Modification Method of High-Efficiency Organic Bentonite for Drilling Fluids: A Review
Abstract
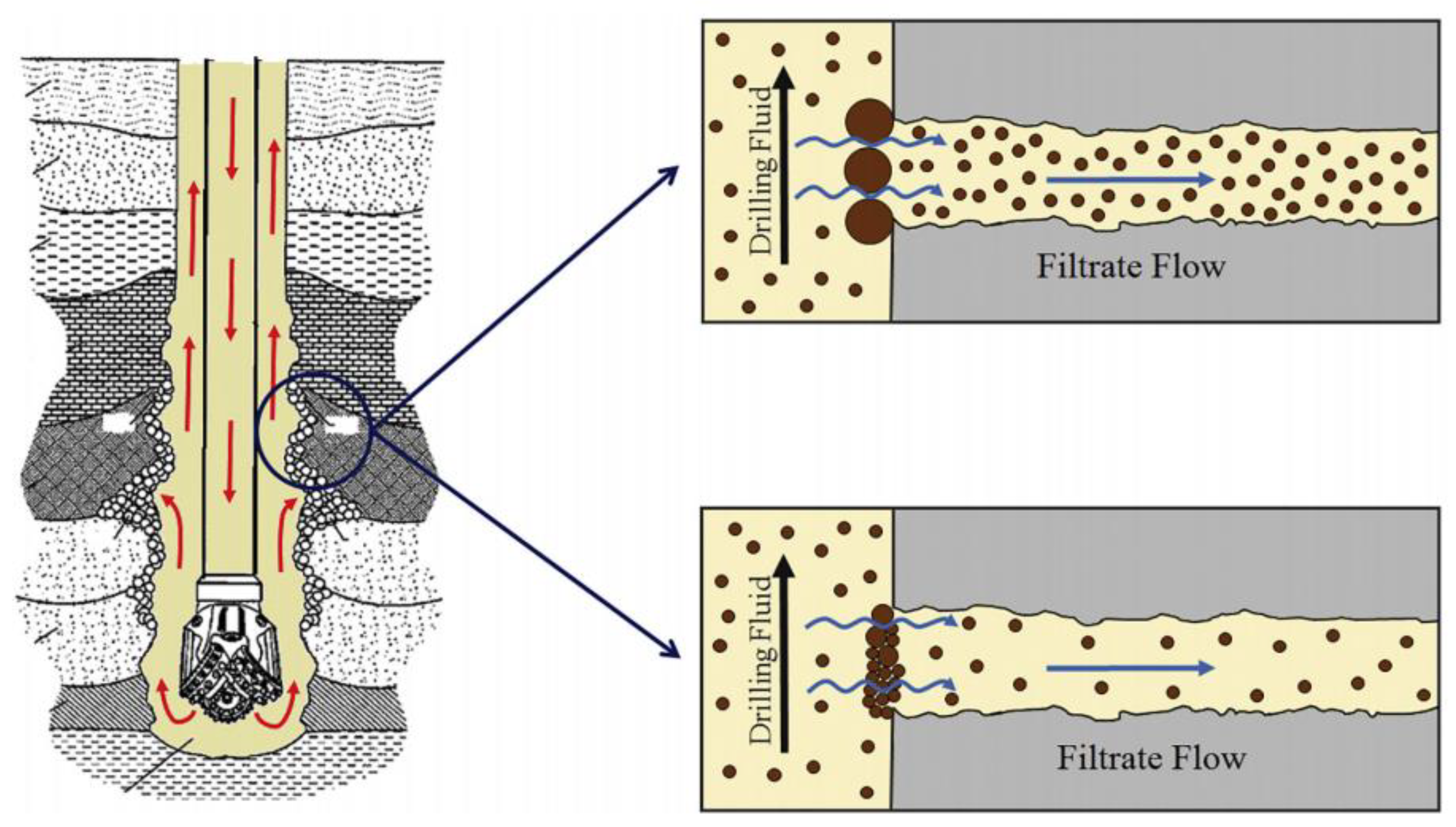
:1. Introduction
2. Organic Modification Mechanism of Bentonite
2.1. Intercalation
2.2. Coupling
2.3. Graft
3. Intercalation-Modified Bentonite
3.1. The Method of Solution Intercalation
3.2. The Method of Melt Intercalation
4. Grafting Modified Bentonite
4.1. The Method of Physical Grafting
4.2. The Method of Chemical Grafting
4.2.1. The Method of Coupling Grafting
4.2.2. The Method of Graft Copolymerization
4.2.3. Other Chemical Reaction Methods
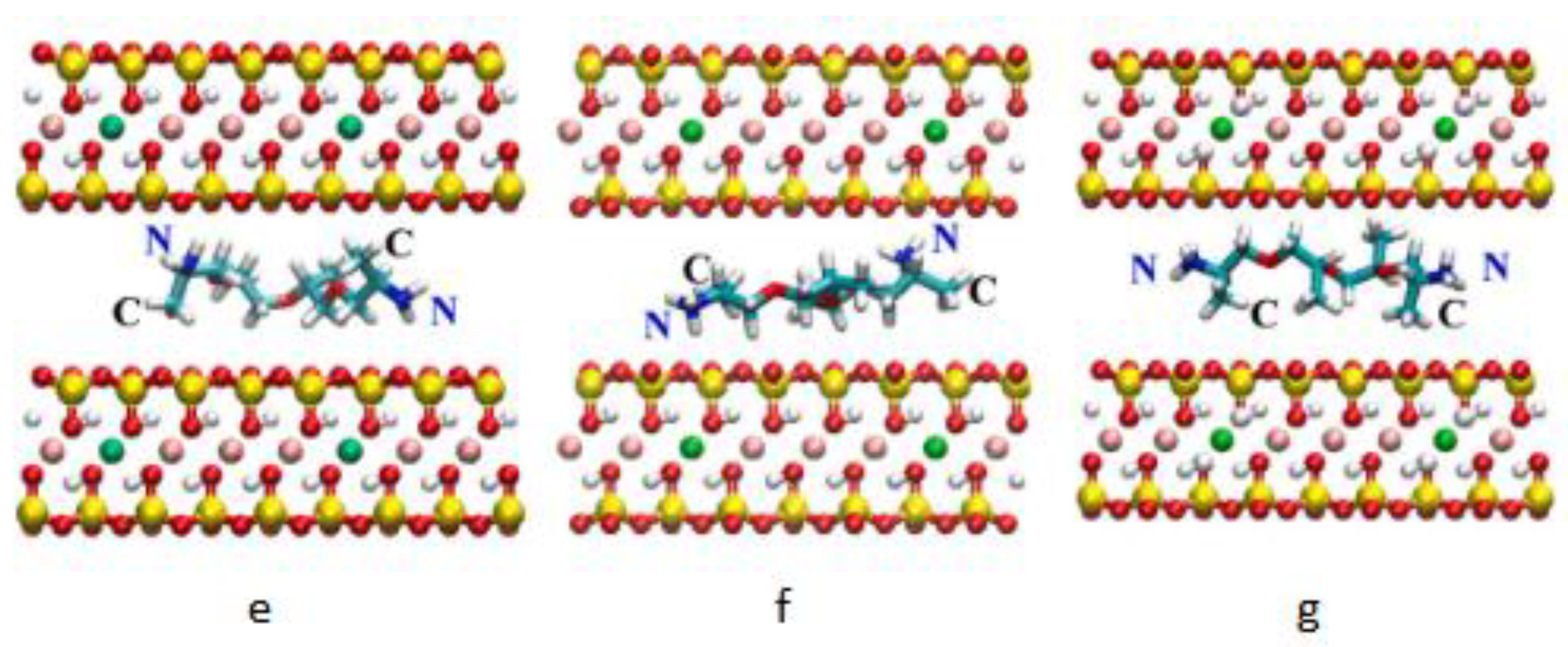
5. Molecular Simulation of Organic Bentonite Modification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Mt | Montmorillonite |
| API | American Petroleum Institute |
| ROP | Rate of penetration |
| XRD | X-ray diffraction |
| TGA | Thermogravimetric analysis |
| CTAB | Hexadecyltrimethylammonium bromide |
| DTAB | N,N,N-trimethyl-1-dodecanaminium bromide |
| OTAB | Octadecyl trimethyl ammonium bromide |
| TTAB | Tetradecyltrimethylammonium bromide |
| PLSN | Polymer/layered silicate nanocomposites |
| PAM | Polyacrylamide |
| CPNm | Clay–polymer nanocomposites |
| FT-IR | Fourier transform infrared spectrometer |
| HDTMA | Cetyltrimethylammonium chloride |
| PPG | Polypropylene glycol |
| HDTMS | Hexadecyltrimethoxysilane |
| PAMAM | Amine-terminated polyamidoamine |
| TEM | Transmission electron microscopy |
| WBDF | Water-based drilling fluids |
| PEA | Amino-terminated polyoxypropylene |
| HAES | Amidosilanols |
| MPS | Methacryloxypropyltrimethoxysilane |
| ILs | Green ionic liquids |
| PLA | Polylactic acid |
| PS | Polystyrene |
| SEM | Scanning electron microscopy |
| PEO | Polyethylene oxide |
| DSC | Differential scanning calorimetry |
| PB | Polycationic bentonite |
| PP | Polypropylene |
| PP-MA | Maleic anhydride PP |
| Si-HPEI | Silane coupling agent |
| HPEI | Hyperbranched polyethyleneimine |
| Si–SL | Silane coupling agent |
| SL | Sulfonated lignin |
| NBR | Nitrile butadiene rubber |
| KH570 | γ-methacryloyloxytrimethoxysilane |
| PAC | Polyaluminium hloride |
| SDBS | Sodium dodecylbenzylsulfonate |
| PVC | Polyvinyl chloride |
| CMC | Carboxymethyl cellulose |
| AM | Acrylamide |
| CMCS | Carboxymethyl chitosan |
| WBDMS | Water-based drilling mud system |
| PMAA | Polymethacrylic acid |
| IR | Infrared spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| AMPS | 2-acrylamide-2-methylpropanesulfonic acid |
| DMDAAC | Diallyldimethylammonium chloride |
| DMAA | N,N-dimethyl Acrylamide |
| SSS | Sodium p-styrene sulfonate |
| SMP | Sulfobenzaldehyde |
| MSP | 3-vinyltriethoxysilane |
| CMS | Carboxymethyl starch |
| CS | Corn starch |
| CES | Compound etherified starch |
References
- Cui, X.; Lv, X. Properties of bentonite and its application. China Mon-Met. Mines. 2000, 2, 6–9. [Google Scholar]
- Bergaya, F.; Jaber, M.; Lambert, J.F. Clays and clay minerals as layered nanofillers for (bio) polymers. In Environmental Silicate Nano-Biocomposites; Springer: Berlin/Heidelberg, Germany, 2012; pp. 41–75. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structure and mineralogy of clay minerals. In Developments in Clay Science; Bergaya, F., Theng, G.B.K., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 21–81. [Google Scholar] [CrossRef]
- Qiu, J.; Cui, X.; Lv, X.; Song, J. Measurement of Montmorillonite Layer Charge. Mod. Min 2005, 21, 6–9. [Google Scholar]
- Lv, F.; Dai, S.; Hou, L.; Chen, Y. Status quo of the sodium-modified processing technology of ca-bentonite and applicatios of its products. China Min. 1997, 6, 65–67. [Google Scholar]
