NMR Structural Study of Syndecan-4 Transmembrane Domain with Cytoplasmic Region
Abstract
:1. Introduction
2. Results and Discussion
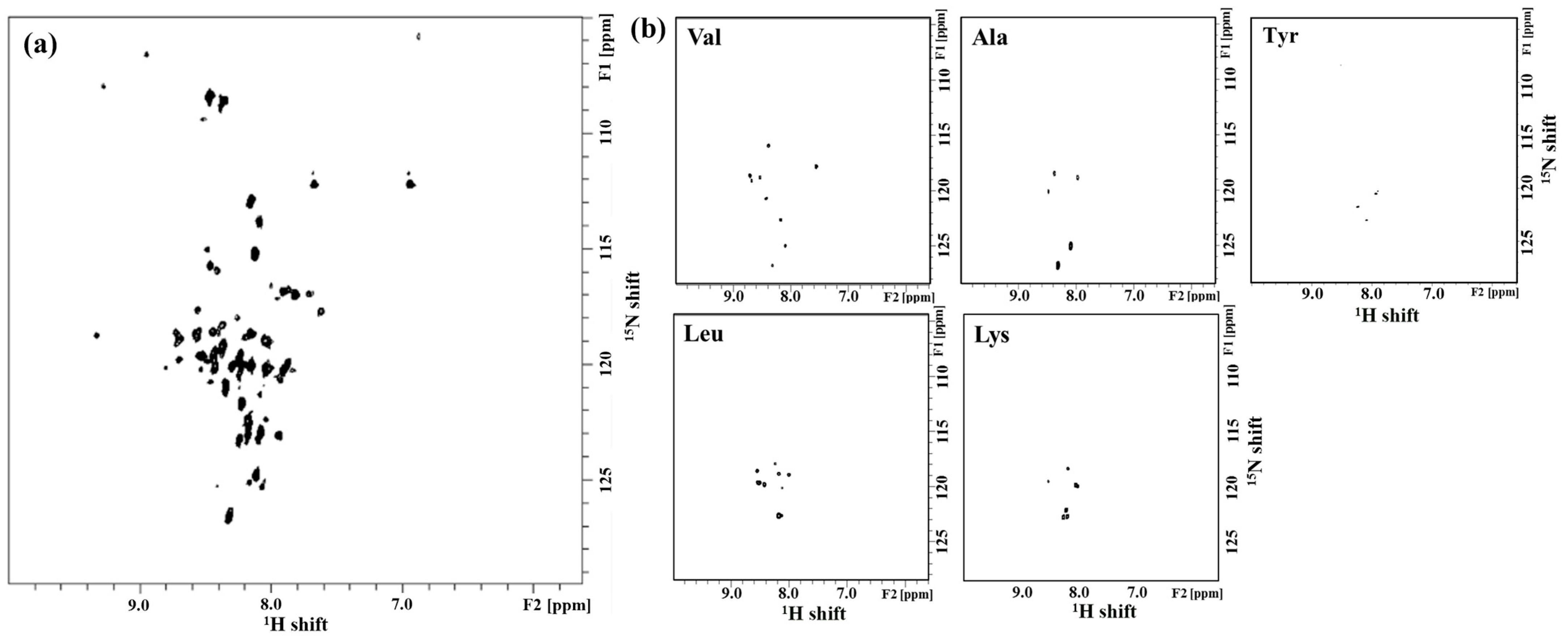
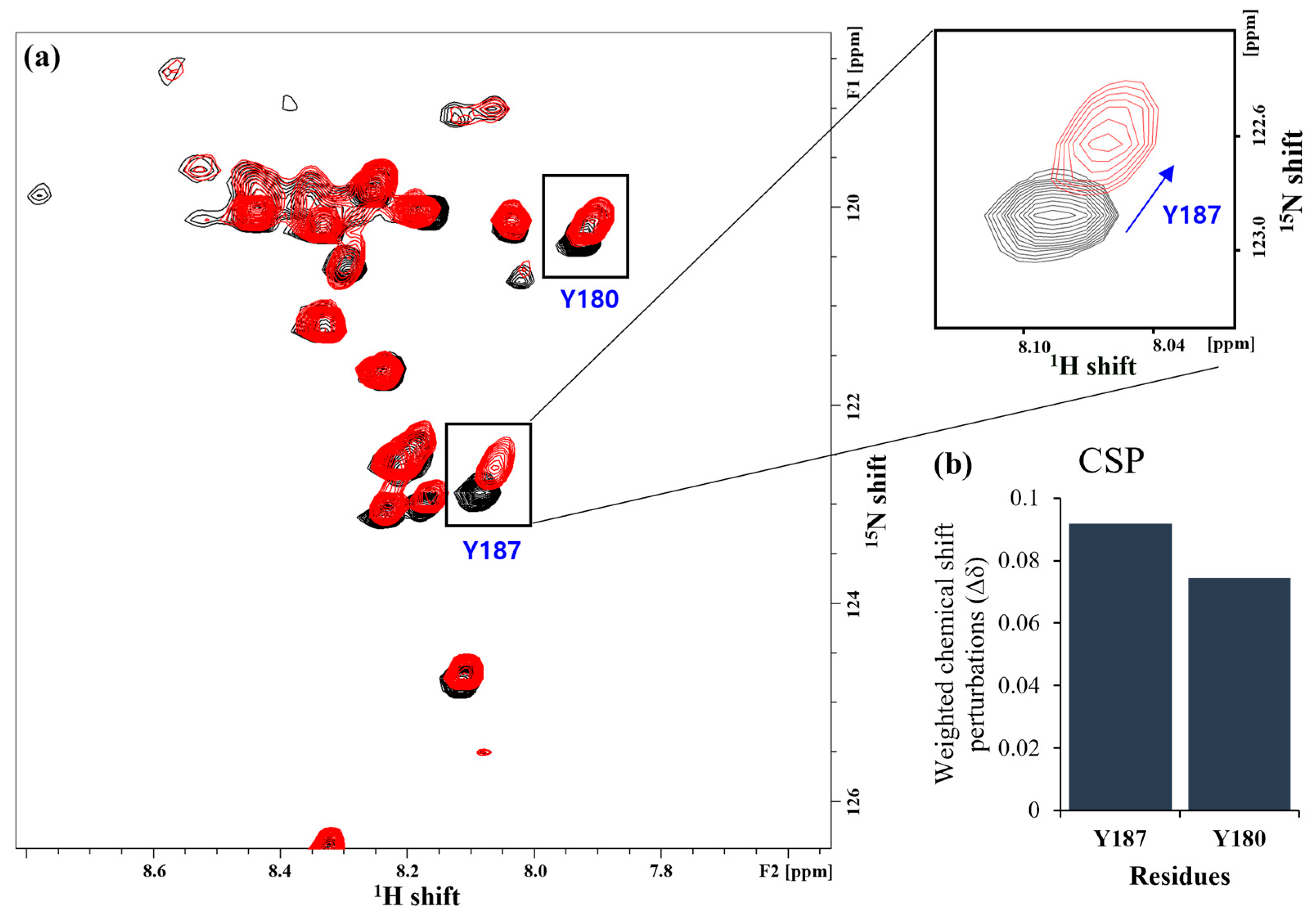
2.1. Solution-State NMR Spectroscopy
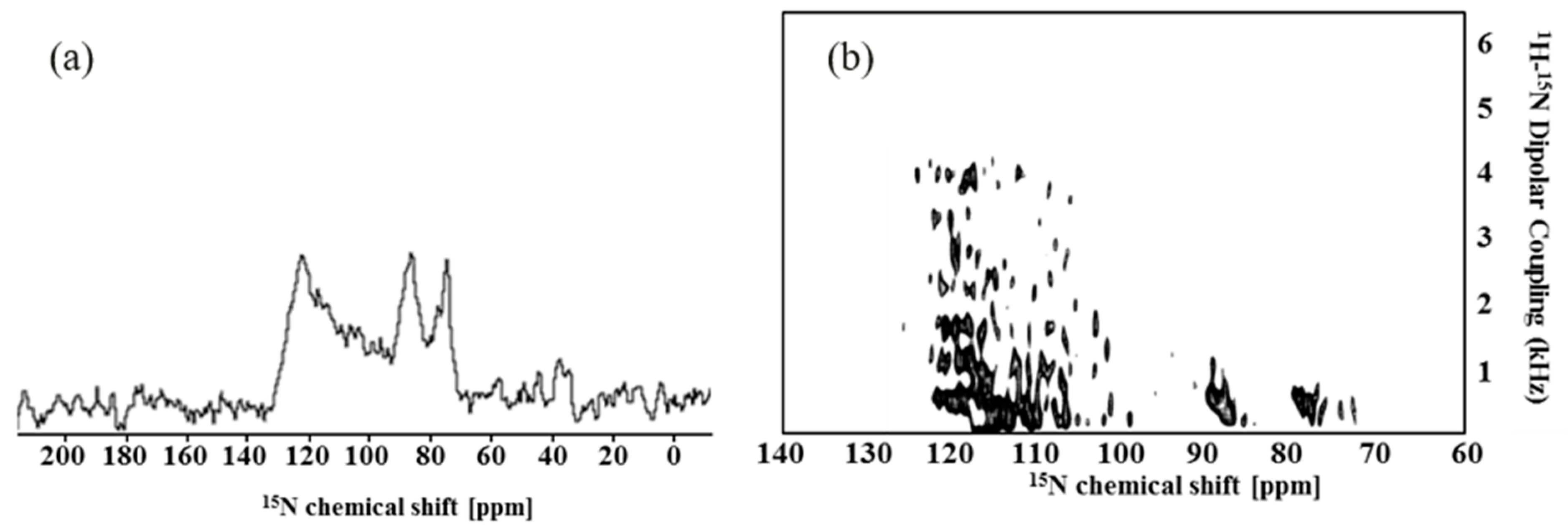
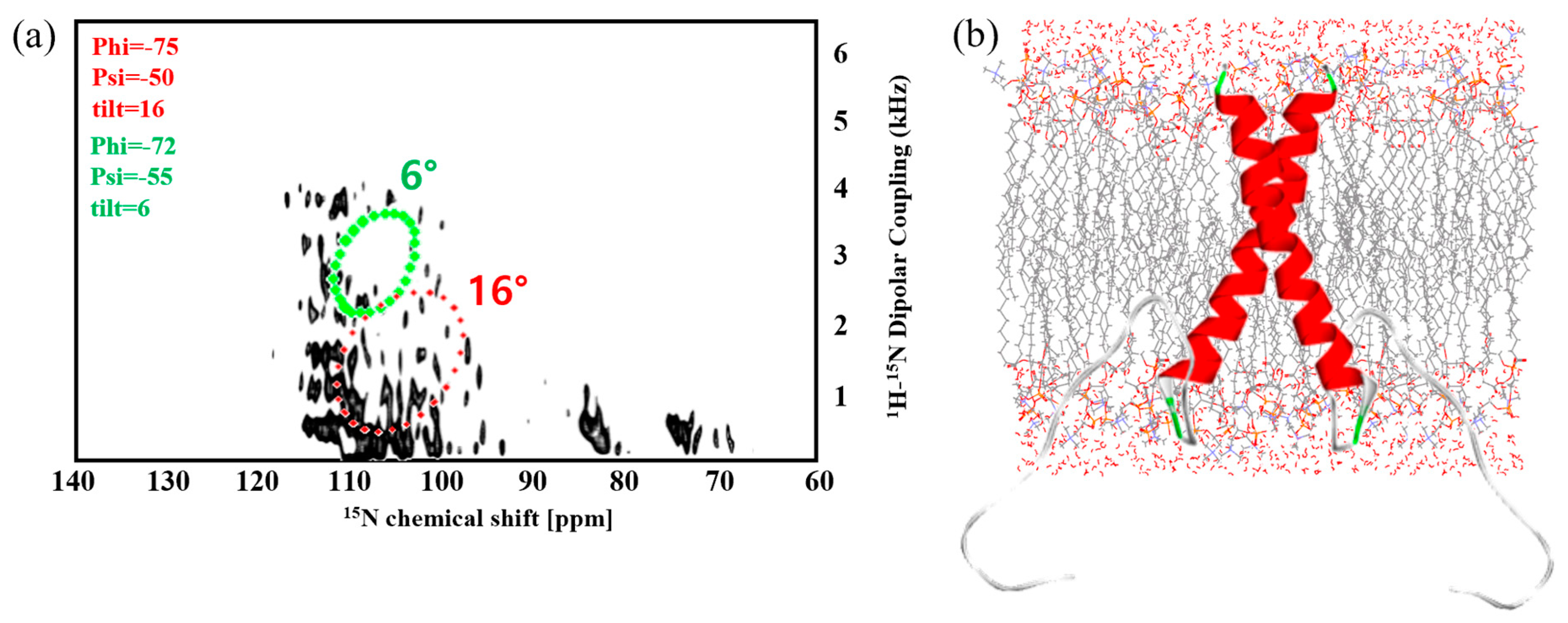
2.2. Solid-State NMR Spectroscopy
3. Materials and Methods
3.1. Expression and Purification of Syd4-eTC
3.2. Solution-State NMR Spectroscopy
3.3. Solid-State NMR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dreyfuss, J.L.; Regatieri, C.V.; Jarrouge, T.R.; Cavalheiro, R.P.; Sampaio, L.O.; Nader, H.B. Heparan sulfate proteoglycans: Structure, protein interactions and cell signaling. An Acad. Bras. Cienc. 2009, 81, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.M.; da Silva, D.A.; Sartorio, P.V.; Silva, T.D.; Saad, S.S.; Nader, H.B.; Forones, N.M.; Toma, L. Heparan Sulfate Proteoglycans in Human Colorectal Cancer. Anal. Cell. Pathol. 2018, 2018, 8389595. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Kokenyesi, R.; Kato, M.; Hinkes, M.T.; Spring, J.; Gallo, R.L.; Lose, E.J. Biology of the syndecans: A Family of Transmembrane Heparan Sulfate Proteoglycans. Annu. Rev. Cell Biol. 1992, 8, 365–393. [Google Scholar] [CrossRef] [PubMed]
- Fears, C.Y.; Woods, A. The role of syndecans in disease and wound healing. Matrix Biol. 2006, 25, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; David, G. The syndecans, tuners of transmembrane signaling. FASEB J. 1999, 13, S91–S100. [Google Scholar] [CrossRef] [PubMed]
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007. [Google Scholar] [CrossRef]
