Antifungal and Phytotoxic Activities of Isolated Compounds from Helietta parvifolia Stems
Abstract
1. Introduction
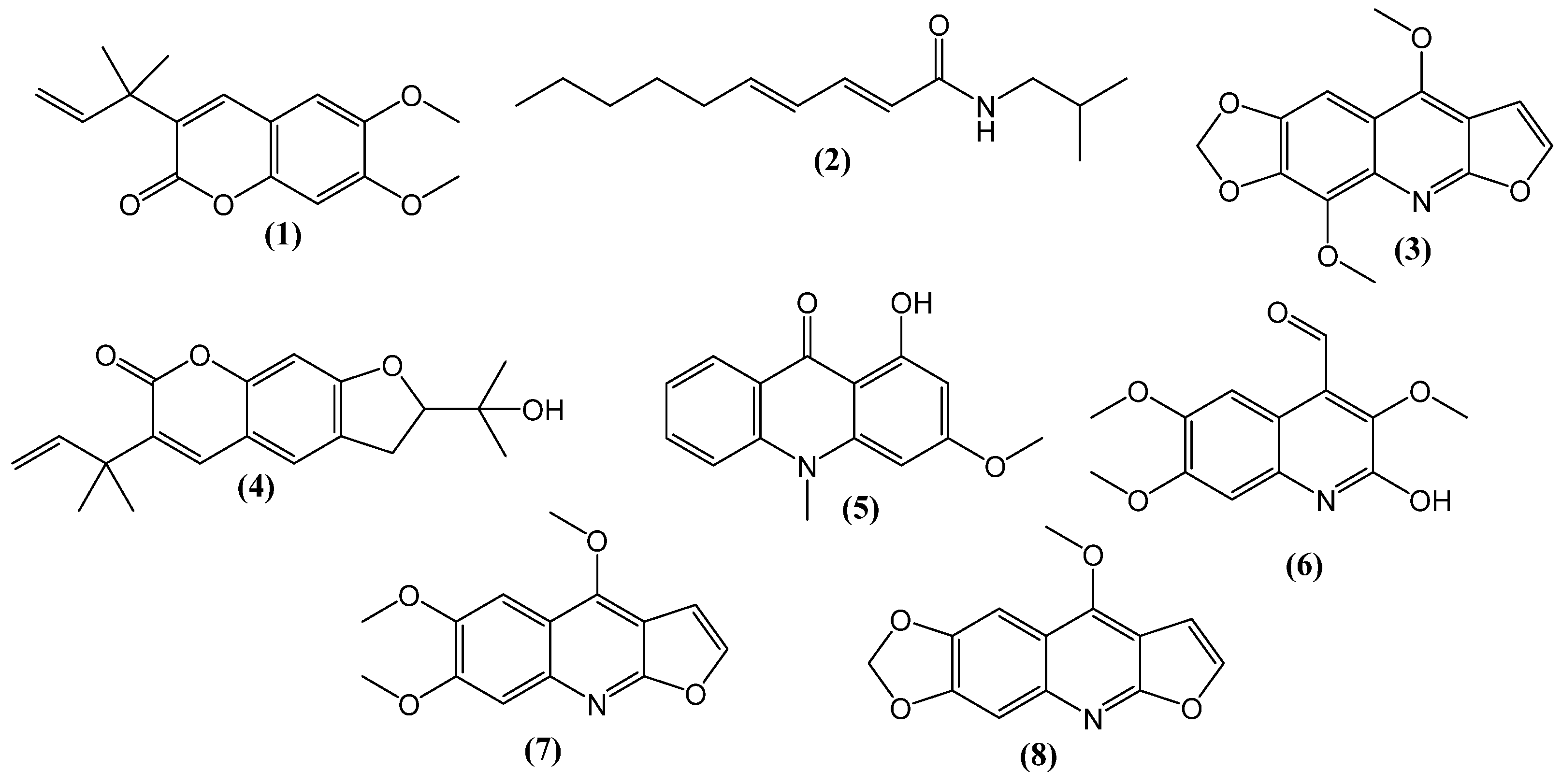
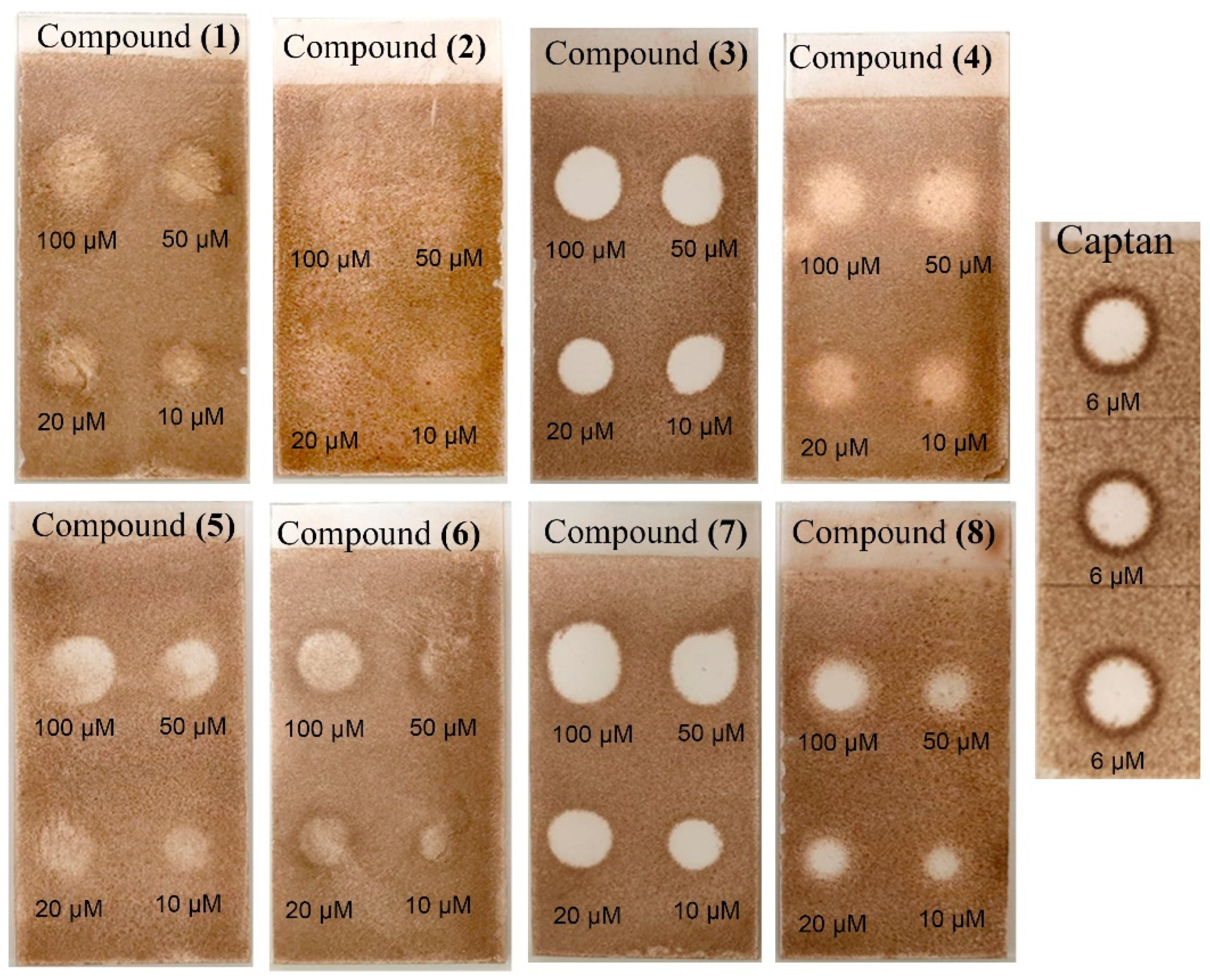
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Extraction of Plant Material
3.3. Isolation of Phytotoxic Compounds
3.4. Bioassay for Phytotoxicity Evaluation with Lactuca sativa and Agrostis stolonifera
3.5. Phytotoxicity Assay with Lemna paucicostata
3.6. Antifungal Bioautography Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baky, N.A.; Amara, A.A.A.F. Recent Approaches towards control of fungal diseases in plants: An updated review. J. Fungi 2021, 7, 900. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–159. [Google Scholar] [CrossRef]
- Heap, I. International Herbicide-Resistant Weed Database. 2023. Available online: www.weedscience.org (accessed on 27 September 2023).
