Simple HPLC-UV Method for Therapeutic Drug Monitoring of 12 Antiepileptic Drugs and Their Main Metabolites in Human Plasma
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Analytical Method
2.2. Method Validation
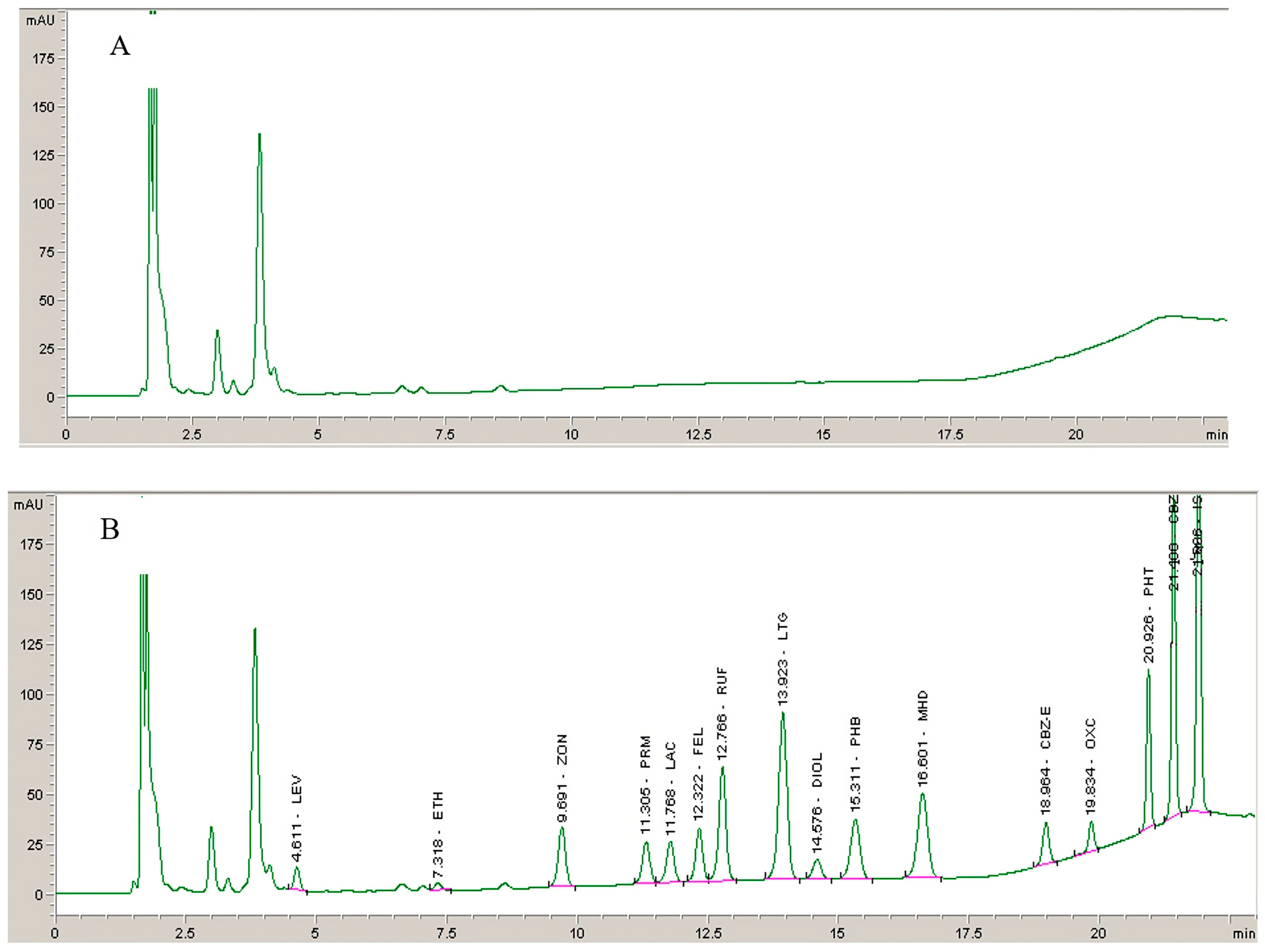
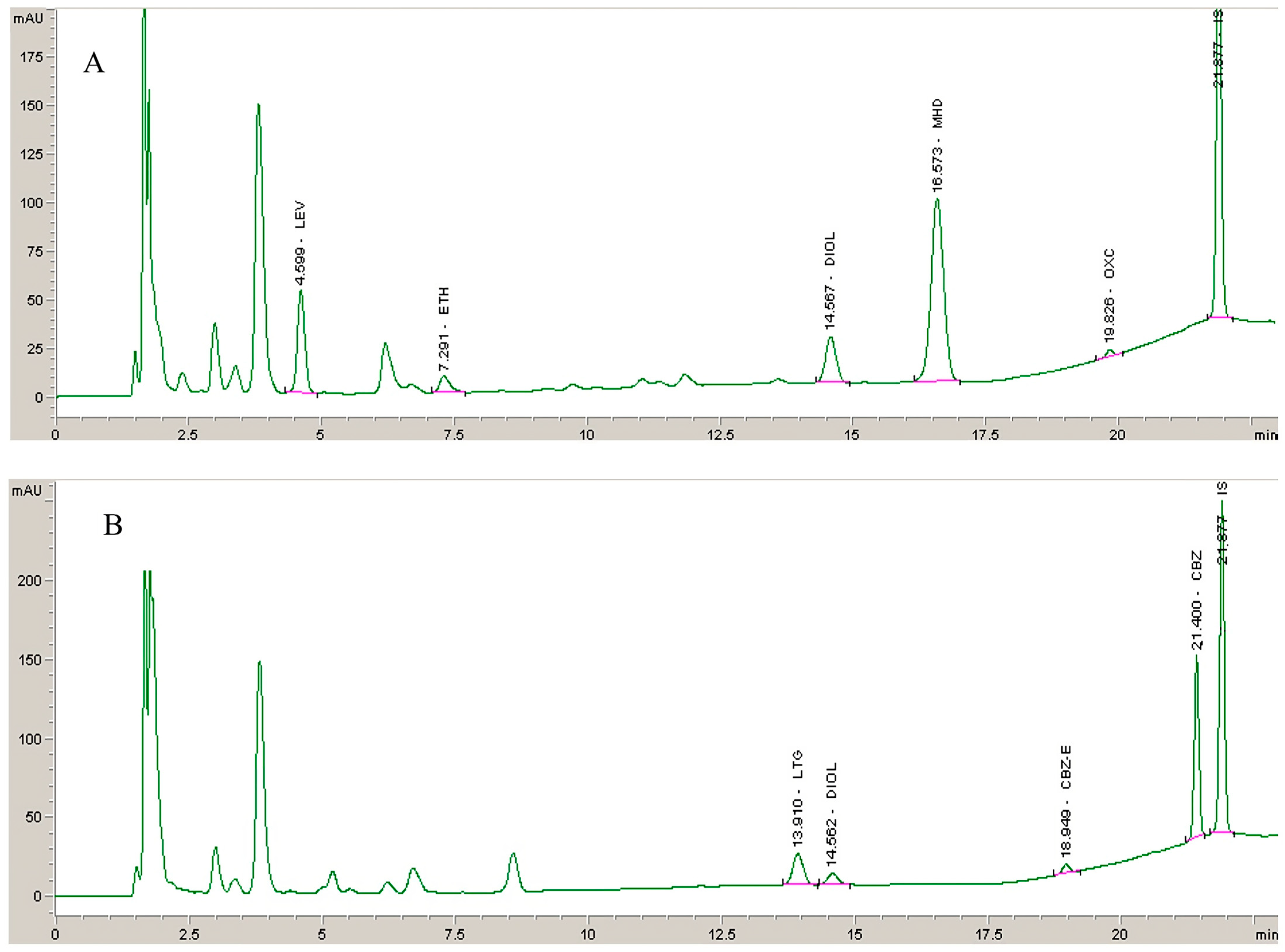
2.2.1. Selectivity
2.2.2. Linearity
2.2.3. Accuracy and Precision
2.2.4. Recovery and Stability
2.3. Clinical Application of the Method
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation and Chromatographic Conditions
3.3. Preparation of Solutions
3.4. Sample Preparation
3.5. Method Validation
3.5.1. Selectivity
3.5.2. Linearity
3.5.3. Accuracy and Precision
3.5.4. Recovery
3.5.5. Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johannessen Landmark, C.; Johannessen, S.I.; Patsalos, P.N. Therapeutic Drug Monitoring of Antiepileptic Drugs: Current Status and Future Prospects. Expert Opin. Drug Metab. Toxicol. 2020, 16, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Berry, D.J. Pharmacotherapy of the Third-Generation AEDs: Lacosamide, Retigabine and Eslicarbazepine Acetate. Expert Opin. Pharmacother. 2012, 13, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, S.I.; Tomson, T. Pharmacokinetic Variability of Newer Antiepileptic Drugs. Clin. Pharmacokinet. 2006, 45, 1061–1075. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Review of Chromatographic Methods Coupled with Modern Detection Techniques Applied in the Therapeutic Drugs Monitoring (TDM). Molecules 2020, 25, 4026. [Google Scholar] [CrossRef]
- Opuni, K.F.M.; Boadu, J.A.; Amponsah, S.K.; Okai, C.A. High Performance Liquid Chromatography: A Versatile Tool for Assaying Antiepileptic Drugs in Biological Matrices. J. Chromatogr. B 2021, 1179, 122750. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.A.; Bakr, M.F.; Rageh, A.H.; Mostafa, A.M. The Use of Separation Techniques in the Analysis of Some Antiepileptic Drugs: A Critical Review. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 783–798. [Google Scholar] [CrossRef]
- Sommerfeld-Klatta, K.; Zielińska-Psuja, B.; Karaźniewcz-Łada, M.; Główka, F.K. New Methods Used in Pharmacokinetics and Therapeutic Monitoring of the First and Newer Generations of Antiepileptic Drugs (AEDs). Molecules 2020, 25, 5083. [Google Scholar] [CrossRef]
- Fortuna, A.; Sousa, J.; Alves, G.; Falcão, A.; Soares-da-Silva, P. Development and Validation of an HPLC-UV Method for the Simultaneous Quantification of Carbamazepine, Oxcarbazepine, Eslicarbazepine Acetate and Their Main Metabolites in Human Plasma. Anal. Bioanal. Chem. 2010, 397, 1605–1615. [Google Scholar] [CrossRef]