- Multhaupt, H.A.B.; Yoneda, A.; Whiteford, J.R.; Oh, E.S.; Lee, W.; Couchman, J.R. Syndecan signaling: When, where and why? J. Physiol. Pharmacol. 2009, 60, 31–38. [Google Scholar]
- Couchman, J.R. Syndecans: Proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 2003, 4, 926–937. [Google Scholar] [CrossRef]
- Beauvais, D.M.; Rapraeger, A.C. Syndecans in tumor cell adhesion and signaling. Reprod. Biol. Endocrinol. 2004, 2, 3. [Google Scholar] [CrossRef]
- Stringer, S.E.; Gallagher, J.T. Heparan Sulphate. Int. J. Biochem. Cell Biol. 1997, 29, 709–714. [Google Scholar] [CrossRef]
- David, G.; Schueren, B.; Marynen, P.; Cassiman, J.J.; Berghe, H.J. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. Cell Biol. 1992, 118, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Elenius, K.; Kalkanen, M. Function of the syndecans-a family of cell surface proteoglycans. J. Cell Sci. 1994, 107, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chiquet-Ehrismann, R.; Moyano, J.V.; Garcia-Pardo, A.; Orend, G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001, 61, 8586–8594. [Google Scholar] [PubMed]
- Chiquet-Ehrismann, R.; Kalla, P.; Pearson, C.A.; Beck, K.; Chiquet, M. Tenascin interferes with fibronectin action. Cell 1998, 53, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Woods, A.; Lim, S.T.; Theibert, A.W.; Couchman, J.R. Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4, 5-bisphosphate coordinately regulate protein kinase C activity. J. Biol. Chem. 1998, 273, 10624–10629. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Murakami, M.; Gao, Y.; Simons, M. Phosphatidylinositol-4, 5-bisphosphate mediates the interaction of syndecan-4 with protein kinase C. Biochemistry 1999, 38, 15871–15877. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Vogt, S.; Lim, S.T.; Oh, E.S.; Prestwich, G.D.; Theibert, A.; Lee, W.; Woods, A. Regulation of Inositol Phospholipid Binding and Signaling through Syndecan-4. J. Biol. Chem. 2002, 277, 49296–49303. [Google Scholar] [CrossRef]