- Wu, X.; Wang, L.; Xie, X.; Zhang, K. Organic activation and Characterization of Calcium Type Bentonite. Multipurpose Util. Miner. Resour. 2007, 2, 13–16. [Google Scholar]
- Li, C.; Yang, X. Modification of bentonite and its application in drilling fluids. China Pet. Chem. Stand. Qual. 2019, 39, 2. [Google Scholar]
- Liu, F.; Jiang, G.; Wang, K.; Wang, X.; Wang, J. Research on the application of new nano materials in shale gas water-based drilling fluids. Drill. Complet. Fluids 2018, 35, 27–33. [Google Scholar]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Wang, Y.; Forson, K.; Cao, L.; Zhang, C.; Zhou, C.; Zhao, B.; Chen, J. Experimental investigation on the high-pressure sand suspension and adsorption capacity of guar gum fracturing fluid in low-permeability shale reservoirs: Factor analysis and mechanism disclosure. Environ. Sci. Pollut. Res. 2022, 29, 53050–53062. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Ouyang, Y.; Han, Q. Synthesis of hydrophobically modified polyampholyte based on epoxidized soybean oil as shale inhibitor in water-based drilling fluid. Colloid. Surf. A Physicochem. Eng. Asp. 2021, 622, 126664. [Google Scholar] [CrossRef]
- Yan, J. Drilling Fluid Technology; Petroleum University Press: Qingdao, China, 2001; pp. 63–104. [Google Scholar]
- Gautam, S.; Guria, C.; Rajak, D.K.; Pathak, A.K. Functionalization of fly ash for the substitution of bentonite in drilling fluid. J. Pet. Sci. Eng. 2018, 166, 63–72. [Google Scholar] [CrossRef]
- Hassan, A.; Elkatatny, S.; Al-Majed, A. Coupling rate of penetration and mechanical specific energy to Improve the efficiency of drilling gas wells. J. Nat. Gas Sci. Eng. 2020, 83, 103558. [Google Scholar] [CrossRef]
- Silva, I.A.; Sousa, F.K.A.; Menezes, R.R.; Neves, G.A.; Santana, L.N.L.; Ferreira, H.C. Modification of bentonites with nonionic surfactants for use in organic-based drilling fluids. Appl. Clay Sci. 2014, 95, 371–377. [Google Scholar] [CrossRef]
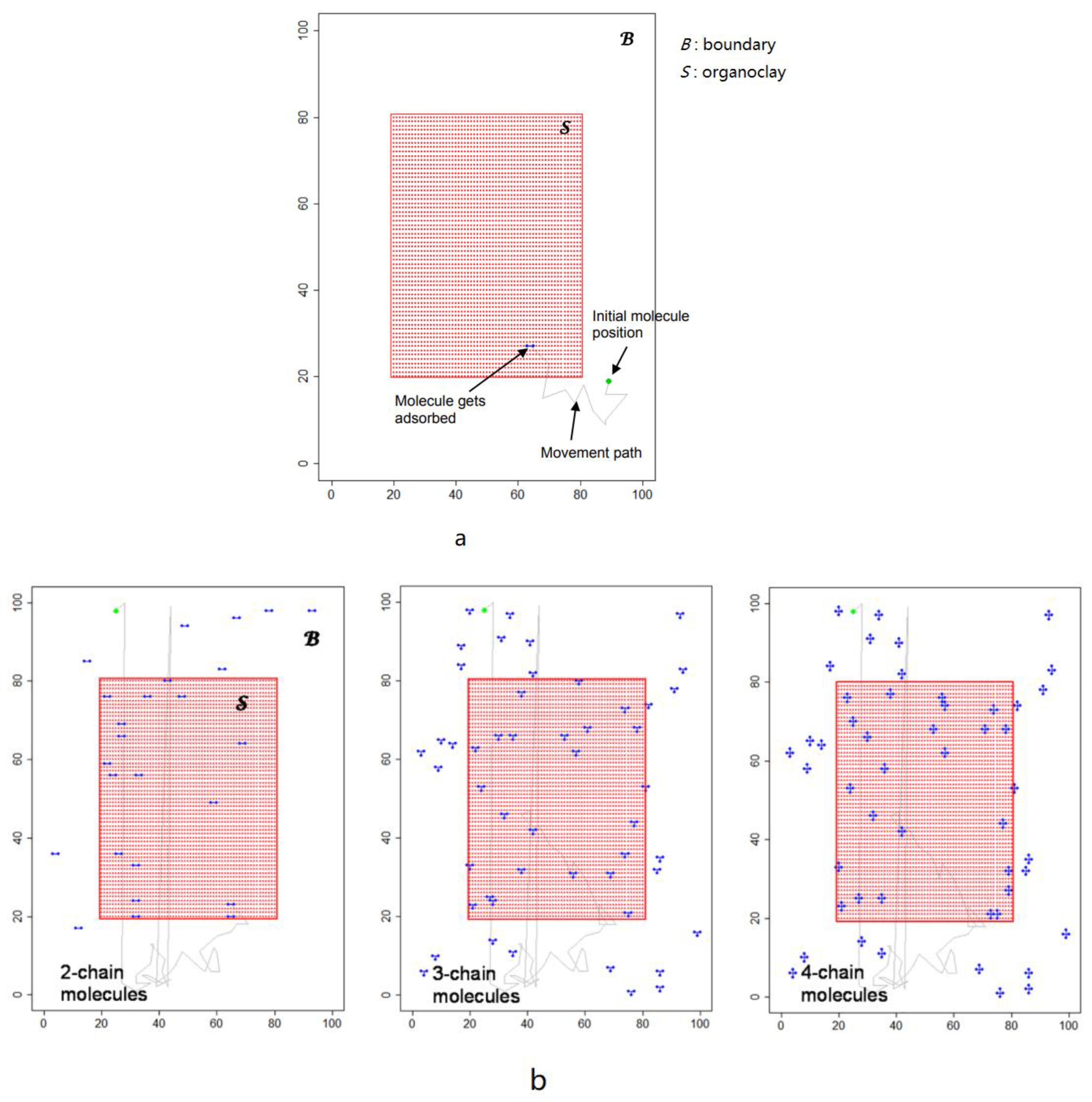
- Kania, D.; Yunus, R.; Omar, R.; Rashid, S.A.; Jan, B.M.; Aulia, A. Adsorption of non-ionic surfactants on organoclays in drilling fluid investigated by molecular descriptors and Monte Carlo random walk simulations. Appl. Surf. Sci. 2021, 538, 148154. [Google Scholar] [CrossRef]
- Lan, L. Study on Treatment of Wastewater Containing cr(vi) by Modified Bentonite. Master’s Thesis, Guangxi University, Nanning, China, 2005. [Google Scholar]
- Mao, J.; Xue, G.; Xu, X.; Guo, X. Organic Modification of Na-montmorillonite. Pap. Chem. 2008, 20, 11–13. [Google Scholar]
- Wu, L. The Study of Preparation, Characterization and Properties of Poiypropylene/Montmorillonite Nanocomposites. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2003. [Google Scholar]
- Feng, Z. Study on synthesis of organic bentonite by bentonite. Chen. Eng. 2005, 4, 57–58. [Google Scholar]
- Li, X. Study of the Pe/Mt Nanocomposite. Master’s Thesis, Harbin University of Science and Technology, Harbin, China, 2003. [Google Scholar]
- Kang, R.; Yang, T.; Wang, B.; Yang, L. Preparation and Structural Properties of 1827 Organic Bentonite. Mon-Met. Mines 2006, 29, 47–49. [Google Scholar]
- Liu, D.; Fan, L.; Chou, X.; Nie, Y.; Qu, B. Preparation of Hexadecyl Trimethyl Ammonium Type Bentonite and Investigation of its Suspending Power. In Proceedings of the 2007 Academic Exchange Meeting on Uniform Experimental Design, Beijing, China, 18 August 2007; pp. 223–228. [Google Scholar]
- Ge, Y.; Zhu, L. Sorption of nitrobenzene in water by cationic-nonionic organobentonites. China Environ. Sci. 2004, 24, 192–195. [Google Scholar]