- Duke, S.O. Biotechnology: Herbicide-Resistant Crops. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N., Ed.; Elsevier: San Diego, CA, USA, 2014; Volume 2, pp. 94–116. [Google Scholar]
- Pratley, J.E.; Urwin, N.A.R.; Stanton, R.A.; Baines, P.R.; Broster, J.C.; Cullis, K.; Schafer, D.E.; Bohn, J.A.; Krueger, R.W. Resistance to glyphosate in Lolium rigidum: I. Bioevaluation. Weed Sci. 1999, 47, 405–411. [Google Scholar] [CrossRef]
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest. Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef]
- HRAC. Herbicide Resistance Action Committee. 2023. Available online: https://www.hracglobal.com/ (accessed on 27 September 2023).
- Duke, S.O.; Dayan, F.E. New herbicide modes of action for new commercial herbicides—Searching for the “Holy Grail”. Pest. Manag. Sci. 2022, 78, 1303–1313. [Google Scholar] [CrossRef]
- Hollomon, D.W. Fungicide resistance: Facing the challenge—A review. Plant Protect. Sci. 2015, 51, 170–176. [Google Scholar] [CrossRef]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide resistance evolution and detection in plant pathogens: Plasmopara viticola as a case study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest. Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest. Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Sparks, J.M.; Duke, S.O. Natural product-based crop protection compounds—Origins and future prospects. J. Agric. Food Chem. 2023, 71, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Duan, C.-B.; Jin, B.; Ali, A.S.; Han, X.; Zhang, H.; Zhang, M.-Z.; Zhang, W.H.; Gu, Y.C. Recent advances in the natural products-based lead discovery for new agrochemicals. Adv. Agrochem, 2023; in press. [Google Scholar] [CrossRef]
- Goloubkova, T.; Heckler, E.; Rates, S.M.; Henriques, J.A.; Henriques, A. Inhibition of cytochrome P450-dependent monooxygenases by an alkaloid fraction from Helietta apiculata markedly potentiate the hypnotic action of pentobarbital. J. Ethnopharmacol. 1998, 60, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ferronatto, R.; Carraro, C.; Marins, K.; Flach, A.; Moura, N.E. Chemical composition and antibacterial activity of the essential oils from Helietta apiculata Benth. (Rutaceae). J. Essent. Oil Res. 2012, 24, 13–17. [Google Scholar] [CrossRef][Green Version]
- Ferreira, M.E.; Arias, A.R.; Yaluff, G.; Bilbao, N.V.; Nakayama, H.; Torres, S.; Schinini, A.; Guy, I.; Heinzen, H.; Fournet, A. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine 2010, 17, 375–378. [Google Scholar] [CrossRef]
- Monjarás-Barrera, J.I.; Salvador, O.S.; Heinz-Castro, R.T.Q.; López-Sánchez, I.V.; Pedro-Méndez, J.G.; Chacón-Hernández, J.C. Two New Hosts of Tetranychus merganser Boudreaux1 in Northeastern Mexico: Pittosporum tobira (Pittosporaceae) and Helietta parvifolia (Rutaceae). Southwest. Entomol. 2020, 45, 819–822. [Google Scholar] [CrossRef]
- Marroquin-Segura, R.; Flores-Pimentel, M.; Carreon-Sanchez, R.; Garcia-Burciaga, M.M.; Mora-Guevara, J.L.A.; Aguilar-Contreras, A.; Hernandez-Abad, V.J. The effect of the aqueous extract of Helietta parvifolia A. Gray (Rutaceae) stem bark on carrageenan-induced paw oedema and granuloma tissue formation in mice. J. Ethnopharmacol. 2009, 124, 639–641. [Google Scholar] [CrossRef]
