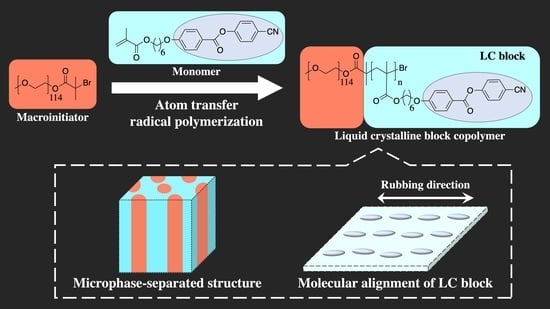
Synthesis and Characterization of Side-Chain Liquid-Crystalline Block Copolymers Containing Cyano-Terminated Phenyl Benzoate Moieties
Abstract
:1. Introduction
2. Results and Discussion
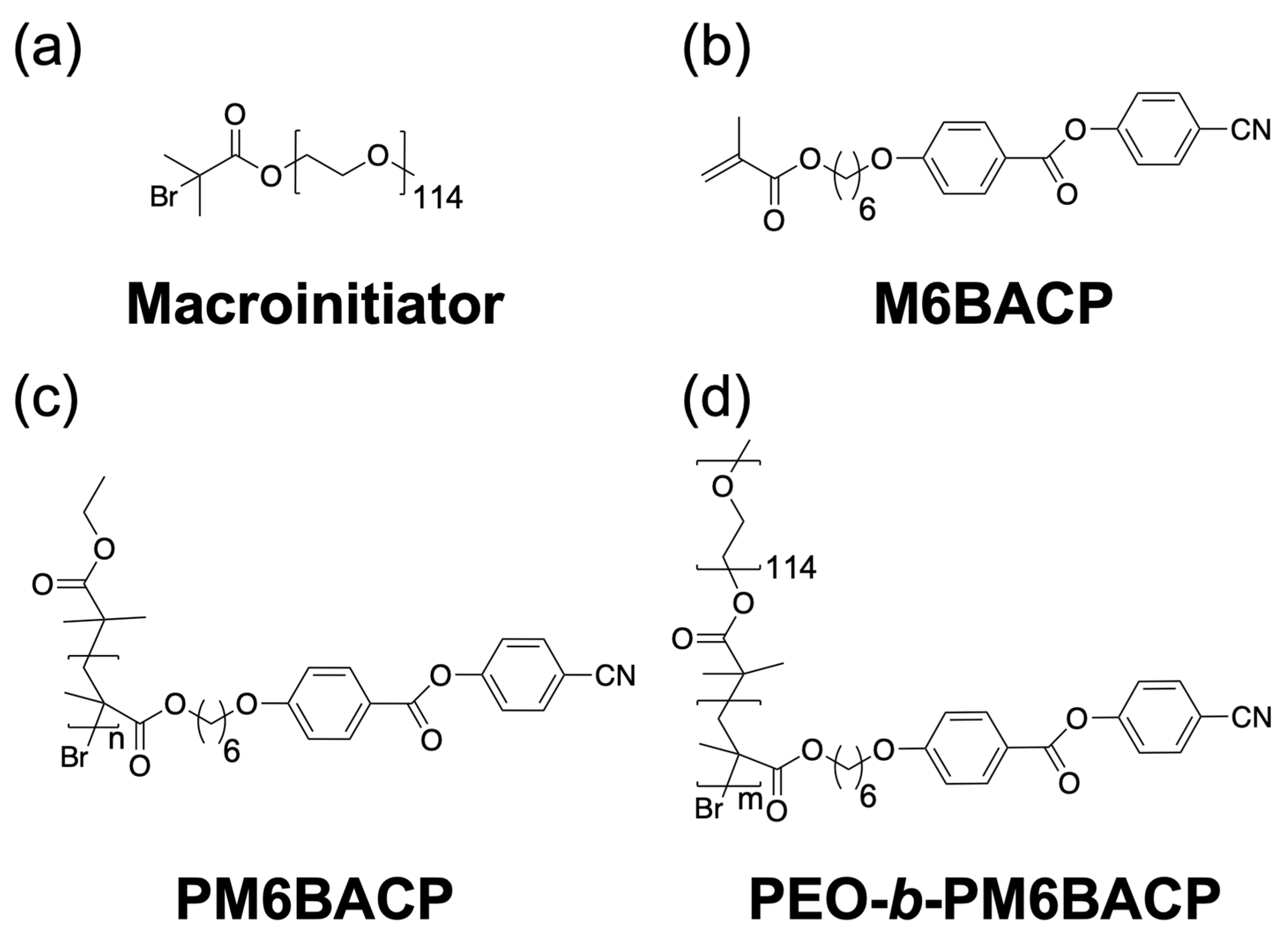
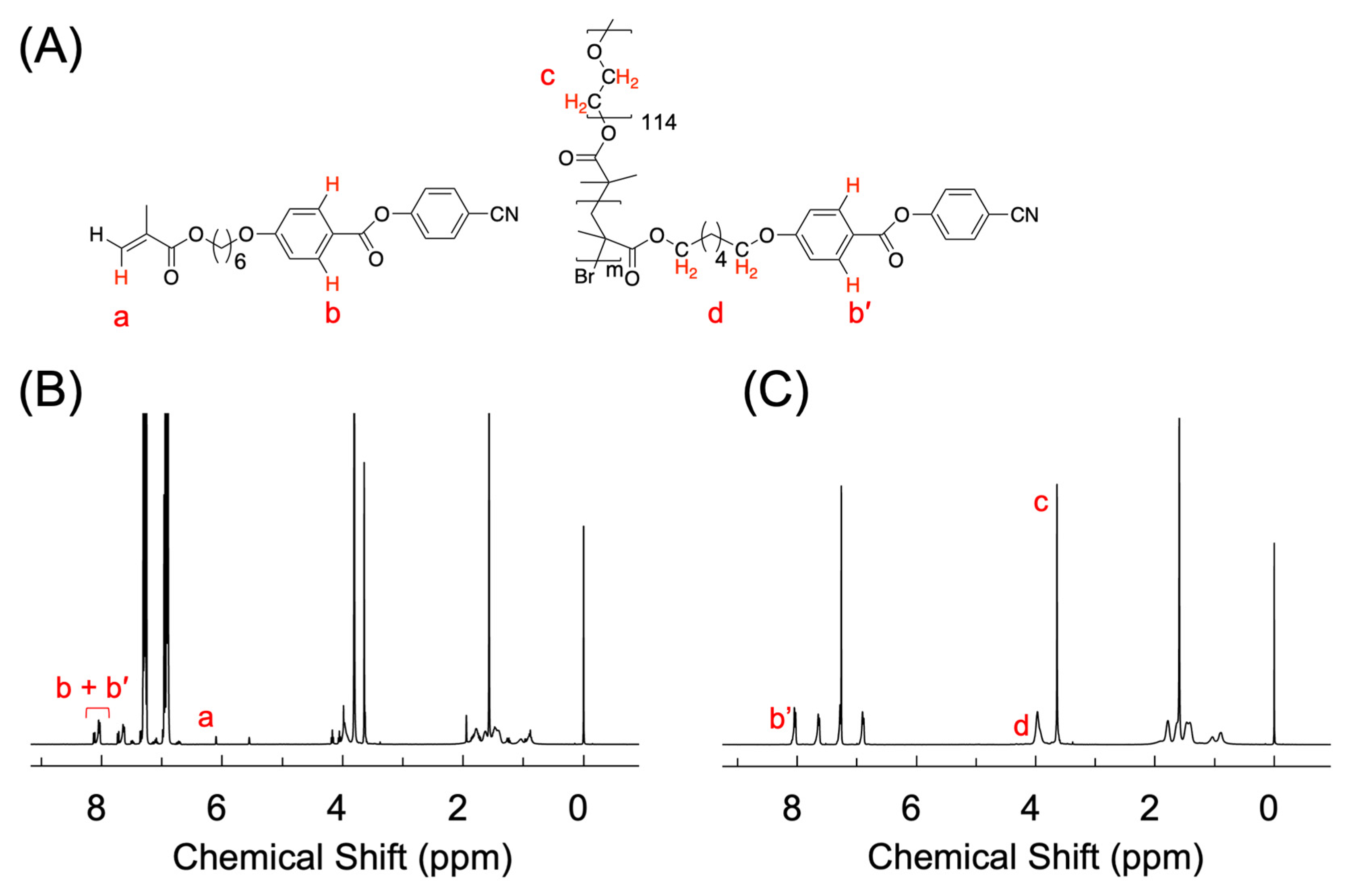
2.1. Synthesis of LC Homopolymer and LC Diblock Copolymers with Cyano-Terminated Phenyl Benzoate Moieties
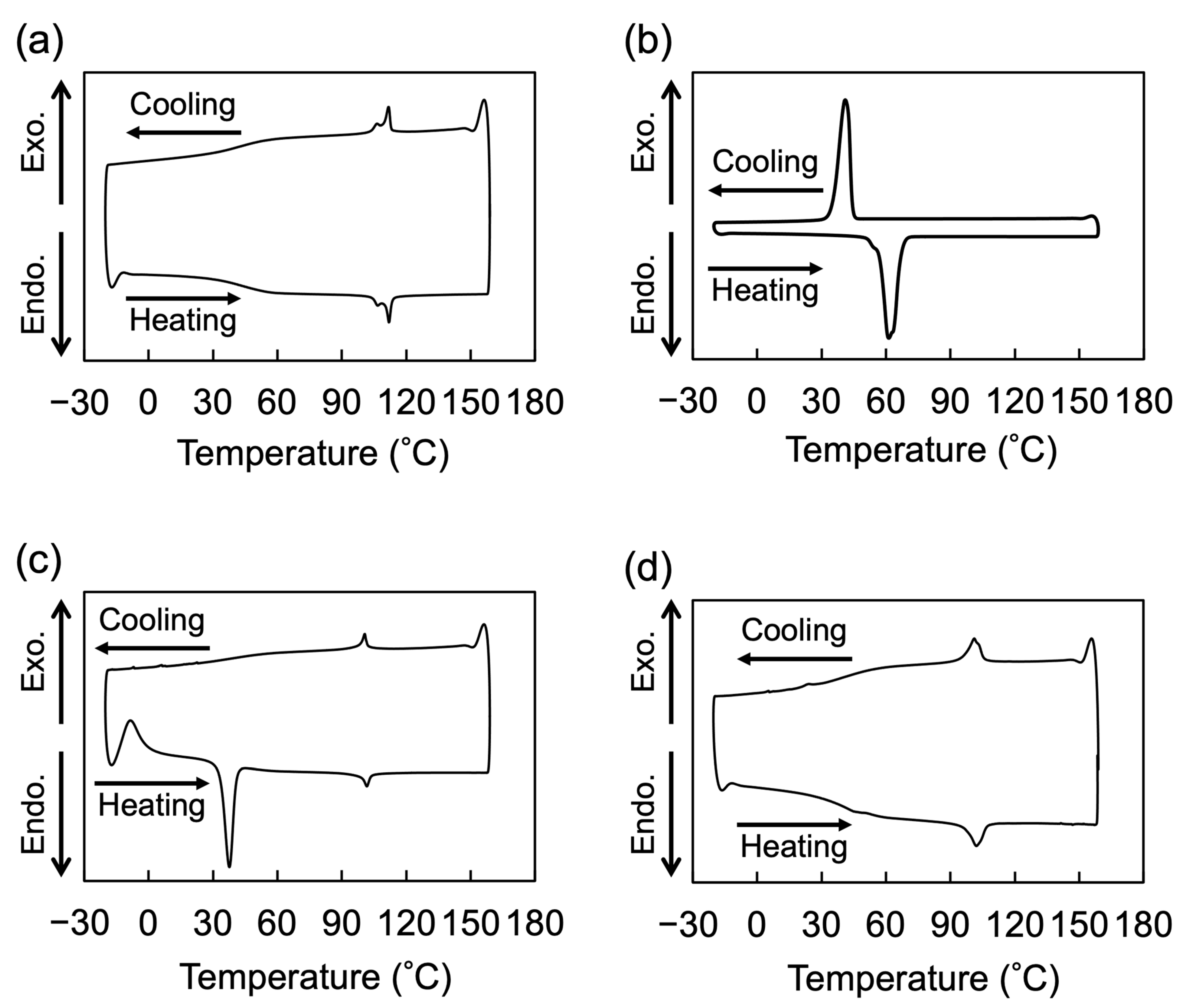
2.2. LC Properties
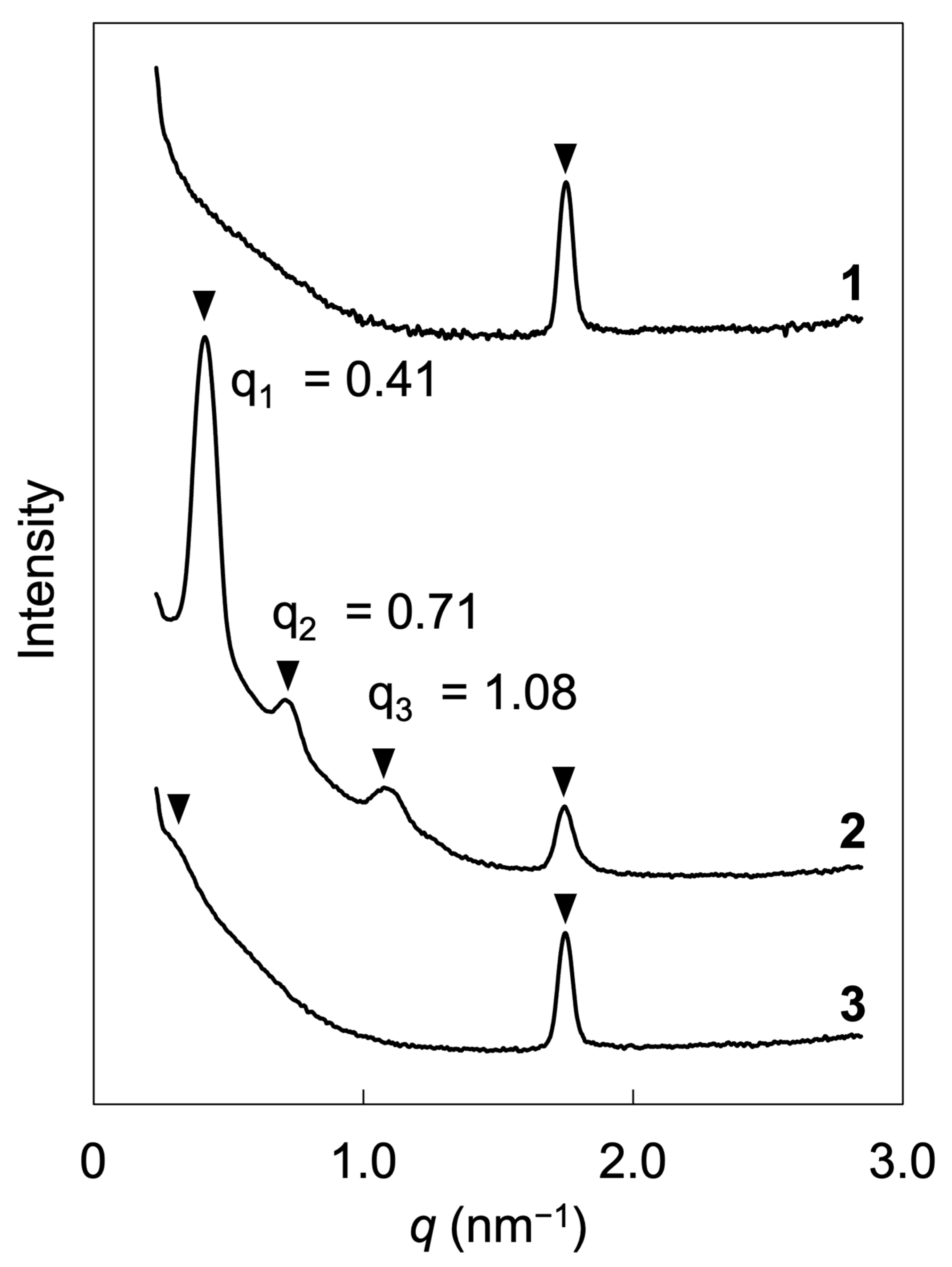
2.3. Microphase-Separated Structures and LC Layers
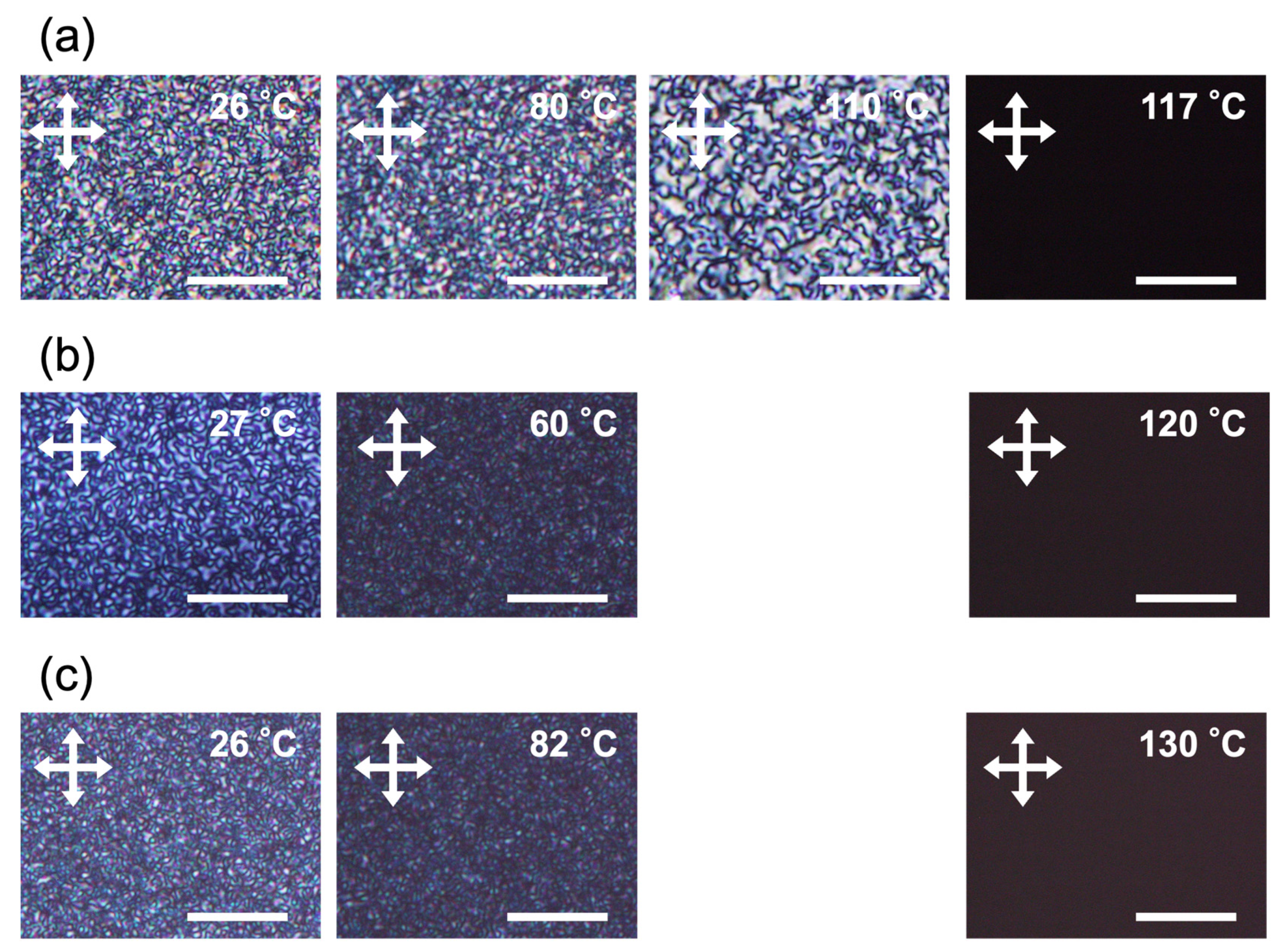
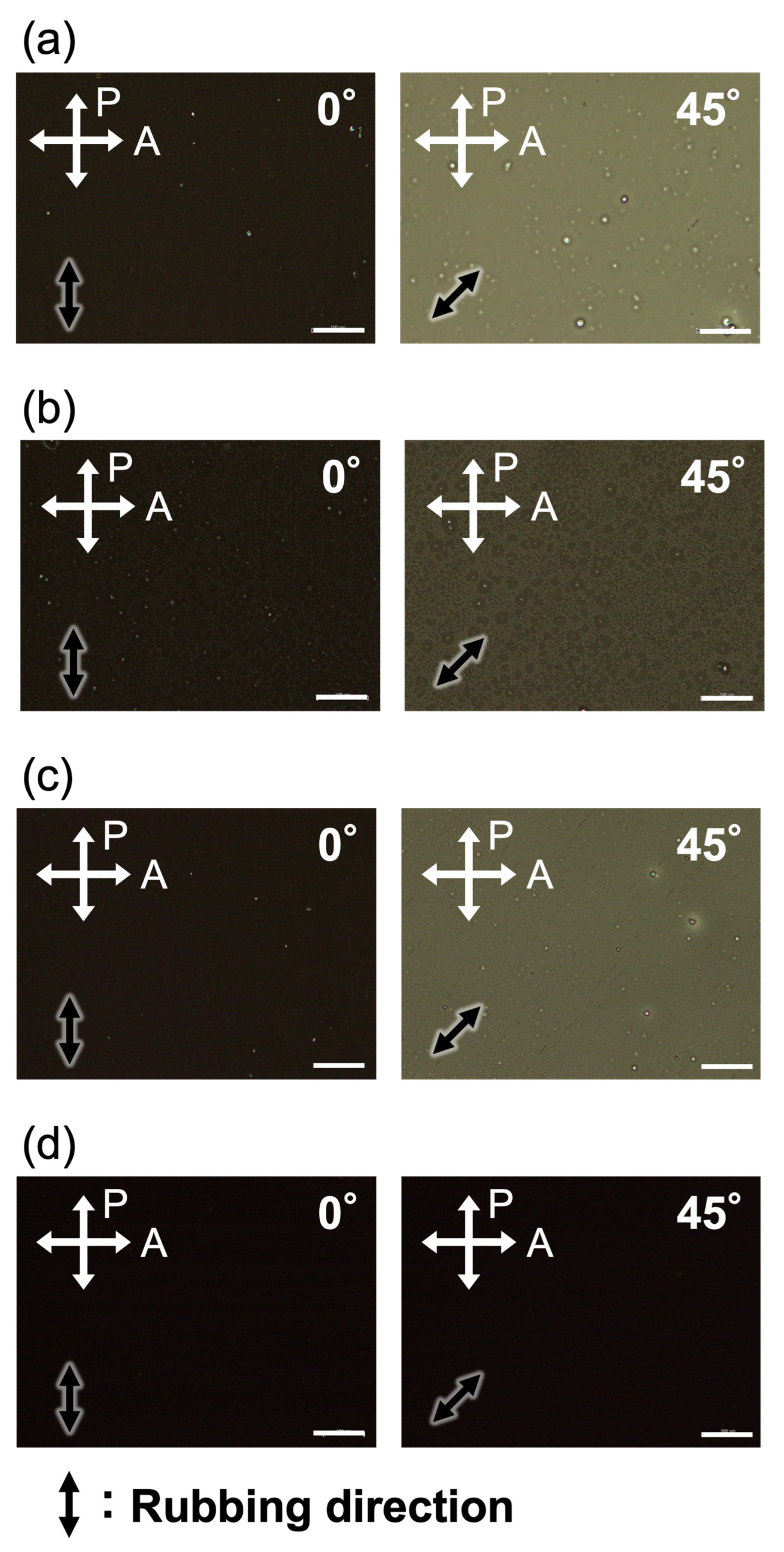
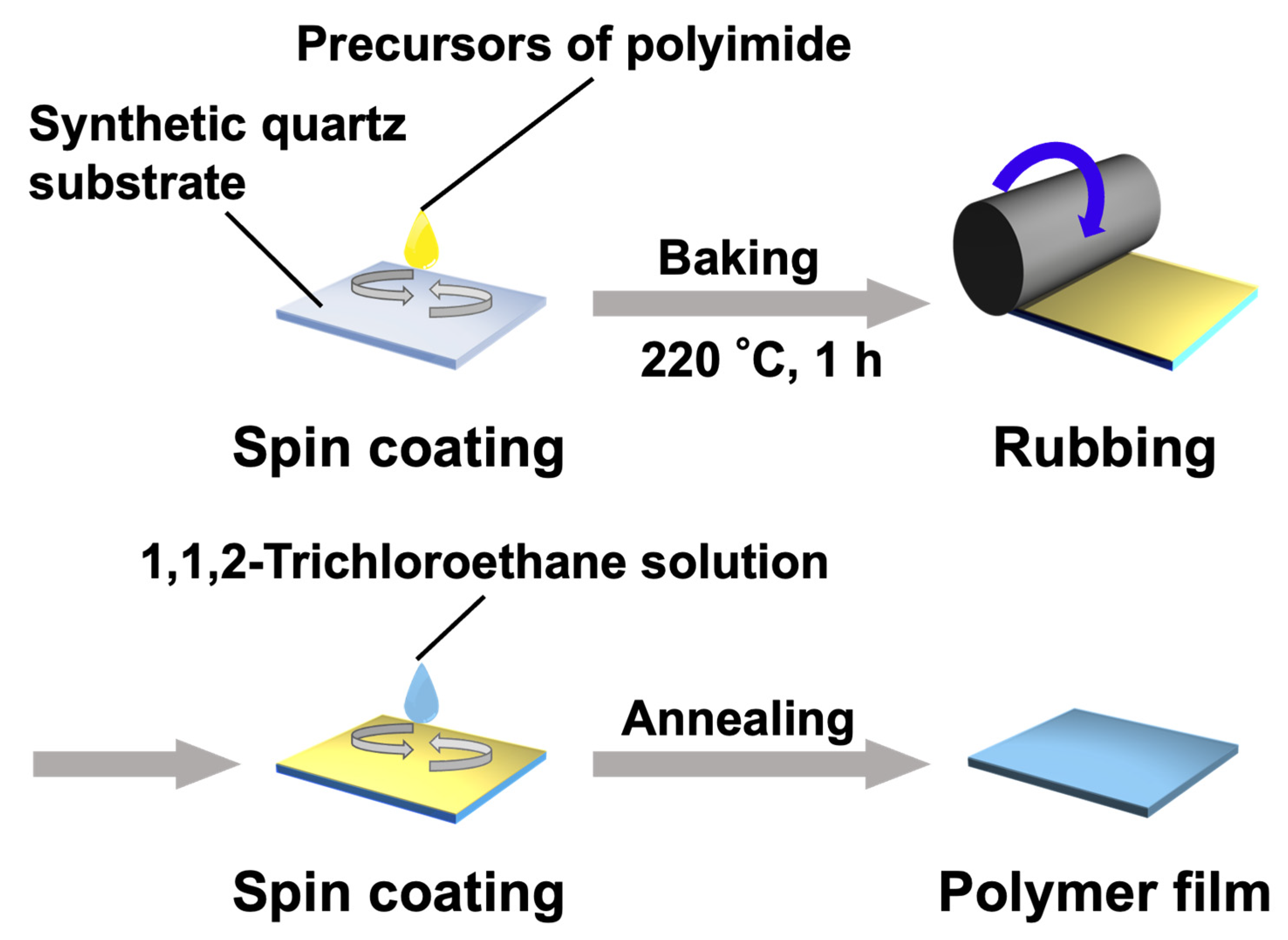
2.4. Alignment Behaviors of LC Polymers
3. Materials and Methods
3.1. Materials
3.2. Film Preparation
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsen, M.W.; Bates, F.S. Unifying weak- and strong-segregation block copolymer theories. Macromolecules 1996, 29, 1091–1098. [Google Scholar] [CrossRef]
- Khandpur, A.K.; Förster, S.; Bates, F.S.; Hamley, I.W.; Ryan, A.J.; Bras, W.; Almdal, K.; Mortensenet, K. Polyisoprene-poly styrene diblock copolymer phase diagram near the order-disorder transition. Macromolecules 1995, 28, 8796–8806. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.; Kim, B.H.; Chang, T.; Lim, J.; Jin, H.M.; Mun, J.H.; Choi, Y.J.; Chung, K.; Shin, J.; et al. Highly tunable refractive index visible-light metasurface from block copolymer self-assembly. Nat. Commun. 2016, 7, 12911. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.W.; Stamm, M. Spatially correlated metallic nanostructures on self-assembled diblock copolymer templates. Langmuir 2005, 21, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jia, X.; Lin, Z.; Guarini, K.W.; Russell, T.P. Growth of silicon oxide in thin film block copolymer scaffolds. Adv. Mater. 2004, 16, 702–706. [Google Scholar] [CrossRef]