- Zhu, L.; Wang, Q.; Chen, B. Sorption of Aniline and Phenol to Anion—Cation Organobentonites from Water. Environ. Sci. 2000, 21, 5. [Google Scholar]
- Yu, Y.; Yu, J.; Zhang, J. Study on Experiment and Characterization of Organic Bentonite Prepared by Bentonite Raw Minierals of High Content of Salt. Mon-Met. Mines 2009, 32, 5–7. [Google Scholar]
- Yang, H.; Zheng, Q. Progress in melt intercalation preparation of polymer-layered silicates nanocomposites. J. Funct. Mater. 2003, 34, 3. [Google Scholar]
- Vaia, R.A.; Price, G.; Ruth, P.N.; Nguyen, H.T.; Lichtenhan, J. Polymer/layered silicate nanocomposites as high performance ablative materials. Appl. Clay Sci. 1999, 15, 67–92. [Google Scholar] [CrossRef]
- Park, C.I.; Kim, M.H.; Park, O.O. Effect of heat treatment on the microstructural change of syndiotactic polystyrene/poly (styrene-co-vinyloxazolin)/clay nanocomposite. Polymer 2004, 45, 1267–1273. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Z.K.; Yin, J.; Wang, X.Y.; Qi, Z.E. Preparation and properties of hybrids of organo-soluble polyimide and montmorillonite with various chemical surface modification methods. Polymer 1999, 40, 4407–4414. [Google Scholar] [CrossRef]
- Dutta, D.; Das, B.M. Development of smart bentonite drilling fluid introducing iron oxide nanoparticles compatible to the reservoirs of Upper Assam. Upstream Oil Gas Technol. 2021, 7, 100058. [Google Scholar] [CrossRef]
- Wu, Y. Research and Application of Modified Nano Silica in Water Based Drilling Fluid. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2017. [Google Scholar]
- Sun, J. Studies on Surface Treatment of Co3O4 Nano-meter Particles. China Powder Sci. Technol. 2007, 13, 23–26. [Google Scholar]
- Xu, S.; Wang, S.; Xu, Z.; Dong, B.; Sun, W. The surface graft modification and characterization of nanoparticles. New Chem. Mater. 2005, 33, 44–46. [Google Scholar]
- Sarkar, A.; Biswas, D.R.; Datta, S.C.; Dwivedi, B.S.; Bhattacharyya, R.; Kumar, R.; Patra, A.K. Preparation of novel biodegradable starch/poly (vinyl alcohol)/bentonite grafted polymeric films for fertilizer encapsulation. Carbohydr. Polym. 2021, 259, 117679. [Google Scholar] [CrossRef] [PubMed]
- Saboori, R.; Sabbaghi, S.; Kalantariasl, A. Improvement of rheological, filtration and thermal conductivity of bentonite drilling fluid using copper oxide/polyacrylamide nanocomposite. Powder Technol. 2019, 353, 257–266. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, F.; Zhang, W. Development and application of water-soluble drilling fluid filtration control agent. J. Qilu Univ. Nat. Sci. Ed. 2010, 24, 58–62. [Google Scholar]
- Zhuang, G. Development and Application of Water-Soluble Drilling Fluid Filtration Control Agent. Ph.D. Thesis, China University of Geosciences (Beijing), Beijing, China, 2019. [Google Scholar]
- Lu, H. Research On Polymer/Clay Composites For The Drilling Fluid. Master’s Thesis, Northeast Petroleum University, Daqing, China, 2011. [Google Scholar]
- Gilman, J.W. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Sun, J.; Qu, Y.; Liu, F.; Zhou, C. Preparation and Properties of Nanometer Bentonite Composite. D & Fluid 2006, 23, 8–10. [Google Scholar]
- Shen, L. Research and Application of Nanometer Bentonite Composite for Drilling Fluid. Ph.D. Thesis, China University of Petroleum (East China), Qingdao, China, 2010. [Google Scholar]
- Kim, S.; Palomino, A.M. Factors influencing the synthesis of tunable clay–polymer nanocomposites using bentonite and polyacrylamide. Appl. Clay Sci. 2011, 51, 491–498. [Google Scholar] [CrossRef]