- Serralheiro, A.; Alves, G.; Fortuna, A.; Rocha, M.; Falcão, A. First HPLC–UV Method for Rapid and Simultaneous Quantification of Phenobarbital, Primidone, Phenytoin, Carbamazepine, Carbamazepine-10,11-Epoxide, 10,11-Trans-Dihydroxy-10,11-Dihydrocarbamazepine, Lamotrigine, Oxcarbazepine and Licarbazepine in Human Plasma. J. Chromatogr. B 2013, 925, 1–9. [Google Scholar] [CrossRef]
- Ferreira, A.; Rodrigues, M.; Oliveira, P.; Francisco, J.; Fortuna, A.; Rosado, L.; Rosado, P.; Falcão, A.; Alves, G. Liquid Chromatographic Assay Based on Microextraction by Packed Sorbent for Therapeutic Drug Monitoring of Carbamazepine, Lamotrigine, Oxcarbazepine, Phenobarbital, Phenytoin and the Active Metabolites Carbamazepine-10,11-Epoxide and Licarbazepine. J. Chromatogr. B 2014, 971, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.E., Jr.; Sherrod, S.D.; Gant-Branum, R.L.; Colby, J.M.; McLean, J.A. Targeted Strategy to Analyze Antiepileptic Drugs in Human Serum by LC-MS/MS and LC-Ion Mobility-MS. Anal. Chem. 2020, 92, 14648–14656. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, G. A UPLC–MS/MS Method for Simultaneous Determination of Nine Antiepileptic Drugs in Human Plasma and Its Application in TDM. Biomed. Chromatogr. 2021, 35, e5090. [Google Scholar] [CrossRef] [PubMed]
- Taibon, J.; Schmid, R.; Lucha, S.; Pongratz, S.; Tarasov, K.; Seger, C.; Timm, C.; Thiele, R.; Herlan, J.M.; Kobold, U. An LC-MS/MS Based Candidate Reference Method for the Quantification of Carbamazepine in Human Serum. Clin. Chim. Acta 2017, 472, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, T.; Shi, M.; Zhang, Y.; Zhao, X.; Yang, Y.; Gu, J. Simultaneous Determination of Ten Antiepileptic Drugs in Human Plasma by Liquid Chromatography and Tandem Mass Spectrometry with Positive/Negative Ion-Switching Electrospray Ionization and Its Application in Therapeutic Drug Monitoring. J. Sep. Sci. 2016, 39, 964–972. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.J.; Schmutz, M.; Wamil, A.W.; Olpe, H.-R.; Portet, C.; Feldmann, K.F. Oxcarbazepine: Mechanisms of Action. Epilepsia 1994, 35, S5–S9. [Google Scholar] [CrossRef]
- Bring, P.; Ensom, M.H.H. Does Oxcarbazepine Warrant Therapeutic Drug Monitoring? Clin. Pharmacokinet. 2008, 47, 767–778. [Google Scholar] [CrossRef]