- Kumar, N.; Hahn, J. Nanoscale protein patterning using self-assembled diblock copolymers. Langmuir 2005, 21, 6652–6655. [Google Scholar] [CrossRef]
- Schulze, M.W.; McIntosh, L.D.; Hillmyer, M.A.; Lodge, T.P. High-modulus, high-conductivity nanostructured polymer electrolyte membranes via polymerization-induced phase separation. Nano Lett. 2014, 14, 122–126. [Google Scholar] [CrossRef]
- Majewski, P.W.; Gopinadhan, M.; Jang, W.-S.; Lutkenhaus, J.L.; Osuji, C.O. Anisotropic ionic conductivity in block copolymer membranes by magnetic field alignment. J. Am. Chem. Soc. 2010, 132, 17516–17522. [Google Scholar] [CrossRef]
- Yang, S.Y.; Ryu, I.; Kim, H.Y.; Kim, J.K.; Jang, S.K.; Russell, T.P. Nanoporous membranes with ultrahigh selectivity and flux for the filtration of viruses. Adv. Mater. 2006, 18, 709–712. [Google Scholar] [CrossRef]
- Hibi, Y.; Oguchi, Y.; Shimizu, Y.; Hashimoto, K.; Kondo, K.; Iyoda, T. Self-template–assisted micro-phase segregation in blended liquid-crystalline block copolymers films toward three-dimensional structures. Proc. Natl. Acad. Sci. USA 2020, 117, 21070–21078. [Google Scholar] [CrossRef]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling nanotechnology with self assembled block copolymer patterns. Polymer 2003, 44, 6725–6760. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, S. Directed self-assembly of block copolymers for sub-10 nm fabrication. Int. J. Extrem. Manuf. 2020, 2, 032006. [Google Scholar] [CrossRef]