- Song, Y.; McFarland, D.C.; Velleman, S.G. Critical amino acids in syndecan-4 cytoplasmic domain modulation of turkey satellite cell growth and development. Comp. Biochem. Physiol. Part A 2012, 161, 271–278. [Google Scholar] [CrossRef]
- Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; Andre, F.; Soria, J. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef]
- Oh, E.S.; Woods, A.; Couchman, J.R. Multimerization of the Cytoplasmic Domain of Syndecan-4 Is Required for Its Ability to Activate Protein Kinase C. J. Biol. Chem. 1997, 272, 11805–11811. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Simons, M. Regulation of syndecan-4 phosphorylation in vivo. J. Biol. Chem. 1998, 273, 10914–10918. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Jung, Y.S.; Shin, J.; Han, I.; Mortier, E.; Zimmermann, P.; Whiteford, J.R.; Couchman, J.R.; Oh, E.-S.; Lee, W. Structural basis of syndecan-4 phosphorylation as a molecular switch to regulate signaling. J. Mol. Biol. 2006, 355, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Oh, E.S.; Woods, A.; Couchman, J.R.; Lee, W.J. Solution Structure of a Syndecan-4 Cytoplasmic Domain and Its Interaction with Phosphatidylinositol 4,5-Bisphosphate. Biol. Chem. 1998, 273, 13022–13029. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, J.R.; Ko, S.; Lee, W.; Couchman, J.R. Structural and Cell Adhesion Properties of Zebrafish Syndecan-4 Are Shared with Higher Vertebrates. J. Biol. Chem. 2008, 283, 29322–29330. [Google Scholar] [CrossRef] [PubMed]
