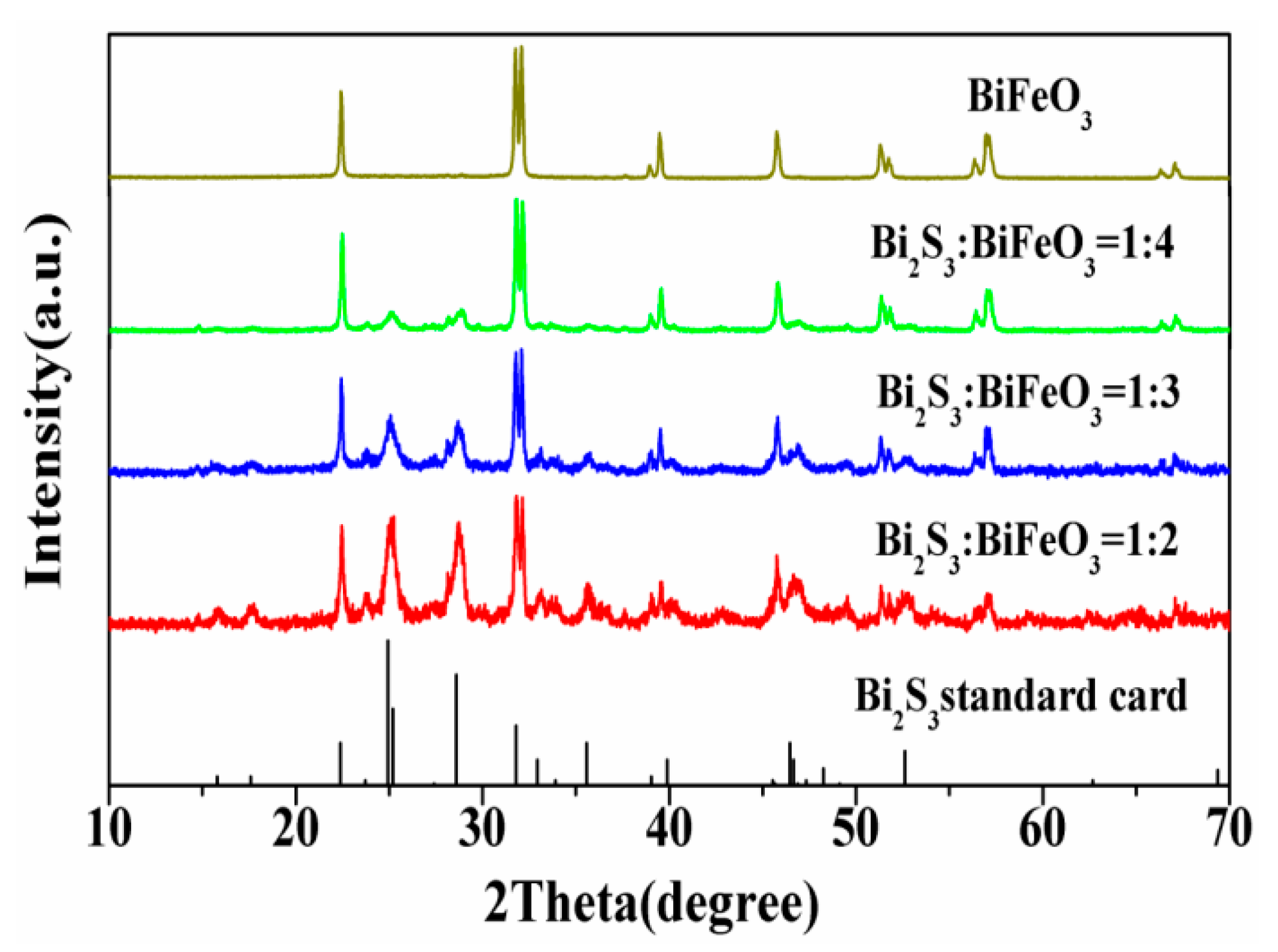
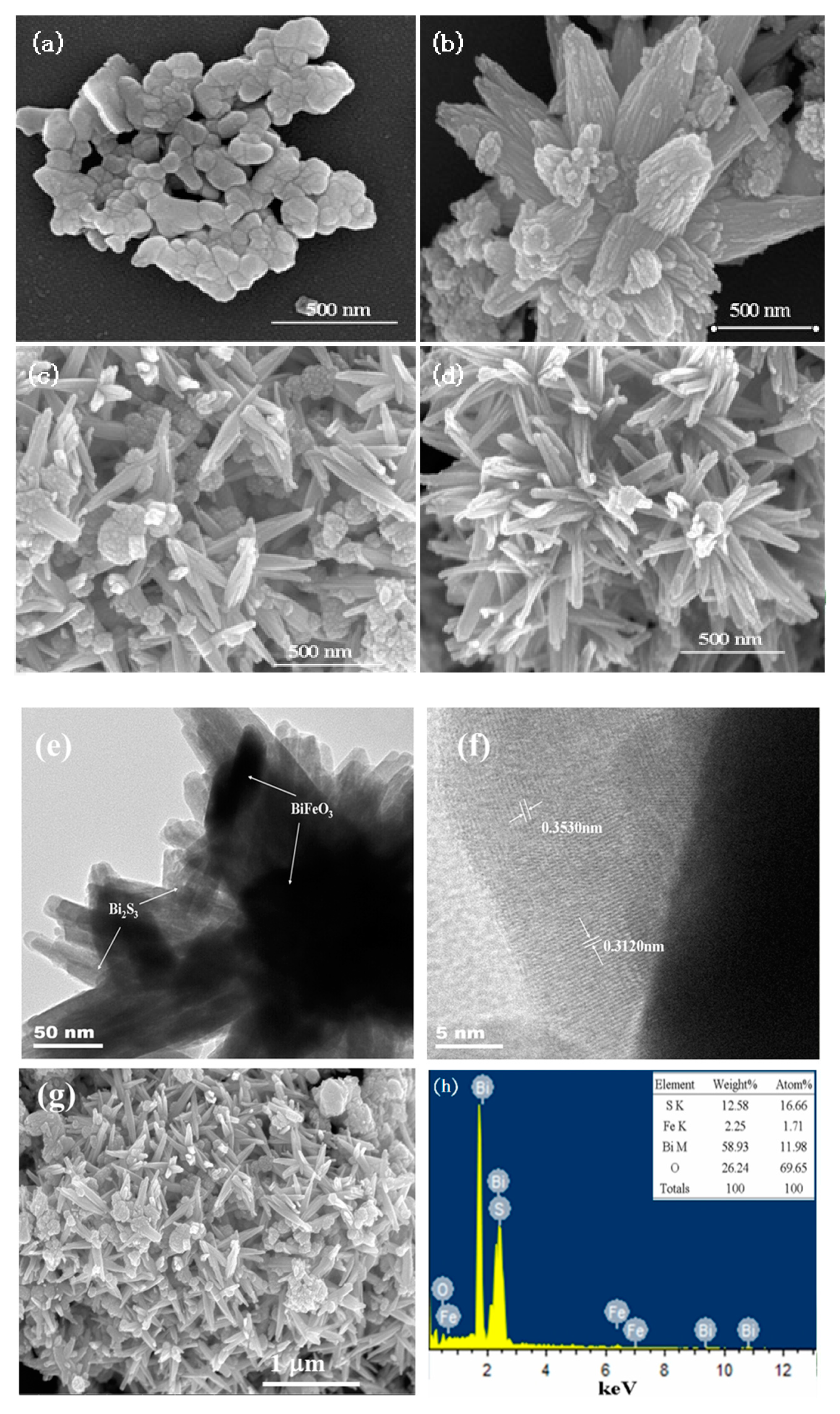
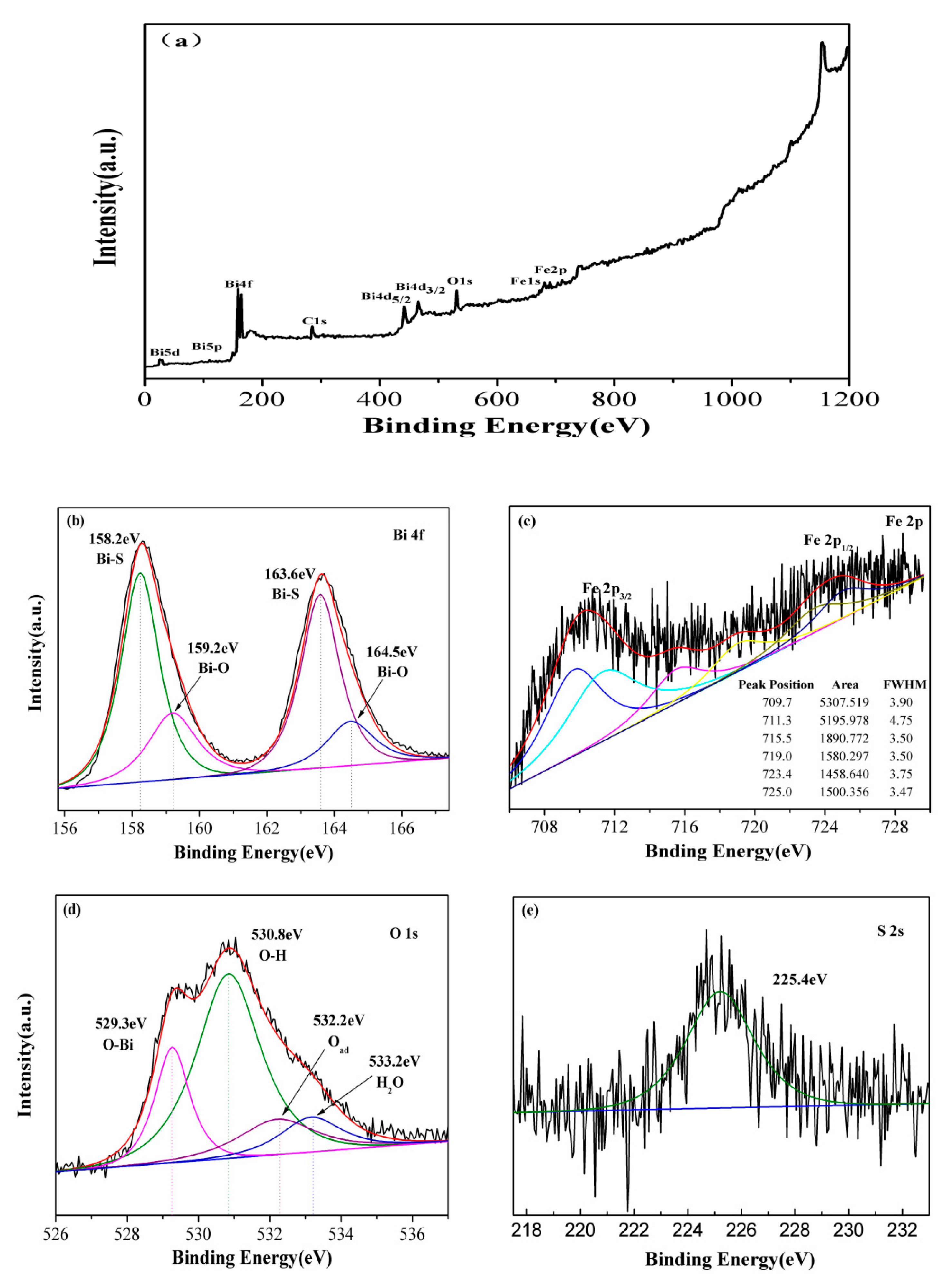
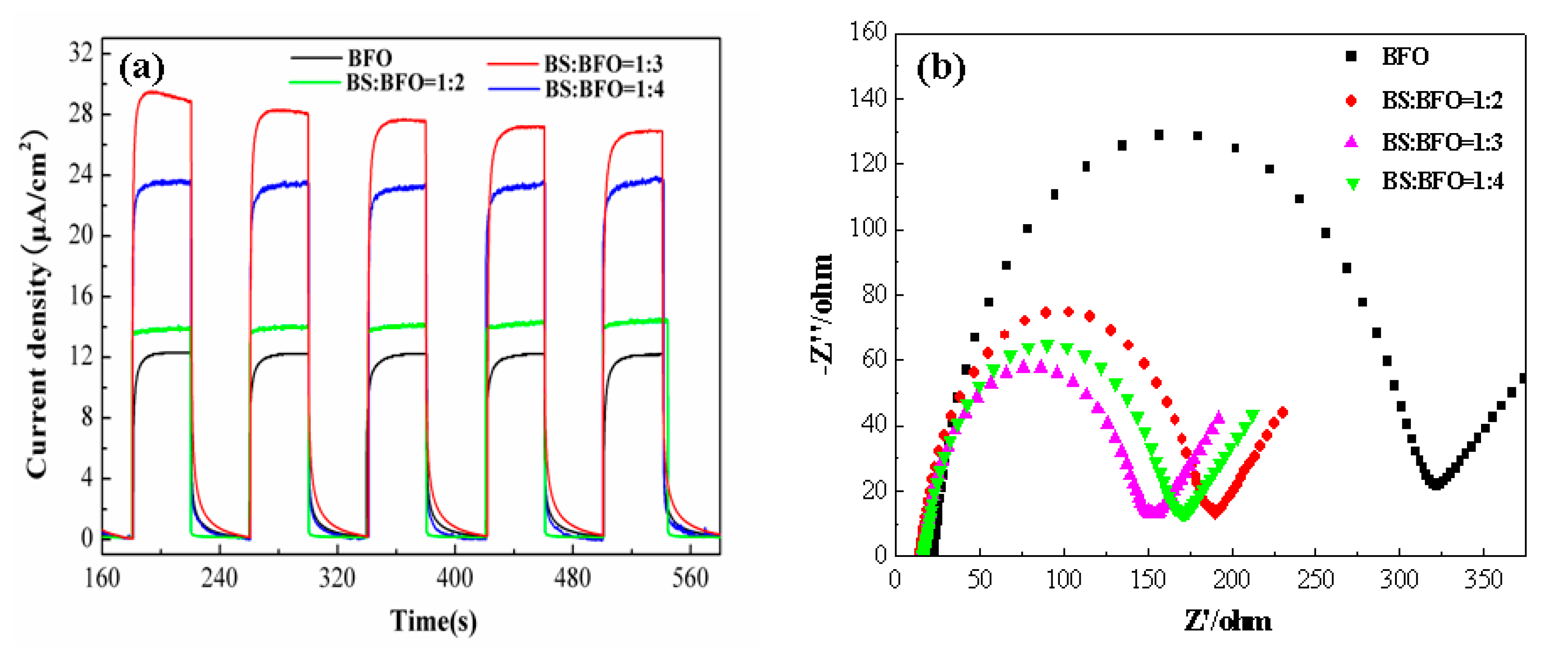
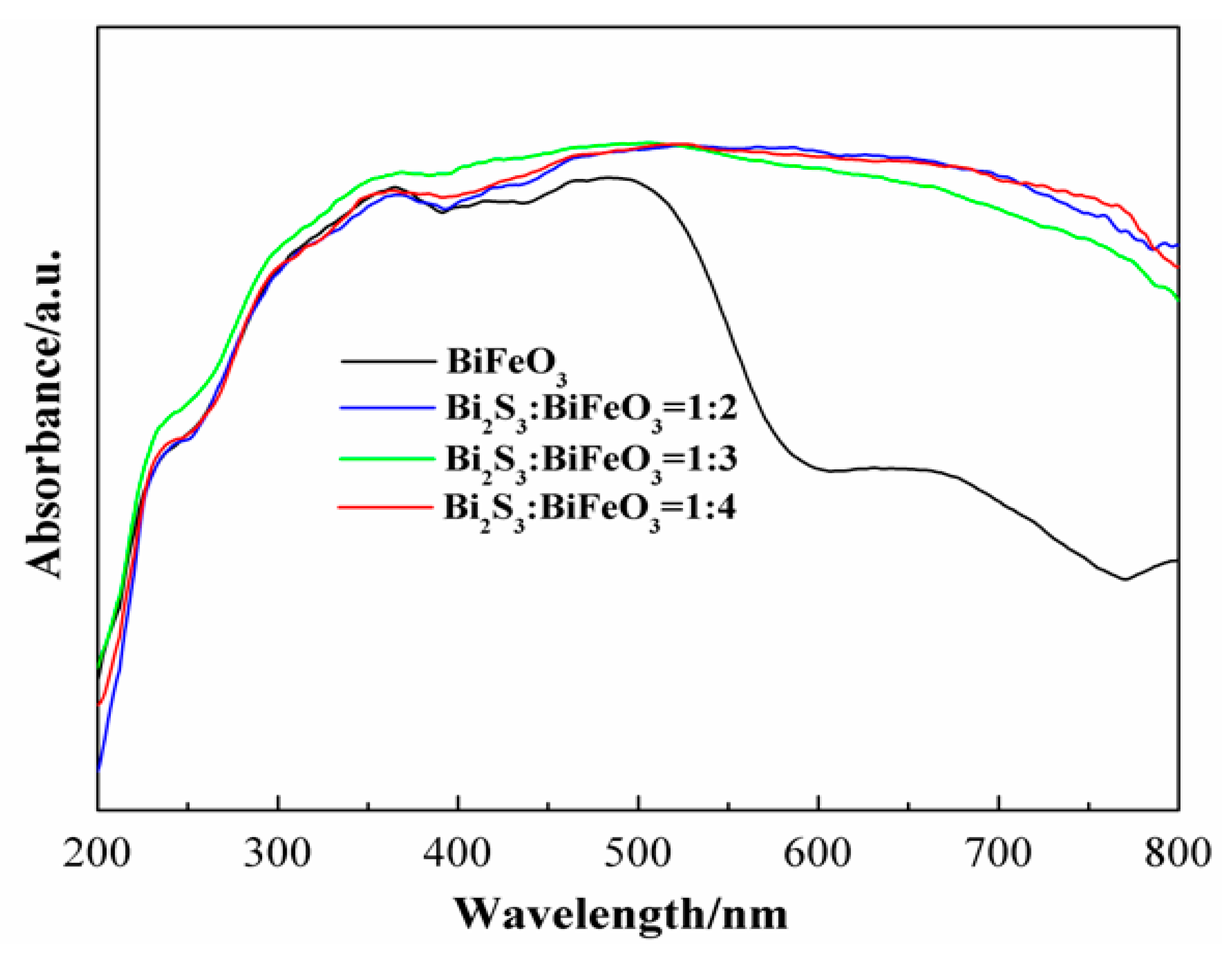
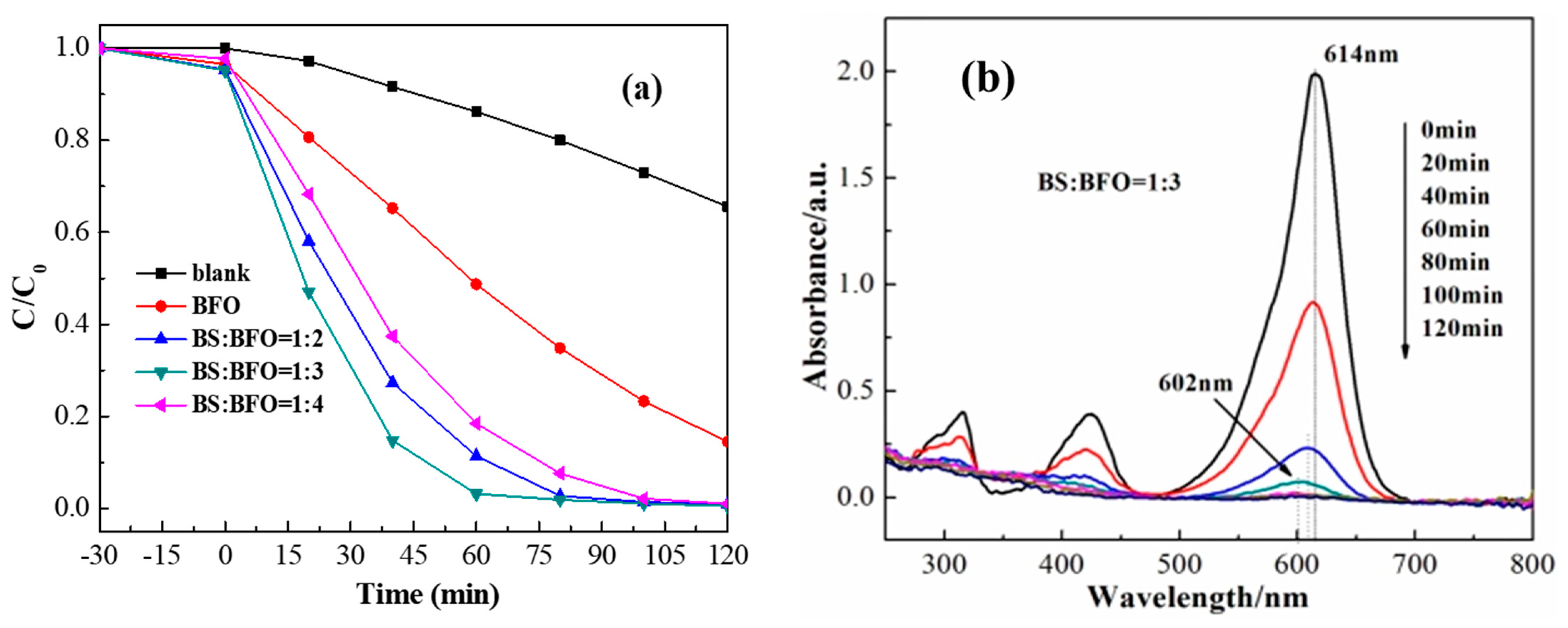
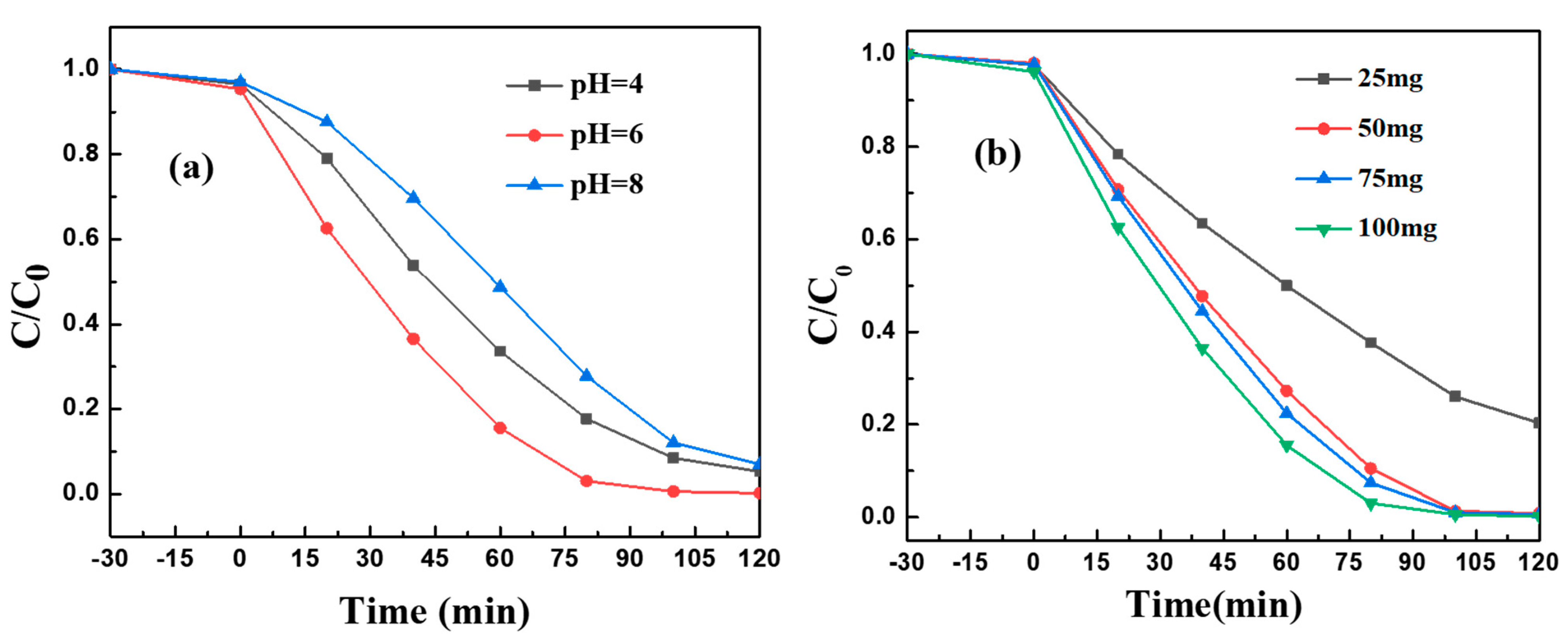
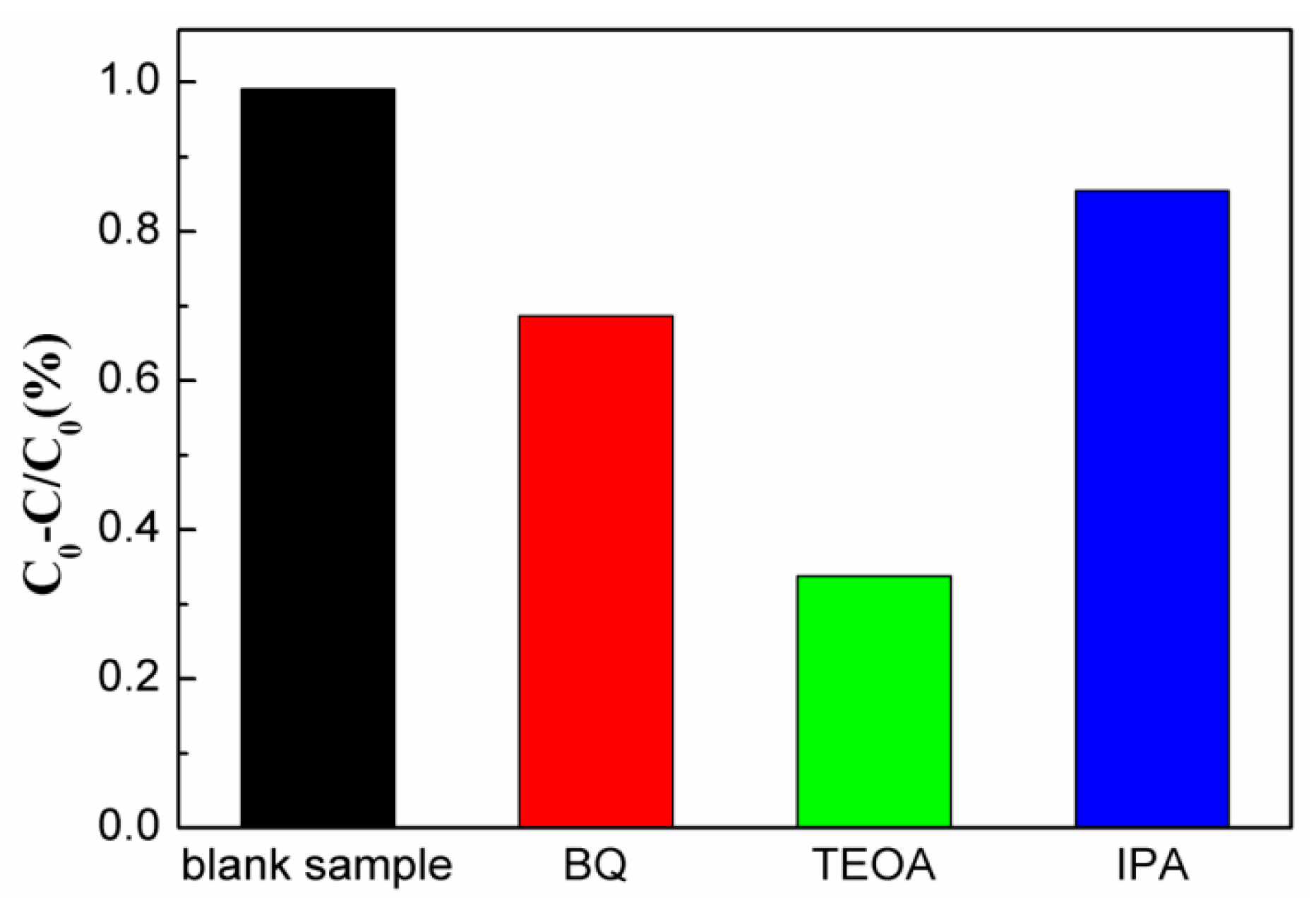
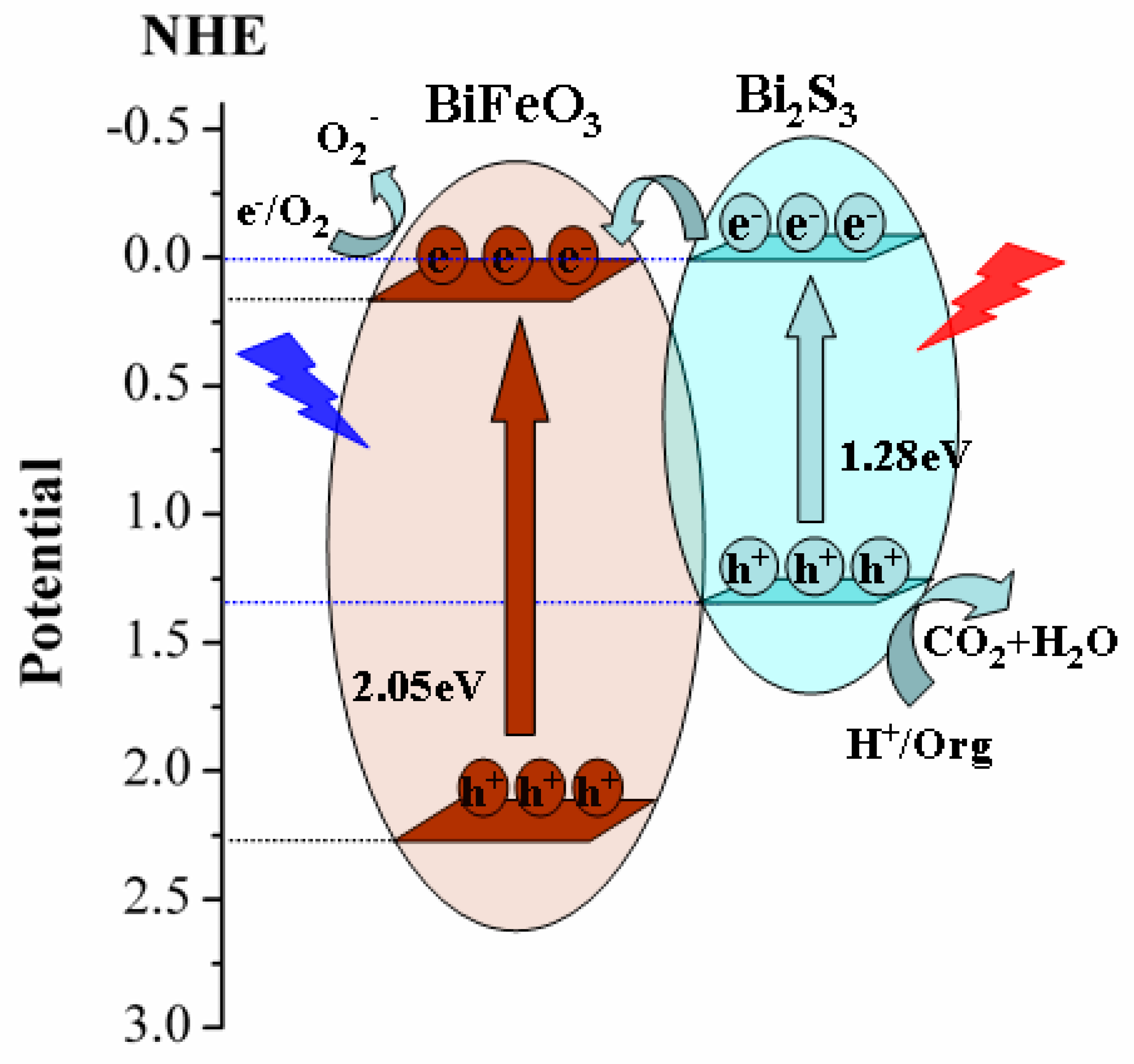
2. Results and Discussion
3. Experimental
3.1. Material Synthesis
3.2. Characterization
3.3. Photocatalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, Z.; Fan, Y.; Quan, X.; Yu, H.; Chen, S.; Zhang, S. Energy-transfer-mediated oxygen activation in carbonyl functionalized carbon nitride nanosheets for high-efficient photocatalytic water disinfection and organic pollutants degradation. Water Res. 2020, 177, 115798. [Google Scholar] [CrossRef]
- Fung, C.-M.; Er, C.-C.; Tan, L.-L.; Mohamed, A.R.; Chai, S.-P. Red phosphorus: An up-and-coming photocatalyst on the horizon for sustainable energy development and environmental remediation. Chem. Rev. 2022, 122, 3879–3965. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.H.; Zhang, B.P.; Wang, Y.; Nan, C.W. Controlled fabrication of BiFeO3 uniform microcrystals and their magnetic and photocatalytic behaviors. J. Phys. Chem. C 2010, 114, 2903–2908. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.G.; Liu, J.M. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Xu, G.; Wei, X.; Ren, Z.; Shen, G.; Han, G. Phase and morphology evolution of bismuth ferrites via hydrothermal reaction route. Mater. Res. Bull. 2013, 48, 1694–1699. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y.; Ding, X.; Jiang, J. Enhanced visible-light-response photocatalytic activity of bismuth ferrite nanoparticles. J. Alloys Compd. 2011, 509, 6585–6588. [Google Scholar] [CrossRef]
- Zhang, J.; Gondal, M.A.; Wei, W.; Zhang, T.; Xu, Q.; Shen, K. Preparation of room temperature ferromagnetic BiFeO3 and its application as an highly efficient magnetic separable adsorbent for removal of Rhodamine B from aqueous solution. J. Alloys Compd. 2012, 530, 107–110. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Dai, J.F.; Wei, Z.Q.; Ma, J.Y.; Feng, W.J. Photocatalytic properties of BiFeO3 nanoparticles with different sizes. Mater. Lett. 2011, 65, 1573–1575. [Google Scholar] [CrossRef]
- He, J.; Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Characterization and visible light photocatalytic mechanism of size-controlled BiFeO3 nanoparticles. Mater. Res. Bull. 2013, 48, 3017–3024. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, Y.; Lu, C. High efficient ultraviolet photocatalytic activity of BiFeO3 nanoparticles synthesized by a chemical coprecipitation process. J. Mater.Sci. Mater. Electron. 2010, 21, 380–384. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Mohan, S.; Subramanian, B. A strategy to fabricate bismuth ferrite (BiFeO3) nanotubes from electro spun nanofibers and their solar light-driven photocatalytic properties. RSC Adv. 2013, 3, 23737–23744. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Zheng, B.; Lin, Y.; Liu, D.; Nan, C.W. Photocatalytic and magnetic behaviors observed in BiFeO3 nanofibers by electrospinning. J. Nanomater. 2013, 2013, 917–948. [Google Scholar] [CrossRef]
- Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Enhanced photocatalytic activity and ferromagnetism in Gd Doped BiFeO3 nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Lin, Y.; Wei, Y.; Nan, C.W.; Deng, X. Influence of La Doping onMagnetic and Optical Properties of Bismuth Ferrite Nanofibers. J. Nanomater. 2012, 2012, 238605. [Google Scholar] [CrossRef]