- Bilgiç, C.; Yazıcı, D.T.; Karakehya, N.; Çetinkaya, H.; Singh, A.; Chehimi, M.M. Surface and interface physicochemical aspects of intercalated organo-bentonite. Int. J. Adhes. Adhes. 2014, 50, 204–210. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.M.; Stasiak, J.; Al-Tabbaa, A. The effect of cationic, non-ionic and amphiphilic surfactants on the intercalation of bentonite. Colloid. Surf. A Physicochem. Eng. Asp. 2014, 444, 330–337. [Google Scholar] [CrossRef]
- Guo, M. Development of Organic Clay and Temperature Sensitive SiO2 for Drilling Fluid and Research on Rheological Properties. Ph.D. Thesis, Henan University, Kaifeng, China, 2020. [Google Scholar]
- Ma, P.; Zhang, L.; Nie, Y.; Chen, H.; Qiu, Z.; Dong, G.; Zhang, S. Efficient Preparation of Nano Starch Particle and Its Effect on the Performance of Drilling Fluid. Oilfield Chem. 2022, 38, 580–583. [Google Scholar]
- Zheng, L.; Su, G.; Li, Z.; Peng, R.; Wang, L.; Wei, P.; Han, S. The wellbore instability control mechanism of fuzzy ball drilling fluids for coal bed methane wells via bonding formation. J. Nat. Gas Sci. Eng. 2018, 56, 107–120. [Google Scholar] [CrossRef]
- Abbas, M.A.; Zamir, A.; Elraies, K.A.; Mahmood, S.M.; Rasool, M.H. A critical parametric review of polymers as shale inhibitors in water-based drilling fluids. J. Pet. Sci. Eng. 2021, 204, 108745. [Google Scholar] [CrossRef]
- Ren, Y.; Zhai, Y.; Wu, L.; Zhou, W.; Qin, H.; Wang, P. Amine-and alcohol-functionalized ionic liquids: Inhibition difference and application in water-based drilling fluids for wellbore stability. Colloid. Surf. A Physicochem. Eng. Asp. 2021, 609, 125678. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Huang, W.; Sun, D.; Zhang, D.; Cao, J. Synergistic stabilization of shale by a mixture of polyamidoamine dendrimers modified bentonite with various generations in water-based drilling fluid. Appl. Clay Sci. 2015, 114, 359–369. [Google Scholar] [CrossRef]
- He, J.; Chen, Q.; Zhao, W.; Chen, F.; Wang, W. Hydrophobically modified cationic oligoacrylamide as a potential shale hydration inhibitor in water-based drilling fluid and mechanism study. J. Nat. Gas Sci. Eng. 2020, 81, 103484. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, X.; Zhang, X.; Qiu, Z.; Zhao, C.; Zhang, X.; Jin, J. Improving the shale stability with nano-silica grafted with hyperbranched polyethyleneimine in water-based drilling fluid. J. Nat. Gas Sci. Eng. 2020, 83, 103624. [Google Scholar] [CrossRef]
- Mao, H.; Huang, Y.; Luo, J.; Zhang, M. Molecular simulation of polyether amines intercalation into Na-montmorillonite interlayer as clay-swelling inhibitors. Appl. Clay Sci. 2021, 202, 105991. [Google Scholar] [CrossRef]
- Chu, Q.; Su, J.; Lin, L. Inhibition performance of amidocyanogen silanol in water-based drilling fluid. Appl. Clay Sci. 2020, 185, 105315. [Google Scholar] [CrossRef]
- Nasser, M.S.; Onaizi, S.A.; Hussein, I.A.; Saad, M.A.; Al-Marri, M.J.; Benamor, A. Intercalation of ionic liquids into bentonite: Swelling and rheological behaviors. Colloid. Surf. A Physicochem. Eng. Asp. 2016, 507, 141–151. [Google Scholar] [CrossRef]
- Sanchez-Solis, A.; Garcia-Rejon, A.; Manero, O. Production of nanocomposites of PET-montmorillonite clay by an extrusion process. Macromol. Symp. 2003, 192, 281–292. [Google Scholar] [CrossRef]
- Vidotti, S.E.; Chinellato, A.C.; Hu, G.H.; Pessan, L.A. Preparation of poly (ethylene terephthalate)/organoclay nanocomposites using a polyester ionomer as a compatibilizer. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3084–3091. [Google Scholar] [CrossRef]
- Vaia, R.A.; Ishii, H.; Giannelis, E.P. Synthesis and properties of two-dimensional nanostructures by direct intercalation of polymer melts in layered silicates. Chem. Mater. 1993, 5, 1694–1696. [Google Scholar] [CrossRef]
