Improvement of the Self-Controlled Hyperthermia Applications by Varying Gadolinium Doping in Lanthanum Strontium Manganite Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
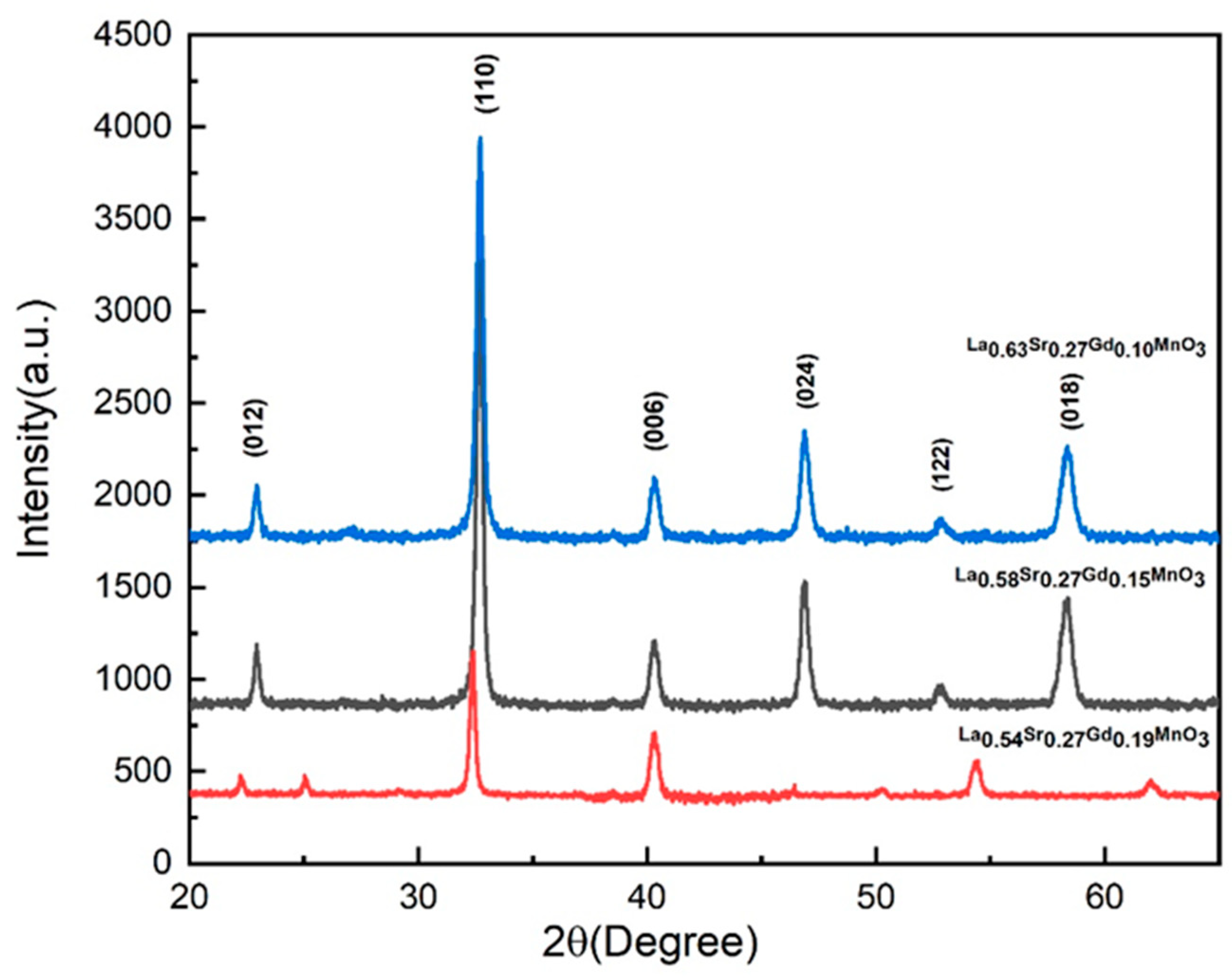
2.1. XRD Study
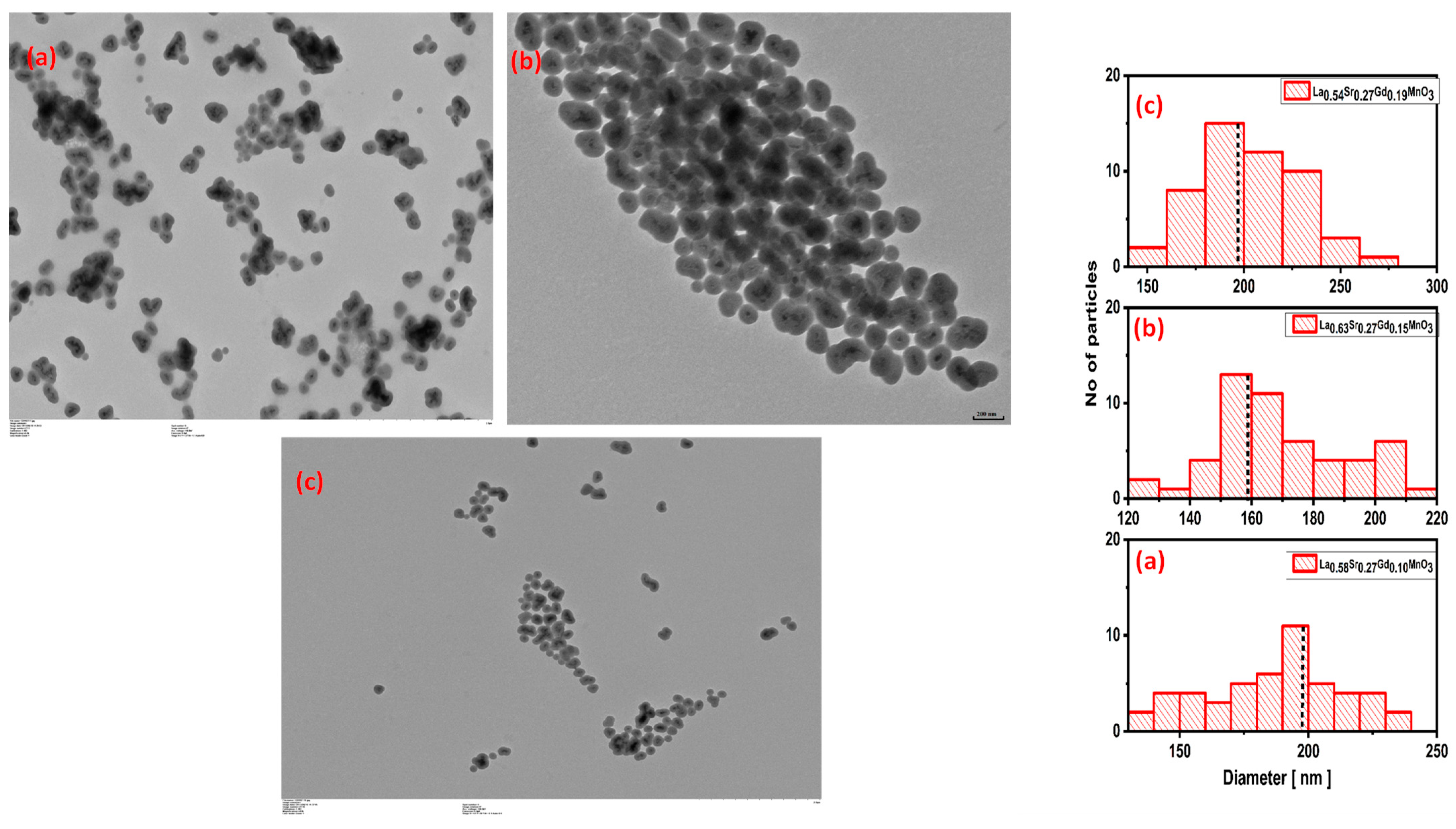
2.2. TEM Studies
2.3. FTIR Studies
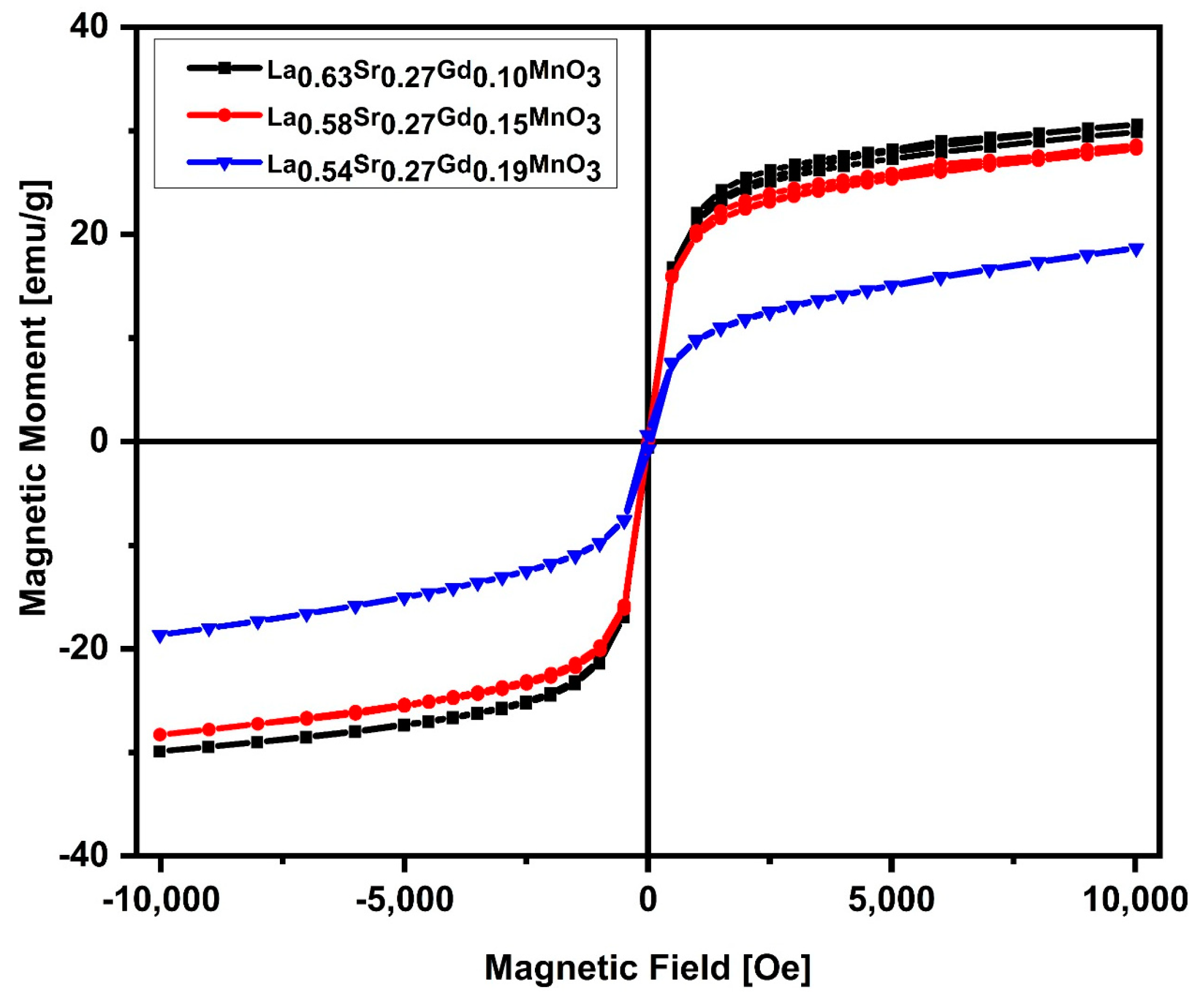
2.4. Magnetic Measurements
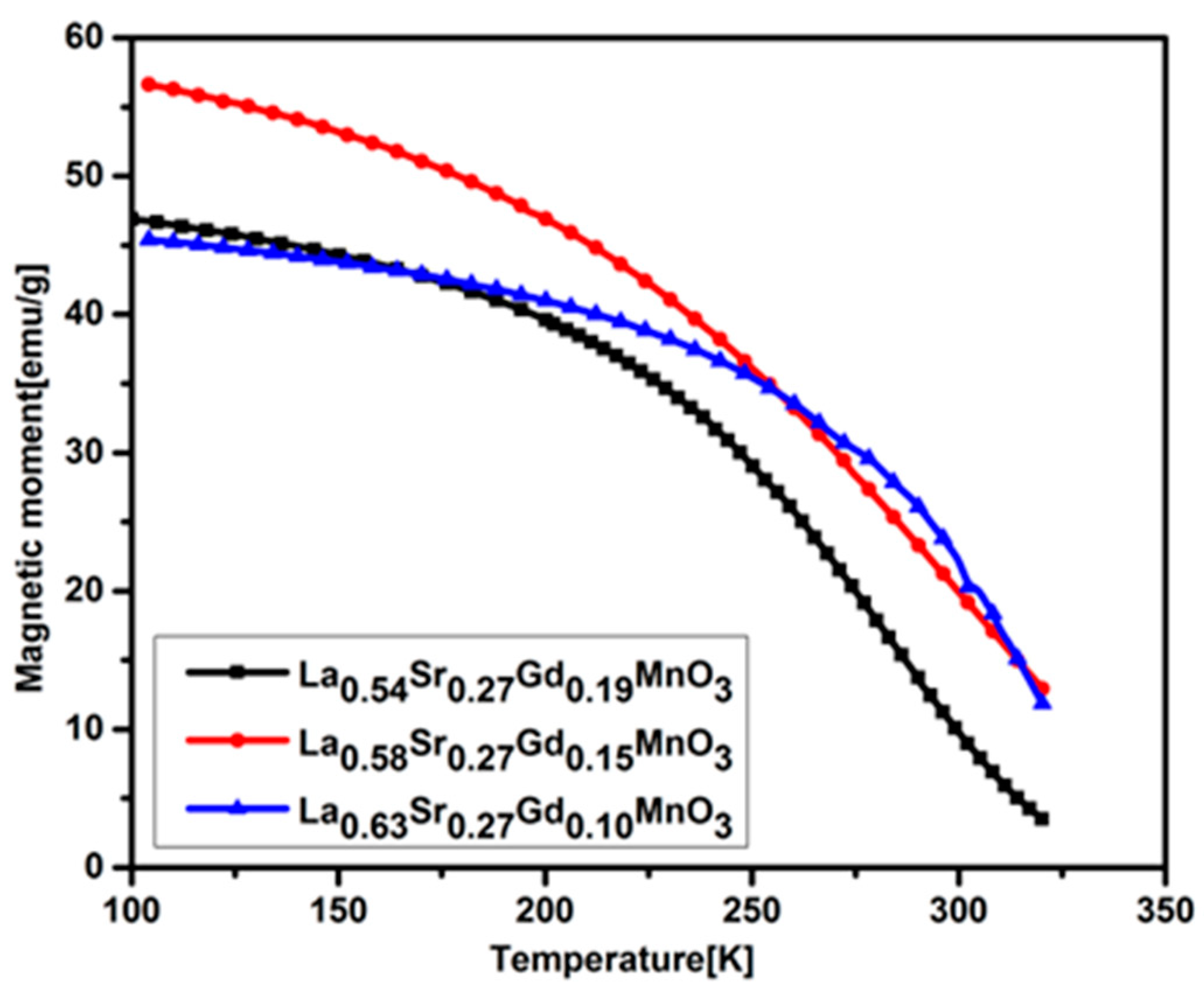
2.5. Curie Temperature Measurements
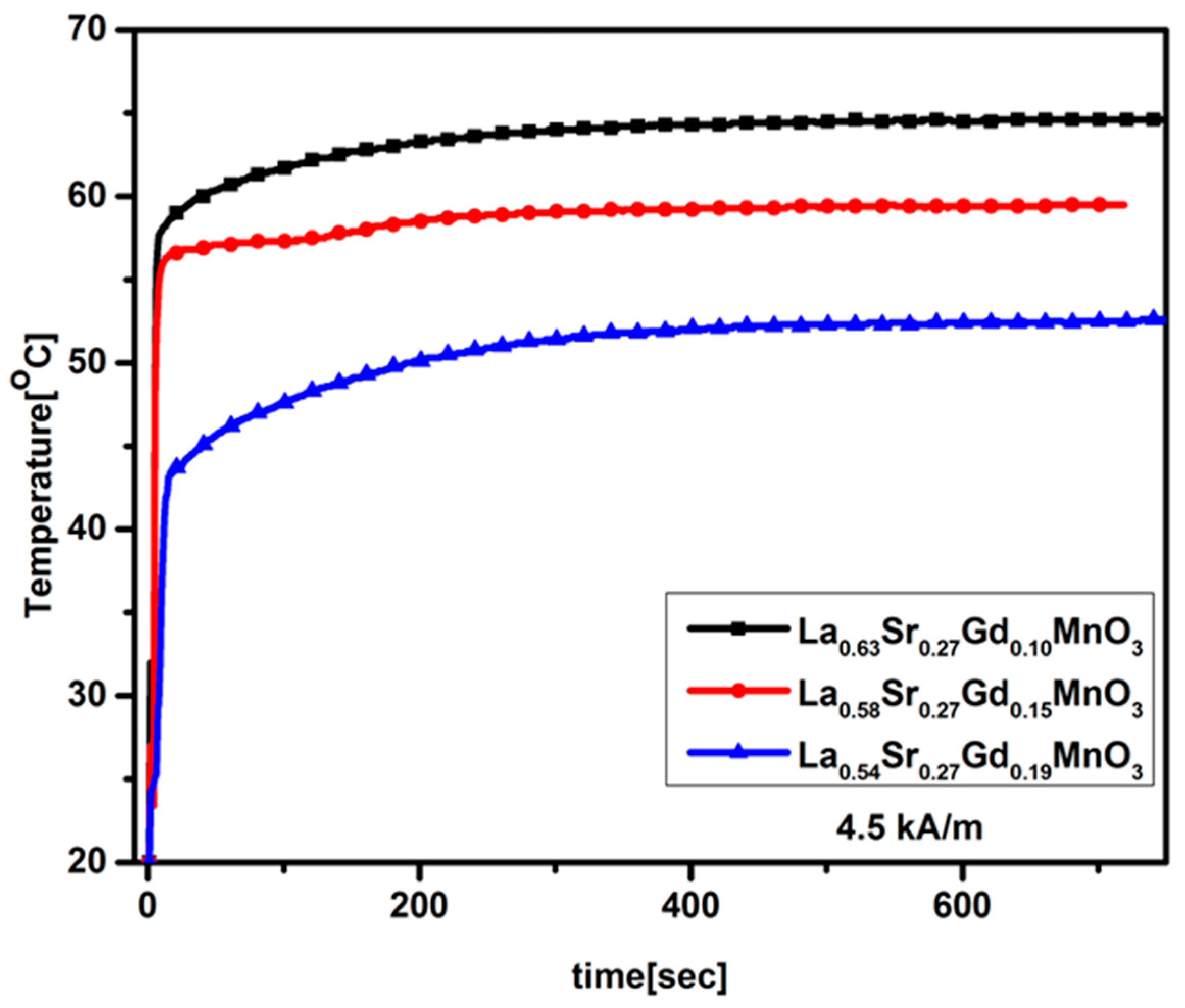
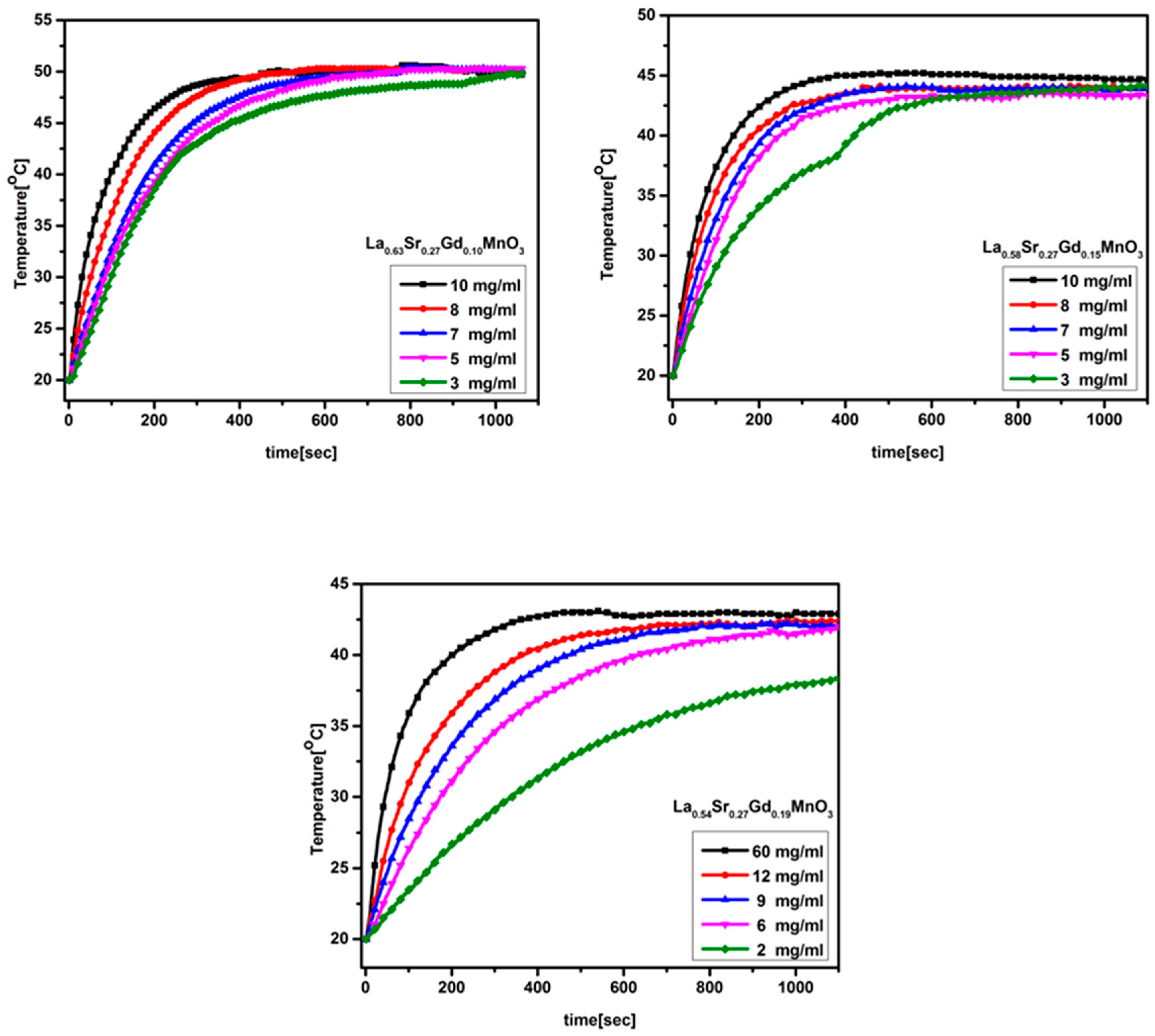
2.6. Magnetic Heating
3. Materials and Methods
3.1. Material Synthesis
3.2. Silica Coating
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, R.; Pal, R.; Bej, S.; Mondal, M.; Banerjee, P. Application of Optical Nanoprobes for Supramolecular Biosensing: Recent Trends and Future Perspectives. In Biosensors Nanotechnology; Wiley Online Library: Hoboken, NJ, USA, 2023; pp. 267–326. [Google Scholar]
- Ale, Y.; Nainwal, N. Progress and Challenges in the Diagnosis and Treatment of Brain Cancer Using Nanotechnology. Mol. Pharm. 2023, 20, 4893–4921. [Google Scholar] [CrossRef]
- Zhao, Y. Co-precipitated Ni/Mn shell coated nano Cu-rich core structure: A phase-field study. J. Mater. Res. Technol. 2022, 21, 546–560. [Google Scholar] [CrossRef]
- Bai, B.; Nie, Q.; Zhang, Y.; Wang, X.; Hu, W. Cotransport of heavy metals and SiO2 particles at different temperatures by seepage. J. Hydrol. 2020, 597, 125771. [Google Scholar] [CrossRef]