- Feng, Y.N.; Wang, H.C.; Luo, Y.D.; Shen, Y.; Lin, Y.H. Ferromagnetic and photocatalytic behaviors observed in Ca-doped BiFeO3 nanofibres. J. Appl. Phys. 2013, 113, 146101. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Kibria, M.G.; Mi, Z.; Chaker, M.; Ma, D.; Nechache, R.; Rosei, F. Remarkably enhanced photocatalytic activity of laser ablated Au nanoparticle decorated BiFeO3 nanowires under visible-light. Chem. Commun. 2013, 49, 5856–5858. [Google Scholar] [CrossRef]
- Schultz, A.M.; Zhang, Y.; Salvador, P.A.; Rohrer, G.S. Effect of Crystal and Domain Orientation on the Visible-Light Photochemical Reduction of Ag on BiFeO3. ACS Appl. Mater. Inter. 2011, 3, 1562–1567. [Google Scholar] [CrossRef]
- Niu, F.; Chen, D.; Qin, L.; Zhang, N.; Wang, J.; Chen, Z.; Huang, Y. Facile Synthesis of Highly Efficient p–n Heterojunction CuO/BiFeO3 Composite Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ChemCatChem 2015, 7, 3279–3289. [Google Scholar] [CrossRef]
- Zhang, Y.; Schultz, A.M.; Salvador, P.A.; Rohrer, G.S. Spatially selective visible light photocatalytic activity of TiO2/BiFeO3 heterostructures. J. Mater. Chem. 2011, 21, 4168–4174. [Google Scholar] [CrossRef]