- Liu, S.; Fan, Q.; Zhou, X. Preparation of polymer/montmorillonite nano-composites by melt intercalation. China Synth. Resin Plast. 2005, 22, 76–79. [Google Scholar]
- Ma, P.; Wang, X.; Xu, G. Research Progress of PLA/Montmorillonite Nanocomposites Prepared by Melting Intercalation. China Plastics. 2009, 7, 6–11. [Google Scholar]
- Limpanart, S.; Khunthon, S.; Taepaiboon, P.; Supaphol, P.; Srikhirin, T.; Udomkichdecha, W.; Boontongkong, Y. Effect of the surfactant coverage on the preparation of polystyrene–clay nanocomposites prepared by melt intercalation. Mater. Lett. 2005, 59, 2292–2295. [Google Scholar] [CrossRef]
- Shen, Z.; Simon, G.P.; Cheng, Y.B. Saturation ratio of poly (ethylene oxide) to silicate in melt intercalated nanocomposites. Eur. Polym. J. 2003, 39, 1917–1924. [Google Scholar] [CrossRef]
- Ramos Filho, F.G.; Mélo, T.J.A.; Rabello, M.S.; Silva, S.M. Thermal stability of nanocomposites based on polypropylene and bentonite. Polym. Degrad. Stab. 2005, 89, 383–392. [Google Scholar] [CrossRef]
- Liborio, P.; Oliveira, V.A.; Maria de Fatima, V.M. New chemical treatment of bentonite for the preparation of polypropylene nanocomposites by melt intercalation. Appl. Clay Sci. 2015, 111, 44–49. [Google Scholar] [CrossRef]
- Flores, J.C.; Torres, V.; Popa, M.; Crespo, D.; Calderón-Moreno, J.M. Variations in morphologies of silver nanoshells on silica spheres. Colloid. Surf. A Physicochem. Eng. Asp. 2008, 330, 86–90. [Google Scholar] [CrossRef]
- Samoshina, Y.; Diaz, A.; Becker, Y.; Nylander, T.; Lindman, B. Adsorption of cationic, anionic and hydrophobically modified polyacrylamides on silica surfaces. Colloid. Surf. A Physicochem. Eng. Asp. 2003, 231, 195–205. [Google Scholar] [CrossRef]
- Lin, Y.J.; Wang, L.; Lin, J.G.; Huang, Y.Y.; Chiu, W.Y. Preparation and properties of poly (acrylic acid)-stabilized magnetite nanoparticles. Synth. Met. 2003, 135, 769–770. [Google Scholar] [CrossRef]
- Li, X.; Liao, J.; Zhai, L.; Lv, H. Study on the surface modifica tion of caco3 with stearic acid by thermal analysis and t he characterization of the surface properties. Chem. Res. Appl. 1997, 9, 478–481. [Google Scholar]
- Chen, J.; He, T.; Wu, W.; Cao, D.; Yun, J.; Tan, C.K. Adsorption of sodium salt of poly (acrylic) acid (PAANa) on nano-sized CaCO3 and dispersion of nano-sized CaCO3 in water. Colloid. Surf. A Physicochem. Eng. Asp. 2004, 232, 163–168. [Google Scholar] [CrossRef]
- Wan, B.; Fei, J.; Gao, W. Research on Surface-modification of Nanoparticles Powder. Dev. Appl. Mater. 2010, 25, 98–104. [Google Scholar]
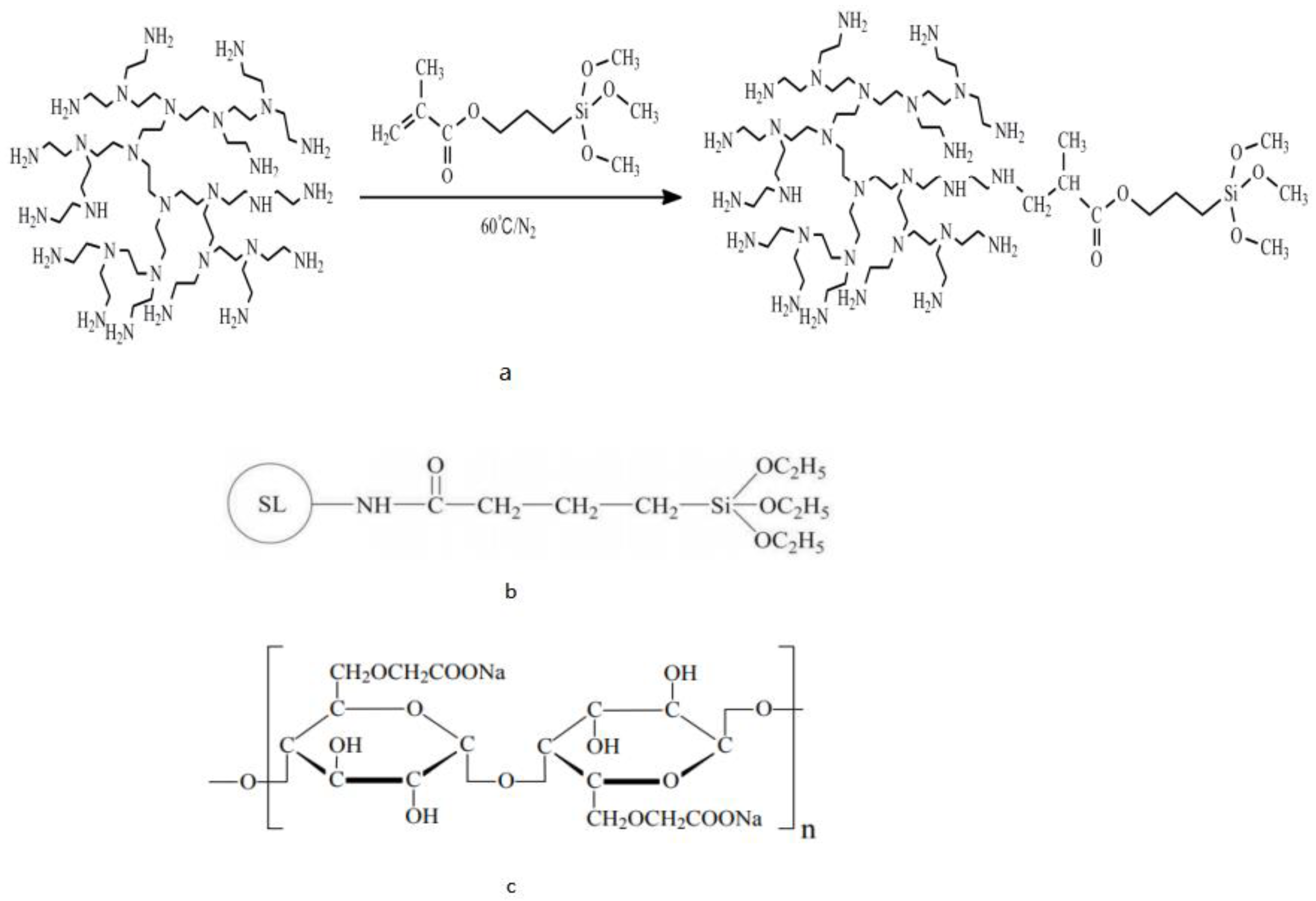
- Chu, Q.; Lin, L.; Zhao, Y. Hyperbranched polyethylenimine modified with silane coupling agent as shale inhibitor for water-based drilling fluids. J. Pet. Sci. Eng. 2019, 182, 106333. [Google Scholar] [CrossRef]
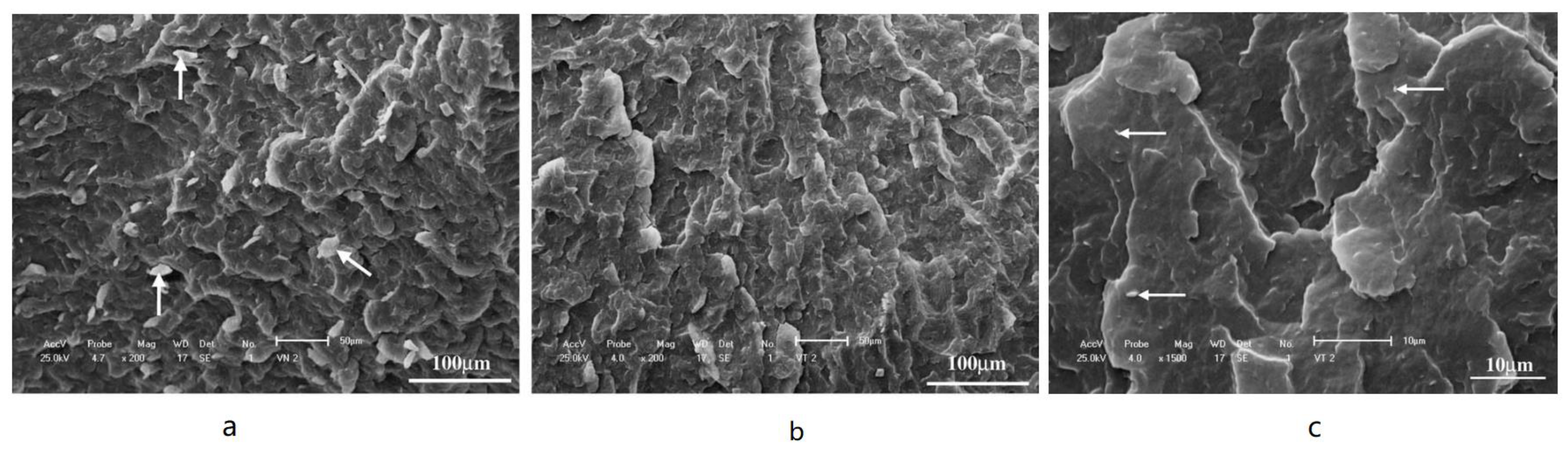
- Su, J.; Liu, M.; Lin, L.; Pu, X.; Ge, C.; Zhang, T.; Liu, G. Sulfonated lignin modified with silane coupling agent as biodegradable shale inhibitor in water-based drilling fluid. J. Pet. Sci. Eng. 2022, 208, 109618. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; He, Y.G.; Ren, F.X.; Wang, G.X. Synthesis, characterization, and applied properties of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr. Polym. 2009, 78, 95–99. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S. A review on clay chemistry, characterization and shale inhibitors for water-based drilling fluids. J. Pet. Sci. Eng. 2021, 206, 109043. [Google Scholar] [CrossRef]