- Anand, M. Hysteresis in a linear chain of magnetic nanoparticles. J. Appl. Phys. 2020, 128, 023903. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, J.; Wang, H.; Yang, K.; Zhu, Y.; Qing, Y.; Ma, Z.; Gao, L.; Liu, Y.; Wei, S.; et al. Air plasma-sprayed high-entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 coating with high thermal protection performance. J. Adv. Ceram. 2022, 11, 1571–1582. [Google Scholar] [CrossRef]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Lai, L.; Gan, M.; Wang, J.; Chen, L.; Liang, X.; Feng, J.; Chong, X. New class of high-entropy rare-earth niobates with high thermal expansion and oxygen insulation. J. Am. Ceram. Soc. 2023, 106, 4343–4357. [Google Scholar] [CrossRef]
- Tong, T.; Wang, L.; You, X.; Wu, J. Nano and microscale delivery platforms for enhanced oral peptide/protein bioavailability. Biomater. Sci. 2020, 8, 5804–5823. [Google Scholar] [CrossRef] [PubMed]
- Herynek, V.; Turnovcová, K.; Veverka, P.; Dědourková, T.; Žvátora, P.; Jendelová, P.; Gálisová, A.; Kosinová, L.; Jiráková, K.; Syková, E. Using ferromagnetic nanoparticles with low Curie temperature for magnetic resonance imaging-guided thermoablation. Int. J. Nanomed. 2016, 11, 3801–3811. [Google Scholar] [CrossRef]