- McMillin, G.A.; Juenke, J.M.; Tso, G.; Dasgupta, A. Estimation of Carbamazepine and Carbamazepine-10,11-Epoxide Concentrations in Plasma Using Mathematical Equations Generated With Two Carbamazepine Immunoassays. Am. J. Clin. Pathol. 2010, 133, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, L.; Tomson, T. Clinical Pharmacokinetics and Pharmacological Effects of Carbamazepine and Carbamazepine-10,11-Epoxide. Clin. Pharmacokinet. 1986, 11, 177–198. [Google Scholar] [CrossRef]
- Bernus, I.; Dickinson, R.G.; Hooper, W.D.; Eadie, M.J. Dose-Dependent Metabolism of Carbamazepine in Humans. Epilepsy Res. 1996, 24, 163–172. [Google Scholar] [CrossRef]
- Flesch, G.; Czendlik, C.; Renard, D.; Lloyd, P. Pharmacokinetics of the Monohydroxy Derivative of Oxcarbazepine and Its Enantiomers after a Single Intravenous Dose Given as Racemate Compared with a Single Oral Dose of Oxcarbazepine. Drug Metab. Dispos. 2011, 39, 1103–1110. [Google Scholar] [CrossRef]
- Louis, E.K.S. The Art of Managing Conversions between Antiepileptic Drugs: Maximizing Patient Tolerability and Quality of Life. Pharmaceuticals 2010, 3, 2956–2969. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhang, D.; Zhao, Z.; Mei, S. Simultaneous Determination of 24 Antiepileptic Drugs and Their Active Metabolites in Human Plasma by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2023, 232, 115437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, L.; You, Y.; Zheng, X.; Du, Y.; Tang, D. Development and Evaluation of a Simple and Easy High-Performance Liquid Chromatography–Ultraviolet System Simultaneously Suitable for Determination of 24 Anti-Epileptic Drugs in Plasma. J. Sep. Sci. 2022, 45, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.A.; El-Yazbi, A.F.; Barary, M.A.; Wagih, M.M. Sensitive Inexpensive HPLC Determination of Four Antiepileptic Drugs in Human Plasma: Application to PK Studies. Bioanalysis 2016, 8, 2219–2234. [Google Scholar] [CrossRef]
- Contin, M.; Mohamed, S.; Candela, C.; Albani, F.; Riva, R.; Baruzzi, A. Simultaneous HPLC-UV Analysis of Rufinamide, Zonisamide, Lamotrigine, Oxcarbazepine Monohydroxy Derivative and Felbamate in Deproteinized Plasma of Patients with Epilepsy. J. Chromatogr. B 2010, 878, 461–465. [Google Scholar] [CrossRef]
- Budakova, L.; Brozmanova, H.; Grundmann, M.; Fischer, J. Simultaneous Determination of Antiepileptic Drugs and Their Two Active Metabolites by HPLC. J. Sep. Sci. 2008, 31, 1–8. [Google Scholar] [CrossRef]