- Udo, H.; Kent, N.; Norbert, P. Heparan sulphate proteoglycans: The sweet side of development. Nat. Rev. Mol. Cell Biol. 2005, 6, 530–541. [Google Scholar]
- Barbouri, D.; Afratis, N.; Gialeli, C.; Vynios, D.H.; Theocharis, A.D.; Karamanos, N.K. Syndecans as Modulators and Potential Pharmacological Targets in Cancer Progression. Front. Oncol. 2014, 4, 4. [Google Scholar] [CrossRef]
- Shin, J.; Lee, W.; Lee, D.; Koo, B.K.; Han, I.; Lim, Y.; Woods, A.; Couchman, J.R.; Oh, E.S. Solution structure of the dimeric cytoplasmic domain of syndecan-4. Biochemistry 2001, 40, 8471–8478. [Google Scholar] [CrossRef]
- Park, T.J.; Lee, M.H.; Kim, J.S.; Kim, Y. Recombinant expression, purification, and characterization of transmembrane domain of syndecan-4. Process Biochem. 2011, 46, 1166–1171. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, J.S.; Song, J.Y.; Kim, Y. High-yield Expression and Characterization of Syndecan-4 Extracellular, Transmembrane and Cytoplasmic Domains. Bull. Korean Chem. Soc. 2013, 34, 1120–1126. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, J.S.; Choi, S.S.; Kim, Y. Structural Effects of the GXXXG Motif on the Oligomer Formation of Transmembrane Domain of Syndecan-4. Bull. Korean Chem. Soc. 2013, 34, 3577–3585. [Google Scholar] [CrossRef]
- Choi, S.S.; Jeong, J.H.; Kim, J.S.; Kim, Y. Structure Determination of Syndecan-4 Transmembrane Domain using PISA Wheel Pattern and Molecular Dynamics simulation. J. Korean Magn. Reson. 2014, 18, 58–62. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, J.S.; Jeong, J.H.; Kim, Y. Structural Change in Transmembrane Region of Syndecan-4 by Mutation. J. Korean Magn. Reson. 2016, 20, 129–137. [Google Scholar] [CrossRef]