- Natividad, E.; Castro, M.; Goglio, G.; Andreu, I.; Epherre, R.; Duguet, E.; Mediano, A. New insights into the heating mechanisms and self-regulating abilities of manganite perovskite nanoparticles suitable for magnetic fluid hyperthermia. Nanoscale 2012, 4, 3954–3962. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Pimentel, B.; Andrade, V.; Zverev, V.; Gimaev, R.R.; Pomorov, A.S.; Pyatakov, A.; Alekhina, Y.; Komlev, A.; Makarova, L.; et al. Understanding the dependence of nanoparticles magnetothermal properties on their size for hyperthermia applications: A case study for la-sr manganites. Nanomaterials 2021, 11, 1826. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Li, J.; Wang, Y.; Xu, H.; Wang, C.; Guo, J.; Du, Y. Ultralow Ru doping induced interface engineering in MOF derived ruthenium-cobalt oxide hollow nanobox for efficient water oxidation electrocatalysis. Chem. Eng. J. 2021, 420, 129805. [Google Scholar] [CrossRef]
- Astefanoaei, I.; Dumitru, I.; Chiriac, H.; Stancu, A. Controlling temperature in magnetic hyperthermia with low Curie temperature particles. J. Appl. Phys. 2014, 115, 17B531. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef]
- Miyamoto, R.; Saito, H.; Suzuki, M.; Yoshimura, N.; Mitobe, K. Accuracy improvement of low-invasive temperature measurement for hyperthermia treatment using a ferromagnetic implant with low Curie temperature. Electron. Commun. Jpn. 2016, 99, 55–62. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, R.; Guan, G.; Liu, H.; Song, G. Renal clearable magnetic nanoparticles for magnetic resonance imaging and guided therapy. In WIREs Nanomedicine and Nanobiotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; p. e1929. [Google Scholar]