- Vermeij, T.A.C.; Edelbroek, P.M. Robust Isocratic High Performance Liquid Chromatographic Method for Simultaneous Determination of Seven Antiepileptic Drugs Including Lamotrigine, Oxcarbazepine and Zonisamide in Serum after Solid-Phase Extraction. J. Chromatogr. B 2007, 857, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Heideloff, C.; Bunch, D.R.; Wang, S. A Novel HPLC Method for Quantification of 10 Antiepileptic Drugs or Metabolites in Serum/Plasma Using a Monolithic Column. Ther. Drug Monit. 2010, 32, 102. [Google Scholar] [CrossRef]
- Martinc, B.; Roškar, R.; Grabnar, I.; Vovk, T. Simultaneous Determination of Gabapentin, Pregabalin, Vigabatrin, and Topiramate in Plasma by HPLC with Fluorescence Detection. J. Chromatogr. B 2014, 962, 82–88. [Google Scholar] [CrossRef]
- Milosheska, D.; Vovk, T.; Grabnar, I.; Roškar, R. Simple and Sensitive High Performance Liquid Chromatography Method with Fluorescence Detection for Therapeutic Drug Monitoring of Topiramate. Acta Chim. Slov. 2015, 62, 411–419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milosheska, D.; Roškar, R. A Novel LC–MS/MS Method for the Simultaneous Quantification of Topiramate and Its Main Metabolites in Human Plasma. J. Pharm. Biomed. Anal. 2017, 138, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Zhang, Z.-Q.; Dong, W.-C.; Jiang, Y. A New Derivatization Method to Enhance Sensitivity for the Determination of Low Levels of Valproic Acid in Human Plasma. J. Chromatogr. Sci. 2014, 52, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fazio, S.; Munch, D.; Drumm, P. Impact of Methanol and Acetonitrile on Separations Based on π–π Interactions with a Reversed-Phase Phenyl Column. J. Chromatogr. A 2005, 1097, 124–129. [Google Scholar] [CrossRef]
- Odi, R.; Bibi, D.; Wager, T.; Bialer, M. A Perspective on the Physicochemical and Biopharmaceutic Properties of Marketed Antiseizure Drugs—From Phenobarbital to Cenobamate and Beyond. Epilepsia 2020, 61, 1543–1552. [Google Scholar] [CrossRef]
- van de Merbel, N.; Savoie, N.; Yadav, M.; Ohtsu, Y.; White, J.; Riccio, M.F.; Dong, K.; de Vries, R.; Diancin, J. Stability: Recommendation for Best Practices and Harmonization from the Global Bioanalysis Consortium Harmonization Team. AAPS J. 2014, 16, 392–399. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in Psychiatry and Neurology: A Comprehensive Summary of the Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology, Update 2017; a Tool for Clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 28 September 2023).