- Li, Q.; Ng, H.Q.; Kang, C. Secondary structure and topology of the transmembrane domain of Syndecan-2 in detergent micelles. FEBS Lett. 2019, 593, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Waugh, J.S. Uncoupling of local field spectra in nuclear magnetic resonance: Determination of atomic positions in solids. Proc. Natl. Acad. Sci. USA 1976, 5, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Nevzorove, A.A.; Opella, S.J. A “Magic Sandwich” pulse sequence with reduced offset dependence for high-resolution separated local field spectroscopy. J. Magn. Reson. 2003, 164, 182–186. [Google Scholar] [CrossRef]
- Marassi, F.M.; Opella, S.J. A Solid-State NMR Index of Helical Membrane Protein Structure and Topology. J. Magn. Reson. 2000, 144, 150–155. [Google Scholar] [CrossRef]
- Lee, W.; Tonelli, M.; Markley, J.L. NMRFAM-SPARKY: Enhanced software for biomolecular NMR spectroscopy. Bioinformatics 2015, 31, 1325–1327. [Google Scholar] [CrossRef]
- Skinner, S.P.; Fogh, R.H.; Boucher, W.; Ragan, T.J.; Mureddu, L.G.; Vuister, G.W. CcpNmr AnalysisAssign: A flexible plat-form for integrated NMR analysis. J. Biomol. NMR 2016, 66, 111–124. [Google Scholar] [CrossRef]
- Maciejewski, M.W.; Schuyler, A.; Gryk, M.R.; Moraru, I.; Romero, P.R.; Ulrich, E.L.; Eghbalnia, H.R.; Livny, M.; Delaglio, F.; Hoch, J.C. NMRbox: A Resource for Biomolecular NMR Computation. Biophys. J. 2017, 112, 1529–1534. [Google Scholar] [CrossRef]
- De Angelis, A.A.; Grant, C.V.; Baxter, M.K.; McGavin, J.A.; Opella, S.J.; Cotten, M.L. Amphipathic antimicrobial piscidin in magnetically aligned lipid bilayers. Biophys. J. 2011, 101, 1086–1094. [Google Scholar] [CrossRef]