- Pan, Y. Research on the Modification Mechanism of Organic Clay Used in Oil-Based Drilling Fluids. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2014. [Google Scholar]
- Ge, X.; Li, M.C.; Li, X.X.; Cho, U.R. Effects of silane coupling agents on the properties of bentonite/nitrile butadiene rubber nanocomposites synthesized by a novel green method. Appl. Clay Sci. 2015, 118, 265–275. [Google Scholar] [CrossRef]
- Wang, W.; Qiu, Z.; Zhong, H.; Huang, X.; Dong, B. Preparation and properties of nanoparticle-based lubricant SD-NR for drilling fluids. Fault-Block Oil Gas Field 2016, 23, 13–116. [Google Scholar]
- Chen, B. Synthesis of the Nano-Modified Polymer Fluid Loss Additive and Research of the Mechanism. Master’s Thesis, China University of Petroleum (Beijing), Beijing, China, 2017. [Google Scholar]
- Chu, Q.; Yang, Z.; Li, T.; Xue, Y.; Liu, S. Preparation and Analyses of Nano SiO2 Plugging Agent Modified with Silane Coupling Agent. D Fluid 2016, 33, 47–50. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, P.; Wang, W. Characterization of emulsion polymeric product of alkyl-nano-SiO2/mma and modifying pc with it. Eng. Plast. Appl. 2002, 30, 1–4. [Google Scholar]
- Yan, L.; Sun, J.; Ji, Y. Development and performance evaluation of nano-silica plugging agent for drilling fluid. In Proceedings of the 2014 National Technical Exchange Seminar on Drilling and Completion Fluids, Chongqing, China, 30 October 2014; Sinopec Publishing House: Beijing, China, 2014. [Google Scholar]
- Jesionowski, T.; Krysztafkiewicz, A. Influence of silane coupling agents on surface properties of precipitated silicas. Appl. Surf. Sci. 2001, 172, 18–32. [Google Scholar] [CrossRef]
- Qiu, X.; Zhong, J.; Long, Z.; Tian, L.; Zan, L.; Gong, C.; Luo, Q. Preparation of Nanometer TiO2/PS Composite Particles. J. Xi’an Wuhan Univ. Nat. Sci. Ed. 2003, 49, 675–679. [Google Scholar]
- Zhang, G.; Liu, G. Organic modificati on of nano-CaCO3 surface. J. Dalian Polytech. Univ. 2006, 25, 12–15. [Google Scholar]
- Demjen, Z.; Pukánszky, B.; Földes, E.; Nagy, J. Interaction of silane coupling agents with CaCO3. J. Colloid Interface Sci. 1997, 190, 427–436. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, D. Review on the methods of surface modification of nanometer calcium carbonate. Ind. Miner. Process. 2007, 36, 32–36. [Google Scholar]
- Ren, Z.; Yu, D. Study on the application of coupling agent of aluminium to the abs/CaCO3, pvc/CaCO3 composites. J. Xi’an Jiaotong Univ. 1994, 28, 104–108. [Google Scholar]
- Lin, M.; Chen, H. Application of aluminate coupling agent in natural rubber/calcium carbonate composite system. China Rubber Ind. 1996, 43, 30–33. [Google Scholar]
- Guo, J. Research and Application of Carboxymethyl Cellulose Modified. Master’s Thesis, Xi’an Shiyou University, Xian, China, 2012. [Google Scholar]
- Jain, R.; Paswan, B.K.; Mahto, T.K.; Mahto, V. Study the effect of synthesized graft copolymer on the inhibitive water based drilling fluid system. Egyptian J. Pet. 2017, 26, 875–883. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Zhao, L.; Zheng, K.; Fang, Y. Synthesis and characterization of thermo-responsive polymer based on carboxymethyl chitosan and its potential application in water-based drilling fluid. Colloid. Surf. A Physicochem. Eng. Asp. 2021, 629, 127478. [Google Scholar] [CrossRef]
- Xu, J.G.; Qiu, Z.S.; Zhao, X.; Zhong, H.Y.; Li, G.R.; Huang, W.A. Synthesis and characterization of shale stabilizer based on polyethylene glycol grafted nano-silica composite in water-based drilling fluids. J. Pet. Sci. Eng. 2018, 163, 371–377. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Yang, X.; Wang, H. Research Advances on Modified Starch with High-temperature Tolerance Used for Drilling Fluid at Home and Abroad. Sino-Global Energ. 2012, 12, 42–47. [Google Scholar]