- Park, S.-M.; Liang, X.; Harteneck, B.D.; Pick, T.E.; Hiroshiba, N.; Wu, Y.; Helms, B.A.; Olynick, D.L. Sub-10 nm nanofabrication via nanoimprint directed self-assembly of block copolymers. ACS Nano 2011, 5, 8523–8531. [Google Scholar] [CrossRef] [PubMed]
- Segalman, R.A.; Yokoyama, H.; Kramer, E.J. Graphoepitaxy of spherical domain block copolymer films. Adv. Mater. 2001, 13, 1152–1155. [Google Scholar] [CrossRef]
- Kim, S.O.; Solak, H.H.; Stoykovich, M.P.; Ferrier, N.J.; de Pablo, J.J.; Nealey, P.F. Epitaxial self-assembly of block copolymers on lithographically defined nanopatterned substrates. Nature 2003, 424, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Kan, H.; Detcheverry, F.A.; Dobisz, E.; Kercher, D.S.; Albrecht, T.R.; de Pablo, J.J.; Nealey, P.F. Density multiplication and improved lithography by directed block copolymer assembly. Science 2008, 321, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.W.; Müller, M.; Stoykovich, M.P.; Solak, H.H.; de Pablo, J.J.; Nealey, P.F. Dimensions and shapes of block copolymer domains assembled on lithographically defined chemically patterned substrates. Macromolecules 2007, 40, 90–96. [Google Scholar] [CrossRef]
- Morkved, T.L.; Lu, M.; Urbas, A.M.; Ehrichs, E.E.; Jaeger, H.M.; Mansky, P.; Russell, T.P. Local control of microdomain orientation in diblock copolymer thin films with electric fields. Science 1996, 273, 931–933. [Google Scholar] [CrossRef]
- Osuji, C.; Ferreira, P.J.; Mao, G.; Ober, C.K.; Vander Sande, J.B.; Thomas, E.L. Alignment of self-assembled hierarchical microstructure in liquid crystalline diblock copolymers using high magnetic fields. Macromolecules 2004, 37, 9903–9908. [Google Scholar] [CrossRef]