- Wang, J.; Denny, J.; Tian, C.; Kim, S.; Mo, Y.; Kovacs, F.; Song, Z.; Nishimura, K.; Gan, Z.; Fu, R.; et al. Imaging Membrane Protein Helical Wheels. J. Magn. Reson. 2000, 144, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.D.; De Angelis, A.A.; Walther, T.H.; Grage, S.L.; Lange, C.; Opella, S.J.; Ulrich, A.S. Structural characterization of the pore forming protein TatAd of the twin-arginine translocase in membranes by solid-state 15N-NMR. Biochim. Biophys. Acta 2007, 1768, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Nevzorov, A.A.; Opella, S.J. Selective averaging for high-resolution solid-state NMR spectroscopy of aligned samples. J. Magn. Reson. 2007, 185, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Mrse, A.A.; Nevzorov, A.A.; Mesleh, M.F.; Oblatt-Montal, M.; Montal, M.; Opella, S.J. Three-dimensional Structure of the Channel-forming Trans-membrane Domain of Virus Protein “u” (Vpu) from HIV-1. J. Mol. Biol. 2003, 333, 409–424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, Y. NMR Structural Study of Syndecan-4 Transmembrane Domain with Cytoplasmic Region. Molecules 2023, 28, 7855. https://doi.org/10.3390/molecules28237855
Kim M, Kim Y. NMR Structural Study of Syndecan-4 Transmembrane Domain with Cytoplasmic Region. Molecules. 2023; 28(23):7855. https://doi.org/10.3390/molecules28237855
Chicago/Turabian StyleKim, Minseon, and Yongae Kim. 2023. "NMR Structural Study of Syndecan-4 Transmembrane Domain with Cytoplasmic Region" Molecules 28, no. 23: 7855. https://doi.org/10.3390/molecules28237855
APA StyleKim, M., & Kim, Y. (2023). NMR Structural Study of Syndecan-4 Transmembrane Domain with Cytoplasmic Region. Molecules, 28(23), 7855. https://doi.org/10.3390/molecules28237855