| Analyte | Therapeutic Range (mg/L) | Analytical Range (mg/L) | Retention Time (min) | LLOQ (mg/L) | CV (%) | Bias (%) | Calibration Parameters | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Equation | SD Slope | SD Intercept | R2 | |||||||
| LEV | 12–46 | 1–50 | 4.6 | 1.06 ± 0.12 | 11.93 | 6.30 | Y = 0.0164x − 0.0027 | 0.0007 | 0.0036 | 0.9998 |
| ETH | 40–100 | 3–150 | 7.3 | 2.86 ± 0.46 | 17.54 | −4.57 | Y = 0.0033x + 0.0029 | 0.0001 | 0.0018 | 0.9993 |
| ZON | 10–40 | 1–50 | 9.7 | 0.94 ± 0.06 | 6.59 | −5.59 | Y = 0.0459x + 0.0063 | 0.0012 | 0.0054 | 0.9998 |
| PRM | 5–10 | 0.5–50 | 11.2 | 0.94 ± 0.03 | 3.98 | −6.31 | Y = 0.0321x + 0.0075 | 0.0057 | 0.0025 | 0.9998 |
| LAC | 10–20 | 1–50 | 11.7 | 0.97 ± 0.12 | 14.05 | −2.86 | Y = 0.0333x + 0.0148 | 0.0009 | 0.0038 | 0.9998 |
| FEL | 30–60 | 2–100 | 12.3 | 1.84 ± 0.12 | 7.21 | −8.21 | Y = 0.0254x + 0.0181 | 0.0002 | 0.0061 | 0.9997 |
| RUF | 30–40 | 1–50 | 12.7 | 0.96 ± 0.08 | 9.06 | −4.49 | Y = 0.0647x + 0.0197 | 0.0005 | 0.0087 | 0.9998 |
| LTG | 2.5–15 | 0.5–50 | 13.9 | 0.44 ± 0.04 | 10.12 | −11.55 | Y = 0.1213x + 0.0227 | 0.0024 | 0.0132 | 0.9998 |
| DIOL | NE * | 0.2–10 | 14.5 | 0.17 ± 0.01 | 9.25 | −13.00 | Y = 0.0846x + 0.0099 | 0.0022 | 0.0004 | 0.9994 |
| PHB | 10–40 | 1–50 | 15.2 | 0.97 ± 0.08 | 8.87 | −3.32 | Y = 0.0522x + 0.0072 | 0.0017 | 0.0041 | 0.9998 |
| MHD | 3–35 | 0.5–50 | 16.5 | 0.45 ± 0.04 | 10.71 | −9.64 | Y = 0.0859x + 0.0140 | 0.0045 | 0.0105 | 0.9998 |
| CBZ-E | NE * | 0.2–10 | 18.9 | 0.16 ± 0.01 | 4.96 | −19.69 | Y = 0.1173x + 0.0060 | 0.0033 | 0.0071 | 0.9996 |
| OXC | NE * | 0.1–10 | 19.8 | 0.11 ± 0.02 | 19.64 | 7.19 | Y = 0.0619x−0.0003 | 0.0089 | 0.0063 | 0.9995 |
| PHT | 10–20 | 1–50 | 20.9 | 0.91 ± 0.08 | 9.49 | −9.40 | Y = 0.0594x + 0.0103 | 0.0019 | 0.0057 | 0.9997 |
| CBZ | 4–12 | 0.5–50 | 21.4 | 0.44 ± 0.03 | 7.71 | −12.98 | Y = 0.0915x + 0.0144 | 0.0008 | 0.0071 | 0.9997 |
| Analyte | Cnominal (mg/L) | Intra-Day (n = 5) | Inter-Day (n = 15) | |||||
|---|---|---|---|---|---|---|---|---|
| Cmeasured (mg/L) | CV (%) | Bias (%) | Cmeasured (mg/L) | CV (%) | Bias (%) | |||
| QCL | 3 | 3.20 ± 0.02 | 0.74 | 6.76 | 3.03 ± 0.13 | 4.11 | 0.90 | |