- Wang, Z. Present Situation and Development of Drilling Fluid Technology. Fault-Block Oil Gas Field 2004, 5, 59–62. [Google Scholar]
- Wang, Z. Progress in research and application of starch graft copolymer used for oilfield. Fault-Block Oil Gas Field 2010, 2, 7. [Google Scholar]
- Lin, Z.; Peng, F.; Yu, H. Research on modification of nano-TiO2 grafted by PMAA and its dispersion in aqueous system. Inorg. Chem. Ind. 2008, 40, 4. [Google Scholar]
- Wang, P.; Yan, M.; Tang, L. Surface modification and treatment of inorganic nano-particles. High Polym. Mater. Sci. Eng. 2003, 19, 183–186. [Google Scholar] [CrossRef]
- Xie, G.; Luo, P.; Deng, M.; Wang, Z. Nanoplugging performance of hyperbranched polyamine as nanoplugging agent in oil-based drilling fluid. J. Nanomater. 2015, 2015, 821910. [Google Scholar] [CrossRef]
- Li, X.; Hong, C.Y.; Pan, C.Y. Preparation and characterization of hyperbranched polymer grafted mesoporous silica nanoparticles via self-condensing atom transfer radical vinyl polymerization. Polymer 2010, 51, 92–99. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, X.; Chen, B.; Egwu, S.B.; Huang, Z. Polyanionic cellulose/hydrophilic monomer copolymer grafted silica nanocomposites as HTHP drilling fluid-loss control agent for water-based drilling fluids. Appl. Surf. Sci. 2022, 578, 152089. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, H.; Wang, Y.; Dong, Y. Grafting lignite with sulformethal phenoldehy resin and their performance in controlling rheological and filtration properties of water–bentonite suspensions at high temperatures. J. Pet. Sci. Eng. 2016, 144, 84–90. [Google Scholar] [CrossRef]
- Bialk, M.; Prucker, O.; Rühe, J. Grafting of polymers to solid surfaces by using immobilized methacrylates. Colloid. Surf. A Physicochem. Eng. Asp. 2002, 198, 543–549. [Google Scholar] [CrossRef]
- Shettigar, R.R.; Misra, N.M.; Patel, K. CTAB grafted PAM gel and its application in drilling fluid. J. Pet. Sci. Eng. 2018, 160, 129–135. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Q.; Dai, H.; Huang, R.; Liang, B.; Zhang, X. Synthesis and Characterization of Calcium Lignosulfonate Grafted with p-Styrenesulfonate and Maleic Anhydride. High Polym. Mater. Sci. Eng. 2014, 30, 72–76. [Google Scholar]
- Zhou, L.; Zhao, H. Development of Compound Ionic Modified Starch Fluid Loss Reducer. J. Oil Gas Technol. 2004, 26, 81–82. [Google Scholar]
- Yang, Y.; Li, Z.; Wang, Z.; Pu, C. Synthesis of Modified Maize Starch as Filtrate Loss Reducer for Water Base Drilling Fluid. Oilfield Chem. 2006, 23, 198–200. [Google Scholar]
- Ma, S.; Guo, M.; Pu, C.; Ni, B.; Xu, C. Synthesis of bisphenol AP by using phosphato—Tungstic heteropoly acid as catalyst. J Xi’an Shiyou Univ. Nat. Sci. Ed. 2004, 19, 44–47. [Google Scholar]
- Wang, A.; Liang, G.; Li, L.; Shi, H.; Zheng, C.; Wang, Z. Study on Reductive Dechlorination of Chlorinated Pollutants in Fe/Cu Bimetallic System. Technol. Dev. Chem. 2018, 47, 8–12. [Google Scholar]
- Wei, J.; Yu, H.; He, J.; Tuo, D. Preparation and Performance Evaluation of Cross-linked Carboxymethyl Starch Filtrate Reducer. Oilfield Chem. 2018, 1, 12–15. [Google Scholar]
- Wang, D.; Wang, J.; Song, Z.; Zhuang, J.; Zhao, Y. Preparation and Properties of a Novel Inorganic Silicon Modified Sodium Carboxylmethyl Starch Filtrate Reducer. Petrochem. Techno. 2010, 39, 440–443. [Google Scholar]
- Xie, J.; Zhao, X.; Sheng, J.; Guan, X. Synthesis and Properties of High Temperature-Resistant Starch Filtrate Reducer fbr Drining Fluid. Petrochem. Techno. 2012, 41, 1389–1393. [Google Scholar]