- Angelescu, D.E.; Waller, J.H.; Adamson, D.H.; Deshpande, P.; Chou, S.Y.; Register, R.A.; Chaikin, P.M. Macroscopic orientation of block copolymer cylinders in single-layer films by shearing. Adv. Mater. 2004, 16, 1736–1740. [Google Scholar] [CrossRef]
- Kim, S.H.; Misner, M.J.; Xu, T.; Kimura, M.; Russell, T.P. Solvent-induced ordering in thin film diblock copolymer/homopolymer mixtures. Adv. Mater. 2004, 16, 226–231. [Google Scholar] [CrossRef]
- Tang, C.B.; Wu, W.; Smilgies, D.M.; Matyjaszewski, K.; Kowalewski, T. Robust control of microdomain orientation in thin films of block copolymers by zone casting. J. Am. Chem. Soc. 2011, 133, 11802–11809. [Google Scholar] [CrossRef]
- Majewski, P.W.; Yager, K.G. Millisecond ordering of block copolymer films via photothermal gradients. ACS Nano 2015, 9, 3896–3906. [Google Scholar] [CrossRef]
- Uchida, J.; Soberats, B.; Gupta, M.; Kato, T. Advanced functional liquid crystals. Adv. Mater. 2022, 34, 2109063. [Google Scholar] [CrossRef]
- Tian, Y.; Watanabe, K.; Kong, X.; Abe, J.; Iyoda, T. Synthesis, nanostructures, and functionality of amphiphilic liquid crystalline block copolymers with azobenzene moieties. Macromolecules 2002, 35, 3739–3747. [Google Scholar] [CrossRef]
- Komura, M.; Iyoda, T. AFM cross-sectional imaging of perpendicularly oriented nanocylinder structures of microphase-separated block copolymer films by crystal-like cleavage. Macromolecules 2007, 40, 4106–4108. [Google Scholar] [CrossRef]