| LEV | QCM | 15 | 15.09 ± 0.02 | 0.21 | 0.58 | 15.30 ± 0.39 | 2.53 | 1.98 |
| QCH | 45 | 45.54 ± 0.12 | 0.26 | 1.21 | 45.24 ± 0.28 | 0.62 | 0.54 | |
| QCL | 9 | 8.47 ± 0.13 | 1.48 | −5.86 | 8.73 ± 0.35 | 4.00 | −3.01 | |
| ETH | QCM | 45 | 42.71 ± 0.12 | 0.27 | −5.09 | 45.81 ± 2.70 | 5.09 | 1.79 |
| QCH | 135 | 135.72 ± 0.55 | 0.41 | 0.53 | 136.65 ± 1.53 | 1.12 | 1.22 | |
| QCL | 3 | 3.07 ± 0.01 | 0.10 | 2.42 | 3.07 ± 0.09 | 2.95 | 2.44 | |
| ZON | QCM | 15 | 15.15 ± 0.02 | 0.14 | 0.97 | 15.42 ± 0.45 | 2.91 | 2.81 |
| QCH | 45 | 45.37 ± 0.13 | 0.28 | 0.82 | 44.99 ± 0.33 | 0.98 | −0.02 | |
| QCL | 1.5 | 1.54 ± 0.01 | 0.7 | 2.72 | 1.53 ± 0.04 | 2.58 | 2.12 | |
| PRM | QCM | 15 | 15.21 ± 0.02 | 0.14 | 1.40 | 15.41 ± 0.37 | 2.38 | 2.75 |
| QCH | 45 | 45.44 ± 0.15 | 0.33 | 0.97 | 44.95 ± 0.44 | 0.98 | −0.11 | |
| QCL | 3 | 3.01 ± 0.03 | 1.15 | 0.28 | 3.08 ± 0.08 | 2.57 | 2.57 | |
| LAC | QCM | 15 | 15.14 ± 0.02 | 0.16 | 0.97 | 15.44 ± 0.47 | 3.01 | 2.90 |
| QCH | 45 | 45.41 ± 0.13 | 0.29 | 0.92 | 45.02 ± 0.33 | 0.73 | 0.04 | |
| QCL | 6 | 6.14 ± 0.01 | 0.23 | 2.32 | 6.18 ± 0.24 | 3.92 | 2.97 | |
| FEL | QCM | 30 | 30.41 ± 0.03 | 0.09 | 1.37 | 30.95 ± 0.86 | 2.77 | 3.16 |
| QCH | 90 | 90.74 ± 0.14 | 0.16 | 0.82 | 89.97 ± 0.64 | 0.71 | −0.03 | |
| QCL | 3 | 3.08 ± 0.02 | 0.61 | 2.71 | 3.09 ± 0.09 | 2.99 | 3.16 | |
| RUF | QCM | 15 | 15.14 ± 0.03 | 0.18 | 0.96 | 15.43 ± 0.48 | 3.09 | 2.92 |
| QCH | 45 | 45.38 ± 0.14 | 0.31 | 0.85 | 45.04 ± 0.29 | 0.64 | 0.12 | |
| QCL | 1.5 | 1.55 ± 0.005 | 0.32 | 2.95 | 1.54 ± 0.04 | 2.42 | 2.42 | |
| LTG | QCM | 15 | 15.15 ± 0.02 | 0.16 | 1.02 | 15.43 ± 0.43 | 2.78 | 2.86 |
| QCH | 45 | 45.46 ± 0.14 | 0.32 | 1.02 | 45.04 ± 0.36 | 0.80 | 0.09 | |
| QCL | 0.6 | 0.56 ± 0.04 | 7.64 | −5.97 | 0.60 ± 0.02 | 3.22 | −0.75 | |
| DIOL | QCM | 3 | 3.00 ± 0.08 | 2.59 | −0.09 | 3.07 ± 0.11 | 3.61 | 2.45 |
| QCH | 9 | 9.08 ± 0.11 | 1.21 | 0.90 | 9.05 ± 0.06 | 0.65 | 0.54 | |
| QCL | 3 | 3.04 ± 0.01 | 0.46 | 1.17 | 3.54 ± 0.08 | 2.52 | 2.39 | |
| PHB | QCM | 15 | 15.17 ± 0.05 | 0.34 | 1.12 | 15.46 ± 0.5 | 3.23 | 3.08 |
| QCH | 45 | 45.34 ± 0.12 | 0.27 | 0.75 | 45.06 ± 0.25 | 0.55 | 0.13 | |
| QCL | 1.5 | 3.08 ± 0.005 | 0.17 | 2.67 | 3.07 ± 0.04 | 2.71 | 2.40 | |