- Zhu, A.; Zhao, Q.; Li, X.; Shi, Y. BiFeO3/TiO2 Nanotube Arrays Composite Electrode: Construction, Characterization, and Enhanced Photoelectrochemical Properties. ACS Appl. Mater. Interfaces 2014, 6, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shen, J.; Li, N.; Ye, M. Hydrothermal preparation, characterization and enhanced properties of reduced grapheme-BiFeO3 nanocomposite. Mater. Lett. 2013, 91, 42–44. [Google Scholar] [CrossRef]
- Li, Z.; Shen, Y.; Guan, Y.; Hu, Y.; Lin, Y.; Nan, C.W. Bandgap engineering and enhanced interface coupling of graphene–BiFeO3 nanocomposites as efficient photocatalysts under visible light. J. Mater. Chem. A 2014, 2, 1967–1973. [Google Scholar] [CrossRef]
- Pineda, E.; Nicho, M.E.; Nair, P.K.; Hu, H. Optoelectronic properties of chemically deposited Bi2S3 thin films and the photovoltaic performance of Bi2S3/P3OT solar cells. Sol. Energy 2012, 86, 1017–1022. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.; Lin, H.; Luo, B.; Chen, S. Novel Bi2S3-sensitized BiOCl with highly visible light photocatalytic activity for the removal of rhodamine B. Catal. Commun. 2012, 26, 204–208. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y. A controlled anion exchange strategy to synthesize Bi2S3 nanocrystals/BiOCl hybrid architectures with efficient visible light photoactivity. Chem. Commun. 2012, 48, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Gu, L.; Cao, X. From single ZnO multipods to heterostructured ZnO/ZnS, ZnO/ZnSe, ZnO/Bi2S3 and ZnO/Cu2S multipods: Controlled synthesis and tunable optical and photoelectrochemical properties. CrystEngComm 2010, 12, 3950–3958. [Google Scholar] [CrossRef]
- Calva-Yanez, J.C.; Rincon, M.E.; Solís de la Fuente, M.; Alvarado-Tenorio, G. Structural and photoelectrochemical characterization of MWCNT-TiO2 matrices sensitized with Bi2S3. J. Solid State Electrochem. 2013, 17, 2633–2641. [Google Scholar] [CrossRef]
- Jana, A.; Bhattacharya, C.; Datta, J. Enhanced photoelectrochemical activity of electro-synthesized CdS-Bi2S3 composite films grown with self-designed cross-linked structure. Electrochim. Acta 2010, 55, 6553–6562. [Google Scholar] [CrossRef]
- He, H.; Berglund, S.P.; Xiao, P.; Chemelewski, W.D.; Zhang, Y.; Mullins, C.B. Nanostructured Bi2S3/WO3 heterojunction films exhibiting enhanced photoelectrochemical performance. J. Mater. Chem. A 2013, 1, 12826–12834. [Google Scholar] [CrossRef]