- Asaoka, S.; Uekusa, T.; Tokimori, H.; Komura, M.; Iyoda, T.; Yamada, T.; Yoshida, H. Normally oriented cylindrical nanostructures in amphiphilic PEO-LC diblock copolymers films. Macromolecules 2011, 44, 7645–7658. [Google Scholar] [CrossRef]
- Komiyama, H.; Sakai, R.; Hadano, S.; Asaoka, S.; Kamata, K.; Iyoda, T.; Komura, M.; Yamada, T.; Yoshida, H. Enormously wide range cylinder phase of liquid crystalline PEO-b-PMA(Az) block copolymer. Macromolecules 2014, 47, 1777–1782. [Google Scholar] [CrossRef]
- Watanabe, R.; Kamata, K.; Iyoda, T. Smart block copolymer masks with molecule-transport channels for total wet nanopatterning. J. Mater. Chem. 2008, 18, 5482–5491. [Google Scholar] [CrossRef]
- Li, J.; Kamata, K.; Iyoda, T. Template- and vacuum-ultraviolet-assisted fabrication of a Ag-nanoparticle array on flexible and rigid substrates. Adv. Mater. 2007, 19, 1267–1271. [Google Scholar] [CrossRef]
- Komiyama, H.; Iyoda, T.; Kamata, K. Polypyrrole Nanowire array with high aspect ratio fabricated by block-copolymertemplated electropolymerization. MRS Proc. 2011, 1312, 302. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Ikeda, T.; Iyoda, T. Macroscopic parallel nanocylinder array fabrication using a simple rubbing technique. Adv. Mater. 2006, 18, 2213–2215. [Google Scholar] [CrossRef]
- Morikawa, Y.; Nagano, S.; Watanabe, K.; Kamata, K.; Iyoda, T.; Seki, T. Optical alignment and patterning of nanoscale microdomains in a block copolymer thin film. Adv. Mater. 2006, 18, 883–886. [Google Scholar] [CrossRef]
- Yu, H.; Iyoda, T.; Ikeda, T. Photoinduced alignment of nanocylinders by supramolecular cooperative motions. J. Am. Chem. Soc. 2006, 128, 11010–11011. [Google Scholar] [CrossRef]