- Astefanoaei, I.; Stancu, A. Thermo-fluid porosity-related effects in the magnetic hyperthermia. Eur. Phys. J. Plus 2021, 136, 1–11. [Google Scholar] [CrossRef]
- Sun, L.; Liang, T.; Zhang, C.; Chen, J. The rheological performance of shear-thickening fluids based on carbon fiber and silica nanocomposite. Phys. Fluids 2023, 35, 032002. [Google Scholar] [CrossRef]
- Cao, M.; Cui, T.; Yue, Y.; Li, C.; Guo, X.; Jia, X.; Wang, B. Preparation and characterization for the thermal stability and mechanical property of PLA and PLA/CF samples built by FFF approach. Materials 2023, 16, 5023. [Google Scholar] [CrossRef]
- He, L.; Nong, H.; Tan, J.; Wu, Q.; Zheng, R.; Zhao, S.; Yu, Q.; Wang, J.; Liu, B. Growth of Two-Dimensional Cr2O3-CrN Mosaic Heterostructures with Tunable Room-Temperature Ferromagnetism. Adv. Mater. 2023, 2304946. [Google Scholar] [CrossRef]
- Jia, L.-C.; Wang, Z.-X.; Wang, L.; Zeng, J.-F.; Du, P.-Y.; Yue, Y.-F.; Zhao, L.-H.; Jia, S.-L. Self-standing boron nitride bulks enabled by liquid metals for thermal management. Mater. Horiz. 2023; Online ahead of print. [Google Scholar]