| MHD | QCM | 15 | 15.22 ± 0.02 | 0.16 | 1.44 | 15.46 ± 0.44 | 2.86 | 3.30 |
| QCH | 45 | 45.44 ± 0.14 | 0.30 | 0.97 | 45.00 ± 0.38 | 0.85 | −0.01 | |
| QCL | 0.6 | 0.58 ± 0.10 | 3.12 | 3.09 | 0.57 ± 0.023 | 4.09 | −5.09 | |
| CBZ-E | QCM | 3 | 2.98 ± 0.08 | 0.55 | −0.02 | 3.03 ± 0.065 | 2.16 | 0.89 |
| QCH | 9 | 9.12 ± 0.21 | 0.47 | 1.29 | 9.01 ± 0.090 | 1.00 | 0.13 | |
| QCL | 0.3 | 0.29 ± 0.16 | 10.57 | 0.98 | 0.30 ± 0.01 | 4.10 | −0.63 | |
| OXC | QCM | 3 | 2.83 ± 0.44 | 3.09 | −5.67 | 2.94 ± 0.09 | 3.32 | −2.11 |
| QCH | 9 | 9.36 ± 1.98 | 4.23 | 3.96 | 9.05 ± 0.27 | 2.94 | 0.56 | |
| QCL | 3 | 3.07 ± 0.02 | 0.75 | 2.49 | 3.07 ± 0.09 | 2.81 | 2.35 | |
| PHT | QCM | 15 | 15.19 ± 0.04 | 0.26 | 1.27 | 15.49 ± 0.48 | 3.11 | 3.30 |
| QCH | 45 | 45.26 ± 0.15 | 0.32 | 0.58 | 44.95 ± 0.27 | 0.61 | −0.12 | |
| QCL | 1.5 | 1.55 ± 0.01 | 0.87 | 3.08 | 1.54 ± 0.05 | 3.26 | 2.52 | |
| CBZ | QCM | 15 | 15.15 ± 0.02 | 0.14 | 1.02 | 15.45 ± 0.48 | 3.09 | 2.99 |
| QCH | 45 | 45.33 ± 0.15 | 0.34 | 0.74 | 44.92 ± 0.37 | 0.82 | −0.18 | |
| Analyte | Recovery | Autosampler Stability | Stock Solution | Freeze and Thaw Stability | Short Term Stability | Long-Term Stability |
|---|---|---|---|---|---|---|
| LEV | 96.2 ± 3.3 | 100.6 ± 2.1 | 102.3 ± 4.2 | 100.3 ± 0.9 | 101.3 ± 2.1 | 101.4 ± 1.8 |
| ETH | 93.4 ± 4.3 | 98.9 ± 1.1 | 101.6 ± 6.3 | 104.6 ± 3.9 | 105.7 ± 2.2 | 103.1 ± 4.9 |
| ZON | 99.7 ± 0.7 | 100.6 ± 0.4 | 101.4 ± 1.3 | 99.9 ± 0.2 | 101.0 ± 0.7 | 99.2 ± 6.3 |
| PRM | 99.8 ± 1.1 | 100.7 ± 0.5 | 101.7 ± 0.8 | 99.6 ± 0.3 | 100.9 ± 0.7 | 98.8 ± 6.6 |
| LAC | 101.4 ± 1.0 | 100.4 ± 0.5 | 100.2 ± 0.5 | 100.1 ± 0.4 | 100.9 ±0.8 | 98.7 ± 5.1 |
| FEL | 100.9 ± 0.7 | 100.8 ± 0.4 | 101.3 ± 1.3 | 99.1 ± 0.9 | 100.9 ± 0.7 | 98.1 ± 5.3 |
| RUF | 100.4 ± 0.4 | 100.1 ± 0.8 | 101.6 ± 2.1 | 100.2 ± 0.5 | 101.0 ± 0.8 | 100.2 ± 6.5 |
| LTG | 100.2 ± 0.2 | 100.4 ± 0.4 | 100.5 ± 1.8 | 99.3 ± 0.5 | 100.9 ± 0.7 | 98.0 ± 5.8 |
| DIOL | 102.2 ± 1.8 | 101.8 ± 2.4 | 102.1 ± 4.2 | 102.8 ± 3.4 | 102.1 ± 2.7 | 99.0 ± 4.9 |
| PHB | 100.4 ± 0.5 | 101.0 ± 1.1 | 100.8 ± 1.3 | 99.4 ± 0.6 | 100.4 ± 1.0 | 98.9 ± 6.0 |
| MHD | 99.8 ± 0.4 | 100.2 ± 0.4 | 101.5 ± 1.3 | 99.9 ± 0.02 | 100.6 ± 0.7 | 99.0 ± 6.1 |