- Yu, H. Photoresponsive liquid crystalline block copolymers: From photonics to nanotechnology. Prog. Polym. Sci. 2014, 39, 781–815. [Google Scholar] [CrossRef]
- Morikawa, Y.; Kondo, T.; Nagano, S.; Seki, T. Photoinduced 3D ordering and patterning of microphase-separated nanostructure in polystyrene-based block copolymer. Chem. Mater. 2007, 19, 1540–1542. [Google Scholar] [CrossRef]
- Sano, M.; Nakamura, S.; Hara, M.; Nagano, S.; Shinohara, Y.; Amemiya, Y.; Seki, T. Pathways toward photoinduced alignment switching in liquid crystalline block copolymer films. Macromolecules 2014, 47, 7178–7186. [Google Scholar] [CrossRef]
- Yamada, M.; Iguchi, T.; Hirao, A.; Nakahama, S.; Watanabe, J. Synthesis of side-chain liquid crystalline homopolymers and block copolymers with well-defined structures by living anionic polymerization and their thermotropic phase behavior. Macromolecules 1995, 28, 50–58. [Google Scholar] [CrossRef]
- Tenneti, K.K.; Chen, X.; Li, C.Y.; Tu, Y.; Wan, X.; Zhou, Q.-F.; Sics, I.; Hsiao, B.S. Perforated layer structures in liquid crystalline rod-coil block copolymers. J. Am. Chem. Soc. 2005, 127, 15481–15490. [Google Scholar] [CrossRef]
- Hamley, I.W.; Castelletto, V.; Parras, P.; Lu, Z.B.; Imrie, C.T.; Itoh, T. Ordering on multiple lengthscales in a series of side group liquid crystal block copolymers containing a cholesteryl-based mesogen. Soft Matter 2005, 1, 355–363. [Google Scholar] [CrossRef]
- Yu, H.; Kobayashi, T. Fabrication of stable nanocylinder arrays in highly birefringent films of an amphiphilic liquid-crystalline diblock copolymer. ACS Appl. Mater. Interfaces 2009, 1, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Gopinadhan, M.; Choo, Y.; Ahn, S.