- Manrique-Juarez, M.D.; Rat, S.; Salmon, L.; Molnár, G.; Quintero, C.M.; Nicu, L.; Shepherd, H.J.; Bousseksou, A. Switchable molecule-based materials for micro-and nanoscale actuating applications: Achievements and prospects. Coord. Chem. Rev. 2016, 308, 395–408. [Google Scholar] [CrossRef]
- Tóth, B.; Péter, L.; Révész, Á.; Pádár, J.; Bakonyi, I. Temperature dependence of the electrical resistivity and the anisotropic magnetoresistance (AMR) of electrodeposited Ni-Co alloys. Eur. Phys. J. B 2010, 75, 167–177. [Google Scholar] [CrossRef]
- Arredondo, M.; Martínez, R.; Núñez, M.T.; Ruz, M.; Olivares, M. Inhibition of iron and copper uptake by iron, copper and zinc. Biol. Res. 2006, 39, 95–102. [Google Scholar] [CrossRef]
- Rakhra, G.; Masih, D.; Vats, A.; Vijay, A.; Ashraf, M.Z.; Singh, S.N. Study of metal-metal interactions and their biomarkers using an intestinal human cell line. Biol. Trace Elem. Res. 2019, 195, 95–104. [Google Scholar] [CrossRef]
- Kong, L.; Liu, Y.; Dong, L.; Zhang, L.; Qiao, L.; Wang, W.; You, H. Enhanced red luminescence in CaAl12O9: Mn4+ via doping Ga3+ for plant growth lighting. Dalton Trans. 2020, 49, 1947–1954. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919. [Google Scholar] [CrossRef]
- Miyagawa, T.; Saito, H.; Minamiya, Y.; Mitobe, K.; Takashima, S.; Takahashi, N.; Ito, A.; Imai, K.; Motoyama, S.; Ogawa, J. Inhibition of Hsp90 and 70 sensitizes melanoma cells to hyperthermia using ferromagnetic particles with a low Curie temperature. Int. J. Clin. Oncol. 2013, 19, 722–730. [Google Scholar] [CrossRef]