- Anaya, A.L.; Macías-Rubalcava, M.; Cruz-Ortega, R.; García-Santana, C.; Sánchez-Monterrubio, P.N.; Hernández-Bautista, B.E.; Mata, R. Allelochemicals from Stauranthus perforatus, a Rutaceae tree of the Yucatan Peninsula, Mexico. Phytochemistry 2005, 66, 487–494. [Google Scholar] [CrossRef]
- Gómez-Calvario, V.; Ramírez-Cisneros, M.A.; Acevedo-Quiroz, M.; Rios, M.Y. Chemical composition of Helietta parvifolia and its in vitro anticholinesterase activity. Nat. Prod. Res. 2019, 33, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Johnson, R.D.; Duke, S.O.; Scheffler, B.E. Dose-response relationships between herbicides with different modes of action and growth of Lemna paucicostata: An improved ecotoxicological method. Environ. Toxicol. Chem. 2004, 23, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Nebo, L.; Varela, R.M.; Molinillo, J.M.B.; Sampaio, O.M.; Severino, V.G.P.; Cazal, C.M.; Fernandes, G.M.F.; Fernandes, J.B.; Macías, F.A. Phytotoxicity of alkaloids, coumarins and flavonoids from 11 species belonging to the Rutaceae and Meliaceae families. Phytochem. Lett. 2014, 8, 226–232. [Google Scholar] [CrossRef]
- Grossmann, K. What it takes to get a herbcide’s mode of action. Physionomics, a classical approache in a new complextion. Pest. Manag. Sci. 2005, 61, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Emerole, G.; Thabrew, M.I.; Anosa, V.; Okorie, D.A. Structure-activity relationship in the toxicity of some naturally occurring coumarins-chalepin, imperatorin and oxypeucedanine. Toxicology 1981, 20, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Al-Majmaie, S.; Al-Groshi, A.; Rasul, A.; Sarker, S.D. Chalepin and Chalepensin: Occurance, biosynthesis and therapeutic potential. Molecules 2021, 26, 1609. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Tice, C.M. Selecting the right compounds for screening: Does Lipinski’s rule of 5 for pharmaceuticals apply to agrochemicals. Pest. Manag. Sci. 2001, 57, 3–6. [Google Scholar] [CrossRef]
- Szewczyk, A.; Pęczek, F. Furoquinoline alkaloids: Insights into chemistry, occurrence, and biological properties. Int. J. Mol. Sci. 2023, 24, 12811. [Google Scholar] [CrossRef]
- Fernandes, T.S.; Copetti, D.; Carmo, G.D.; Neto, A.T.; Pedroso, M.; Silva, U.F.; Mostardeiro, M.A.; Burrow, R.E.; Dalcol, I.I.; Morel, A.F. Phytochemical analysis of bark from Helietta apiculata Benth and antimicrobial activities. Phytochemistry 2017, 141, 131–139. [Google Scholar] [CrossRef]
- Adamska-Szewczyk, A.; Glowniak, K.; Baj, T. Furochinoline alkaloids in plants from Rutaceae family—A review. Curr. Issues Pharm. Med. Sci. 2016, 29, 33–38. [Google Scholar] [CrossRef]
- Nna, P.J.; Tor-Anylin, T.A.; Igoli, J.O. Fagaramide and pellitorine from the stem bark of Zanthoxylum zanthoxyloides and their antimicrobial activities. S. Asian Res. J. Nat. Prod. 2019, 2, 1–8. [Google Scholar]
- Biavatti, M.W.; Vieira, P.C.; da Silva, M.F.G.F.; Fernandes, J.B.; Victor, S.R.; Panocca, F.C.; Albuquerque, S.; Caracelli, I.; Zukerman-Schpector, J. Biological activity of quiinonline alkaloids from Raulinoa echinata and X-ray structure of flindersiamine. J. Braz. Chem. Soc. 2002, 13, 66–70. [Google Scholar] [CrossRef]
- Lee, H.-S. Pesticidal constituents derived from Piperacease fruits. Agric. Chem. Biotechnol. 2005, 48, 65–74. [Google Scholar]