-K.; Majewski, P.W.; Yoon, S.Y.; Bakajin, O.; Elimelech, M.; Osuji, C.O.; Kasi, R.M. Molecular design of liquid crystalline brush-like block copolymers for magnetic field directed self-assembly. ACS Macro Lett. 2014, 3, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Kobayashi, S.; Hadano, S.; Komura, M.; Iyoda, T.; Nakagawa, M. Directed self-assembly of nematic liquid crystalline polymers on a rubbed polyimide alignment layer. Jpn. J. Appl. Phys. 2014, 53, 06JC04. [Google Scholar] [CrossRef]
- Fan, Z.X.; Buchner, S.; Haase, W.; Zachmann, H.G. Packing, correlation function, defect structures, and order parameters of liquid-crystal side-chain polymers by X-ray-diffraction investigations. J. Chem. Phys. 1990, 92, 5099–5106. [Google Scholar] [CrossRef]
- Wei, W.; Wu, Z.; Huang, M.; Hsu, C.-H.; Liu, Y.; Zhang, X.; Xiong, H. Discovery of hierarchical superstructures in block copolymers by integrating different liquid crystalline interactions. Soft Matter 2017, 13, 2583–2589. [Google Scholar] [CrossRef]
- Blinov, L.M. Electro-Optical and Magneto-Optical Properties of Liquid Crystals; John Wiley & Sons Ltd.: New York, NY, USA, 1983; pp. 19–22. [Google Scholar]
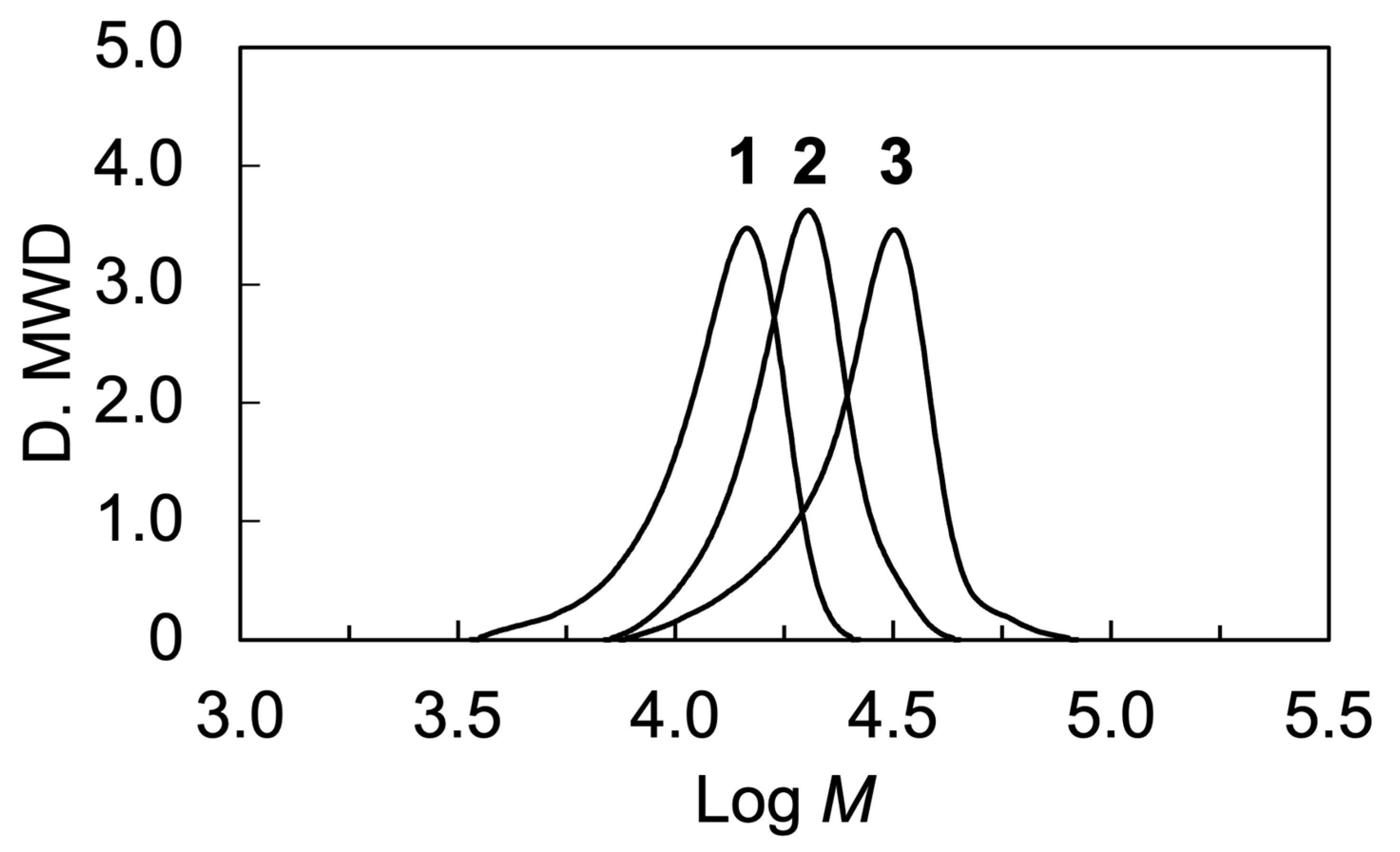

| Sample | [M]0/[I]0 | Conv (%) (a) | DP | Mn(calc) (d) | Mn(NMR) (e) | Mn(SEC) (f) | Mw/Mn (f) | Weight Fraction of LC Block (%) (g) | |
|---|---|---|---|---|---|---|---|---|---|
| PEO | PM6BACP | ||||||||
| PM6BACP | 50 | 47 | – | 23 (b) | 9900 | – | 11,800 | 1.11 | – |
| PEO-b-PM6BACP46 | 50 | 76 | 114 | 46 (c) | 20,700 | 24,000 | 19,200 | 1.09 | 78 |
| PEO-b-PM6BACP106 | 100 | 84 | 114 | 106 (c) | 39,500 | 48,500 | 25,700 | 1.13 | 89 |