- Zhang, J.; Yuan, K.; Wang, Y.P.; Gu, S.J.; Zhang, S.T. Preparation and properties of polyacrylate/bentonite superabsorbent hybrid via intercalated polymerization. Mater. Lett. 2007, 61, 316–320. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ibrahim, M.A. Synthesis of amyl ester grafted on carbon-nanopolymer composite as an inhibitor for cleaner shale drilling. Petroleum 2021, 8, 529–537. [Google Scholar] [CrossRef]
- Caldwell, C.G.; Wurzburg, O.B. Polysaccharide Derivatives of Substituted Dicarboxylic Acids. U.S. Patent U.S.2661349 A, 1 December 1953. [Google Scholar]
- Elion, G.R. Agricultural Biodegradable Plastics. U.S. Patent U.S.5205863 A, 27 April 1993. [Google Scholar]
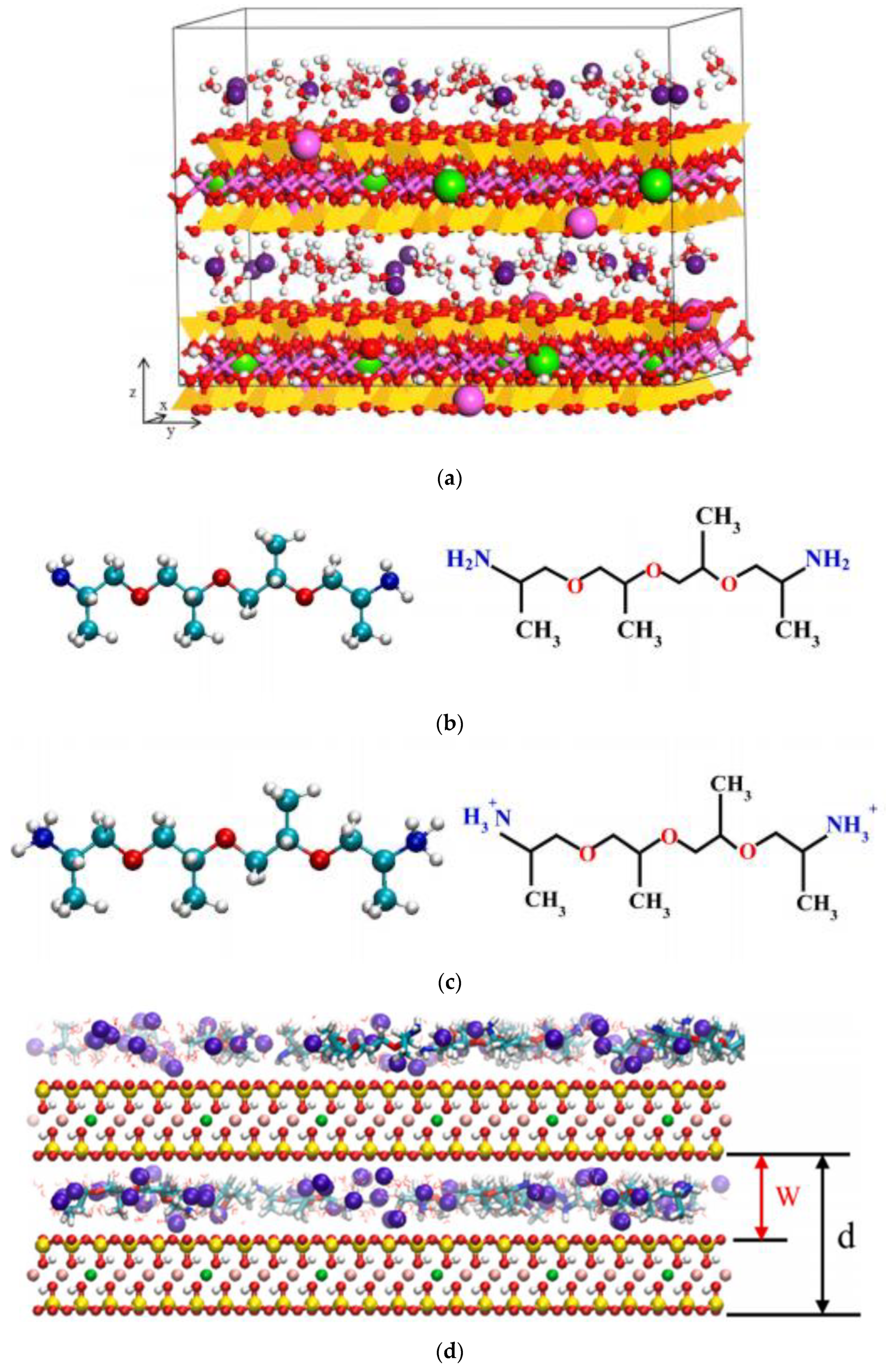
- He, H.; Galy, J.; Gerard, J.F. Molecular simulation of the interlayer structure and the mobility of alkyl chains in HDTMA+/montmorillonite hybrids. J. Phys. Chem. B 2005, 109, 13301–13306. [Google Scholar] [CrossRef]
- Zhao, Q.; Burns, S.E. Molecular simulation of electrokinetics of montmorillonite surface coated with hexadecyltrimethylammonium cations. Colloid. Surf. A Physicochem. Eng. Asp. 2017, 516, 354–361. [Google Scholar] [CrossRef]
- Ngouana, W.B.F.; Kalinichev, A.G. Structural arrangements of isomorphic substitutions in smectites: Molecular simulation of the swelling properties, interlayer structure, and dynamics of hydrated Cs–montmorillonite revisited with new clay models. J. Phys. Chem. C 2014, 118, 12758–12773. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, G.; Wu, L.; Xu, X.; Song, Q.; Yang, Y.; Qi, Z. Synthesis and properties of pet-clay nano-composite. Acta Polym. Sin. 1999, 3, 309–314. [Google Scholar]
- Liu, X.; Fan, J.; Qi, Z. Study on non-isothermal crystallization kinetics of polypropylene/montmorillonite nanocomposites. High Polym. Mater. Sci. Eng. 2001, 17, 103–105. [Google Scholar]
- Alexandre, M.; Dubois, P.; Sun, T.; Garces, J.M.; Jérôme, R. Polyethylene-layered silicate nanocomposites prepared by the polymerization-filling technique: Synthesis and mechanical properties. Polymer 2002, 43, 2123–2132. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, Y.; Yang, X. Molecular Simulation on Swelling/Strctural Behavior of Organoclay with CO2 Intercalation. J. Mater. Sci. Eng. 2016, 2, 5. [Google Scholar]
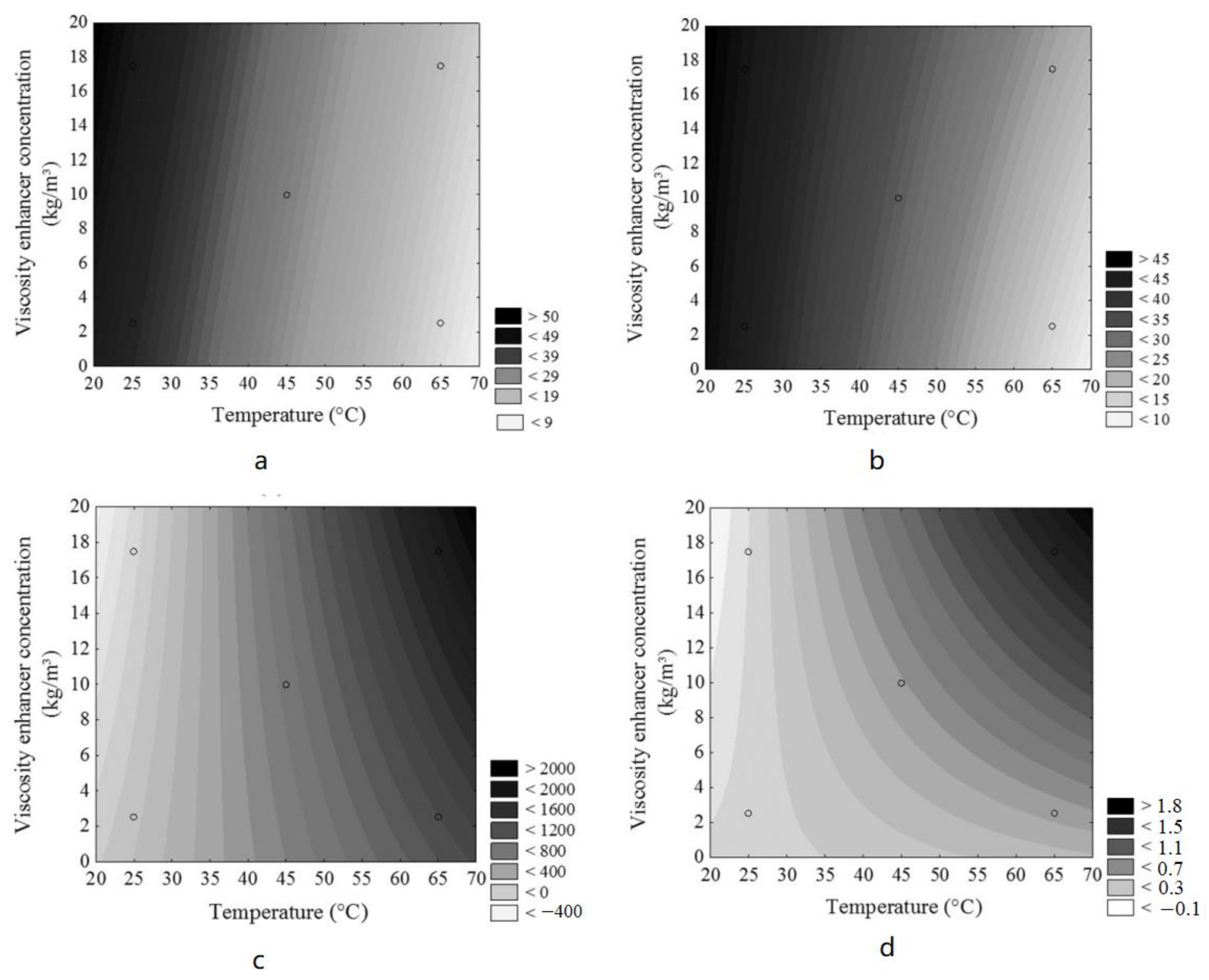
- Ratkievicius, L.A.; Da Cunha Filho, F.J.V.; Neto, E.L.D.B.; Santanna, V.C. Modification of bentonite clay by a cationic surfactant to be used as a viscosity enhancer in vegetable-oil-based drilling fluid. Appl. Clay Sci. 2017, 135, 307–312. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Zhang, X.; Ji, C.; Zhan, Q.; Li, Z.; Guan, J.; Huang, J. Modification Method of High-Efficiency Organic Bentonite for Drilling Fluids: A Review. Molecules 2023, 28, 7866. https://doi.org/10.3390/molecules28237866
Pan Y, Zhang X, Ji C, Zhan Q, Li Z, Guan J, Huang J. Modification Method of High-Efficiency Organic Bentonite for Drilling Fluids: A Review. Molecules. 2023; 28(23):7866. https://doi.org/10.3390/molecules28237866
Chicago/Turabian StylePan, Yi, Xinyue Zhang, Chengcheng Ji, Qianru Zhan, Zhaoxuan Li, Jian Guan, and Jian Huang. 2023. "Modification Method of High-Efficiency Organic Bentonite for Drilling Fluids: A Review" Molecules 28, no. 23: 7866. https://doi.org/10.3390/molecules28237866
APA StylePan, Y., Zhang, X., Ji, C., Zhan, Q., Li, Z., Guan, J., & Huang, J. (2023). Modification Method of High-Efficiency Organic Bentonite for Drilling Fluids: A Review. Molecules, 28(23), 7866. https://doi.org/10.3390/molecules28237866