- Ata, S.; Shaheen, I.; Aslam, H.; Mohsin, I.U.; Alwadai, N.; Al Huwayz, M.; Iqbal, M.; Younas, U. Barium and strontium doped La-based perovskite synthesis via sol-gel route and photocatalytic activity evaluation for methylene blue. Results Phys. 2023, 45, 106235. [Google Scholar] [CrossRef]
- Jiao, S.; Zhao, Y.; Bi, M.; Bi, S.; Li, X.; Wang, B.; Li, C.; Dong, Y. Removal of Methylene Blue from Water by BiFeO3/Carbon Fibre Nanocomposite and Its Photocatalytic Regeneration. Catalysts 2018, 8, 267. [Google Scholar] [CrossRef]
- Tseng, W.J.; Lin, R.D. BiFeO3/α-Fe2O3 core/shell composite particles for fast and selective removal of methyl orange dye in water. J. Colloid Interface Sci. 2014, 428, 95–100. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. A review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xing, Z.; Zhao, H.; Li, Z.; Zhou, W. Recent advances in bismuth-based photocatalysts: Environment and energy applications. Green Energy Environ. 2023, 8, 1232–1264. [Google Scholar] [CrossRef]
- JCPDS 20-0169; Elements of X-ray Diffraction, B. D. Cullinity, 2nd ed., Addison-Wesley, Reading, MA, 1978. JCPDS (Joint Committee on Powder Diffraction Standards): Newtown Square, PA, USA, 2023.