| Sample | d (nm) | Δn | S |
|---|---|---|---|
| PM6BACP | 51 | 0.20 | 0.50 |
| PEO-b-PM6BACP46 | 31 | 0.19 | 0.40 |
| PEO-b-PM6BACP106 | 47 | 0.19 | 0.45 |
| Sample | M6BACP (mg) | Initiator (mg) | Cu(I)Cl (mg) | HMTETA (mg) | Anisole (mL) |
|---|---|---|---|---|---|
| PM6BACP | 310 | 3.0 | 1.5 | 3.5 | 2.5 |
| PEO-b-PM6BACP46 | 310 | 75 | 1.5 | 3.5 | 2.5 |
| PEO-b-PM6BACP106 | 610 | 75 | 1.5 | 3.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Taguchi, D.; Kajitani, T.; Fukushima, T.; Kubo, S.; Shishido, A. Synthesis and Characterization of Side-Chain Liquid-Crystalline Block Copolymers Containing Cyano-Terminated Phenyl Benzoate Moieties. Molecules 2023, 28, 7849. https://doi.org/10.3390/molecules28237849
Takahashi K, Taguchi D, Kajitani T, Fukushima T, Kubo S, Shishido A. Synthesis and Characterization of Side-Chain Liquid-Crystalline Block Copolymers Containing Cyano-Terminated Phenyl Benzoate Moieties. Molecules. 2023; 28(23):7849. https://doi.org/10.3390/molecules28237849
Chicago/Turabian StyleTakahashi, Kaito, Daisuke Taguchi, Takashi Kajitani, Takanori Fukushima, Shoichi Kubo, and Atsushi Shishido. 2023. "Synthesis and Characterization of Side-Chain Liquid-Crystalline Block Copolymers Containing Cyano-Terminated Phenyl Benzoate Moieties" Molecules 28, no. 23: 7849. https://doi.org/10.3390/molecules28237849
APA StyleTakahashi, K., Taguchi, D., Kajitani, T., Fukushima, T., Kubo, S., & Shishido, A. (2023). Synthesis and Characterization of Side-Chain Liquid-Crystalline Block Copolymers Containing Cyano-Terminated Phenyl Benzoate Moieties. Molecules, 28(23), 7849. https://doi.org/10.3390/molecules28237849