- Miyakado, M.; Nakayama, I.; Ohno, N. Insecticides unsaturatred isobutylamides from natural products to agrochemical leads. In Insecticides of Plant Origin; Arnason, J.T., Philogène, B.J.R., Morand, P., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 173–187. [Google Scholar]
- Buxton, T.; Takahashi, S.; Takakura, M.; Niwata, I.; Owusu, E.O.O.; Kim, C.-S. Insecticidal activities of pellitorine isolated from Zanthoxylum zanthooxyloides roots against Sitophilus oryzae L. (Coleoptera: Curculionidae). J. Entomol. Zool. Stud. 2017, 5, 163–168. [Google Scholar]
- Valdez, M.B.; Giménez, D.M.B.; Fernández, L.R.; Musikant, A.D.; Ferri, G.; Sáenz, D.; Di Venosa, G.; Casas, A.; Avigliano, E.; Edreira, M.M.; et al. Antiparasitic derivatives of the furoquinoline alkaloids kokusagenine and flindersiamine. ChemMedChem 2022, 17, e2021007. [Google Scholar]
- Steck, W.; Bailey, B.K.; Shyluk, J.P.; Gamborg, O.L. Coumarins and alkaloids from cell cultures of Ruta graveolens. Phytochemistry 1971, 10, 191–194. [Google Scholar] [CrossRef]
- Rosario, S.L.; Silva, A.J.; Parente, J.P. Alkamides from Cissampelos glaberrima. Planta Med. 1996, 62, 376–377. [Google Scholar] [CrossRef]
- Richardson, J.S.M.; Sethi, G.; Lee, G.S.; Malek, S.N.A. Chalepin: Isolated from Ruta angustifolia L. Pers induces mitochondrial mediated apoptosis in lung carcinoma cells. BMC Complement. Altern. Med. 2016, 16, 389. [Google Scholar] [CrossRef]
- Jolivet, C.; Rivalle, C.; Bisagni, E. Reaction of noracronycine and 1-hydroxy-3-methoxy-10-methylacridone with alkyl- and aryl-lithiums: Formation of quinone methides. J. Chem. Soc. Perkin Trans. 1995, 1, 511–515. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Stappen, I.; Alib, A.; Tabanca, N.; Khanb, I.A.; Wannerc, J.; Gochevd, V.K.; Singhe, V.; Lalf, B.; Jaitakf, V.; Kaulh, V.K. Antimicrobial and repellent activity of the essential oils of two Lamiaceae cultivated in western Himalaya. Curr. Bioact. Compd. 2015, 11, 23–30. [Google Scholar] [CrossRef]


| Compounds | Ranking | |
|---|---|---|
| L. sativa | A. stolonifera | |
| 1 | 1 | 1 |
| 2 | 0 | 2 |
| 3 | 0 | 2 |
| 4 | 0 | 3 |
| 5 | 0 | 0 |
| 6 | 0 | 3 |
| 7 | 0 | 0 |
| 8 | 0 | 0 |
| atrazine | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, V.P.; Bajsa-Hirschel, J.; Tamang, P.; Meepagala, K.; Duke, S.O. Antifungal and Phytotoxic Activities of Isolated Compounds from Helietta parvifolia Stems. Molecules 2023, 28, 7930. https://doi.org/10.3390/molecules28237930
Ribeiro VP, Bajsa-Hirschel J, Tamang P, Meepagala K, Duke SO. Antifungal and Phytotoxic Activities of Isolated Compounds from Helietta parvifolia Stems. Molecules. 2023; 28(23):7930. https://doi.org/10.3390/molecules28237930
Chicago/Turabian StyleRibeiro, Victor Pena, Joanna Bajsa-Hirschel, Prabin Tamang, Kumudini Meepagala, and Stephen O. Duke. 2023. "Antifungal and Phytotoxic Activities of Isolated Compounds from Helietta parvifolia Stems" Molecules 28, no. 23: 7930. https://doi.org/10.3390/molecules28237930
APA StyleRibeiro, V. P., Bajsa-Hirschel, J., Tamang, P., Meepagala, K., & Duke, S. O. (2023). Antifungal and Phytotoxic Activities of Isolated Compounds from Helietta parvifolia Stems. Molecules, 28(23), 7930. https://doi.org/10.3390/molecules28237930