- Dai, Y.; Wang, Y.; Yao, J.; Wang, Q.; Liu, L.; Chu, W.; Wang, G. Phosgene-Free Synthesis of Phenyl Isocyanate by Catalytic Decomposition of Methyl N-Phenyl Carbamate over Bi2O3 Catalyst. Catal. Lett. 2008, 123, 307–316. [Google Scholar] [CrossRef]
- Zeng, Z.; Ye, F.; Deng, S.; Fang, D.; Wang, X.; Bai, Y.; Xiao, H. Accelerated organic pollutants mineralization in interlayer confined single Pt atom photocatalyst for hydrogen recovery. Chem. Eng. J. 2022, 444, 136561. [Google Scholar] [CrossRef]
- Lu, X.; Pu, F.; Xia, Y.; Huang, W.; Li, Z. Facile fabrication of porous thin films of Bi2O3/Bi2S3 nanocomposite semiconductors at gas/liquid interface and their photoelectrochemical performances. Appl. Surface Sci. 2014, 299, 131–135. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Wang, M.; Wang, D.; Tang, H. Sono-enhanced degradation of dye pollutants with the use of H2O2 activated by Fe3O4 magnetic nanoparticles as peroxidase mimetic. Ultrason. Sonochem. 2010, 17, 526–533. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, J.; Anjum, M.N.; Yan, J.; Huang, L.; Zhang, Y.; Chen, J. Synthesis and characterization of wafer-like BiFeO3 with efficient catalytic activity. Solid State Sci. 2011, 13, 1779–1785. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, J.; Li, K.; Guo, H.; Yan, L.; Zeng, Z. Silicotungstic acid as electron transfer mediator in Z-scheme g-C3N4/H4SiW12O40/Ag3PO4: Accelerated charge interface transportation for high-efficient photocatalysis. Process Saf. Environ. 2023, 171, 188–199. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Q.; Ahmad, M.; Dong, S.; Zhang, Y.; Fang, D.; Wang, X.; Peng, H.; Lei, Y.; Wu, G.; et al. Carbonate mediated hole transfer boosting the photocatalytic degradation of organic pollutants over carbon nitride nanosheets. Sep. Purif. Technol. 2023, 306, 122580. [Google Scholar] [CrossRef]
- Marchon, B.; Carrazza, J.; Heinemann, H.; Somorjai, G.A. TPD and XPS studies of O2, CO2, and H2O adsorption on clean polycrystalline graphite. Carbon 1988, 26, 507–514. [Google Scholar] [CrossRef]
- Xu, W.; Fang, J.; Chen, Y.; Lu, S.; Zhou, G.; Zhu, X.; Fang, Z. Novel heterostructured Bi2S3/Bi2Sn2O7 with highly visible light photocatalytic activity for the removal of rhodamine B. Mater. Chem. Phys. 2015, 154, 30–37. [Google Scholar] [CrossRef]
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl. Catal. B Environ. 2016, 180, 219–226. [Google Scholar] [CrossRef]
- Li, X.; Ye, F.; Zhang, H.; Ahmad, M.; Zeng, Z.; Wang, S.; Wang, S.; Gao, D.; Zhang, Q. Ternary rGO decorated W18O49 @g-C3N4 composite as a full-spectrum-responded Z-scheme photocatalyst for efficient photocatalytic H2O2 production and water disinfection. J. Environ. Chem. Eng. 2023, 11, 110329. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, Z. Unusual reactivity of visible-light-responsive AgBr-BiOBr heterojunction photocatalysts. J. Catal. 2012, 293, 116–125. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamental and Application; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Li, X.; Zhu, J. Comparative study on the mechanism in photocatalytic degradation of different-type organic dyes on SnS2 and CdS. Appl. Catal. B Environ. 2012, 123–124, 174–181. [Google Scholar] [CrossRef]
- Xu, M.L.; Lu, M.; Qin, G.Y.; Wu, X.M.; Yu, T.; Zhang, L.N.; Li, K.; Cheng, X.; Lan, Y.Q. Piezo-Photocatalytic Synergy in BiFeO3@COF Z-Scheme Heterostructures for High-Efficiency Overall Water Splitting. Angew. Chem. Int. Ed. 2022, 61, e202210700. [Google Scholar] [CrossRef]
- Tang, J.; Wang, R.; Liu, M.; Zhang, Z.; Song, Y.; Xue, S.; Zhao, Z.; Dionysiou, D.D. Construction of novel Z-scheme Ag/FeTiO3/Ag/BiFeO3 photocatalyst with enhanced visible-light-driven photocatalytic performance for degradation of norfloxacin. Chem. Eng. J. 2018, 351, 1056–1066. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Tu, X.; Zheng, P.; Zhang, L.; Zeng, Z. In Situ Synthesis of Bi2S3/BiFeO3 Nanoflower Hybrid Photocatalyst for Enhanced Photocatalytic Degradation of Organic Pollutants. Molecules 2023, 28, 8007. https://doi.org/10.3390/molecules28248007
Zhou R, Tu X, Zheng P, Zhang L, Zeng Z. In Situ Synthesis of Bi2S3/BiFeO3 Nanoflower Hybrid Photocatalyst for Enhanced Photocatalytic Degradation of Organic Pollutants. Molecules. 2023; 28(24):8007. https://doi.org/10.3390/molecules28248007
Chicago/Turabian StyleZhou, Rentao, Xinman Tu, Peng Zheng, Li Zhang, and Zhenxing Zeng. 2023. "In Situ Synthesis of Bi2S3/BiFeO3 Nanoflower Hybrid Photocatalyst for Enhanced Photocatalytic Degradation of Organic Pollutants" Molecules 28, no. 24: 8007. https://doi.org/10.3390/molecules28248007
APA StyleZhou, R., Tu, X., Zheng, P., Zhang, L., & Zeng, Z. (2023). In Situ Synthesis of Bi2S3/BiFeO3 Nanoflower Hybrid Photocatalyst for Enhanced Photocatalytic Degradation of Organic Pollutants. Molecules, 28(24), 8007. https://doi.org/10.3390/molecules28248007