- Pollert, E.; Knížek, K.; Maryško, M.; Kašpar, P.; Vasseur, S.; Duguet, E. New Tc-tuned magnetic nanoparticles for self-controlled hyperthermia. J. Magn. Magn. Mater. 2007, 316, 122–125. [Google Scholar] [CrossRef]
- Vasseur, S.; Duguet, E.; Portier, J.; Goglio, G.; Mornet, S.; Hadová, E.; Knížek, K.; Maryško, M.; Veverka, P.; Pollert, E. Lanthanum manganese perovskite nanoparticles as possible in vivo mediators for magnetic hyperthermia. J. Magn. Magn. Mater. 2006, 302, 315–320. [Google Scholar] [CrossRef]
- Manzoor, S.; Ahmed, A.; Rashid, A.U.; Ahmad, S.N.; Shaheen, S.A. Study of magnetothermal properties of strontium doped lanthanum manganite nanoparticles for hyperthermia applications. IEEE Trans. Magn. 2013, 49, 3504–3507. [Google Scholar] [CrossRef]
- McBride, K.; Partridge, N.; Bennington-Gray, S.; Felton, S.; Stella, L.; Poulidi, D. Synthesis, characterisation and study of magnetocaloric effects (enhanced and reduced) in manganate perovskites. Mater. Res. Bull. 2017, 88, 69–77. [Google Scholar] [CrossRef]
- Di, W.; Ren, X.; Zhang, L.; Liu, C.; Lu, S. A facile template-free route to fabricate highly luminescent mesoporous gadolinium oxides. CrystEngComm 2011, 13, 4831–4833. [Google Scholar] [CrossRef]
- Kaman, O.; Pollert, E.; Veverka, P.; Veverka, M.; Hadová, E.; Knížek, K.; Maryško, M.; Kašpar, P.; Klementová, M.; Grünwaldová, V.; et al. Silica encapsulated manganese perovskite nanoparticles for magnetically induced hyperthermia without the risk of overheating. Nanotechnology 2009, 20, 275610. [Google Scholar] [CrossRef]
- Vázquez-Olmos, A.; Redón, R.; Rodríguez-Gattorno, G.; Mata-Zamora, M.E.; Morales-Leal, F.; Fernández-Osorio, A.L.; Saniger, J.M. One-step synthesis of Mn3O4 nanoparticles: Structural and magnetic study. J. Colloid Interface Sci. 2005, 291, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kaman, O.; Veverka, P.; Jirák, Z.; Maryško, M.; Knížek, K.; Veverka, M.; Kašpar, P.; Burian, M.; Šepelák, V.; Pollert, E. The magnetic and hyperthermia studies of bare and silica-coated La0.75Sr0.25MnO3 nanoparticles. J. Nanoparticle Res. 2011, 13, 1237–1252. [Google Scholar] [CrossRef]
- Tokura, Y.; Tomioka, Y. Colossal magnetoresistive manganites. J. Magn. Magn. Mater. 1999, 200, 1–23. [Google Scholar] [CrossRef]
- Zener, C. Interaction between the d-shells in the transition metals. II. Ferromagnetic compounds of manganese with perovskite structure. Phys. Rev. B 1951, 82, 403–405. [Google Scholar] [CrossRef]
- Epherre, R.; Pepin, C.; Penin, N.; Duguet, E.; Mornet, S.; Pollert, E.; Goglio, G. Evidence of non-stoichiometry effects in nanometric manganite perovskites: Influence on the magnetic ordering temperature. J. Mater. Chem. 2011, 21, 14990–14998. [Google Scholar] [CrossRef]
- Ahmad, A.; Bae, H.; Rhee, I. Silica-coated gadolinium-doped lanthanum strontium manganite nanoparticles for self-controlled hyperthermia applications. AIP Adv. 2018, 8, 015108. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Akbar, H.; Zada, I.; Anjum, F.; Afzal, A.M.; Javed, S.; Muneeb, M.; Ali, A.; Choi, J.R. Improvement of the Self-Controlled Hyperthermia Applications by Varying Gadolinium Doping in Lanthanum Strontium Manganite Nanoparticles. Molecules 2023, 28, 7860. https://doi.org/10.3390/molecules28237860
Ahmad A, Akbar H, Zada I, Anjum F, Afzal AM, Javed S, Muneeb M, Ali A, Choi JR. Improvement of the Self-Controlled Hyperthermia Applications by Varying Gadolinium Doping in Lanthanum Strontium Manganite Nanoparticles. Molecules. 2023; 28(23):7860. https://doi.org/10.3390/molecules28237860
Chicago/Turabian StyleAhmad, Ashfaq, Hassan Akbar, Imran Zada, Faiza Anjum, Amir Muhammad Afzal, Subhan Javed, Muhammad Muneeb, Asghar Ali, and Jeong Ryeol Choi. 2023. "Improvement of the Self-Controlled Hyperthermia Applications by Varying Gadolinium Doping in Lanthanum Strontium Manganite Nanoparticles" Molecules 28, no. 23: 7860. https://doi.org/10.3390/molecules28237860
APA StyleAhmad, A., Akbar, H., Zada, I., Anjum, F., Afzal, A. M., Javed, S., Muneeb, M., Ali, A., & Choi, J. R. (2023). Improvement of the Self-Controlled Hyperthermia Applications by Varying Gadolinium Doping in Lanthanum Strontium Manganite Nanoparticles. Molecules, 28(23), 7860. https://doi.org/10.3390/molecules28237860