| CBZ-E | 99.6 ± 0.6 | 100.2 ± 1.0 | 100.2 ± 1.3 | 101.7 ± 2.7 | 101.8 ± 0.9 | 101.8 ± 7.2 |
| OXC | 83.9 ± 5.2 | 91.4 ± 3.5 | 95.5 ± 2.5 | 95.3 ± 1.6 | 98.5 ± 4.3 | 101.6 ± 6.2 |
| PHT | 99.7 ± 0.8 | 101.9 ± 1.7 | 102.3 ± 2.2 | 100.8 ± 1.7 | 101.3 ± 0.4 | 99.4 ± 7.1 |
| CBZ | 99.6 ± 1.4 | 100.8 ± 0.9 | 101.6 ± 1.4 | 99.6 ± 0.5 | 100.7 ± 0.6 | 99.5 ± 6.9 |
| IS | 100.2 ± 1.6 | 100.8 ± 3.3 | 103.1 ± 1.3 | 97.8 ± 1.4 | 99.5 ± 4.0 | 98.0 ± 0.6 |
| ID | Therapy (mg/day) | Measured Concentration (mg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTG | LEV | OXC | MHD | DIOL | CBZ | CBZ-E | ETH | PHB | PHT | LAC | ZON | ||
| 1 | OXC (450) LEV (1050) ETH (475) | / | 25 | 0.4 | 12 | 2.7 | / | / | 52 | / | / | / | / |
| 2 | OXC (450) Vigabatrin (500) | / | / | 0.3 | 8.7 | 1.2 | / | / | / | / | / | / | / |
| 3 | OXC (450) Topiramate (200) | / | / | 1.7 | 14 | 1.5 | / | / | / | / | / | / | / |
| 4 | LTG(400) OXC (1200) PHB (50) | 4.6 | / | 0.3 | 11 | 0.6 | / | / | / | 2.2 | / | / | / |
| 5 | LTG (400) OXC (1200) | 2.2 | / | 0.4 | 8.8 | 0.2 | / | / | / | / | / | / | / |
| 6 | LTG (600) PHT (300) Pregabaline (150) | 4.2 | / | / | / | / | / | / | / | / | 1.2 | / | / |
| 7 | LTG (400) PHT (100) | 2.8 | / | / | / | / | / | / | / | / | 1.1 | / | / |
| 8 | LTG (150) ZON (300) Valproic acid (750) | 5.6 | / | / | / | / | / | / | / | / | / | / | 8.3 |
| 9 | LTG (400) LAC (200) | 5.9 | / | / | / | / | / | / | / | / | / | 4.4 | / |
| 10 | LTG (300) CBZ(1200) | 2.1 | / | / | / | 3.9 | 8.0 | 1.1 | / | / | / | / | / |
| 11 | LTG (300) LEV (3500) Clonazepam (1) | 2.8 | 39.7 | / | / | / | / | / | / | / | / | / | / |
| 12 | LTG (250) PHB (250) | 0.9 | / | / | / | / | / | / | / | 7.6 | / | / | / |
| 13 | LTG (300) CBZ (800) | 1.4 | / | / | / | 0.7 | 3.9 | 0.3 | / | / | / | / | / |
| Time (min) | % KH2PO4 (25 mM; pH 5.1) | % Methanol |
|---|---|---|
| 0 | 75 | 25 |
| 5 | 70 | 30 |
| 10 | 60 | 40 |
| 15 | 58 | 42 |
| 19 | 30 | 70 |
| 23 | 30 | 70 |
| 23.1 | 75 | 25 |
| 27 | 75 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosheska, D.; Roškar, R. Simple HPLC-UV Method for Therapeutic Drug Monitoring of 12 Antiepileptic Drugs and Their Main Metabolites in Human Plasma. Molecules 2023, 28, 7830. https://doi.org/10.3390/molecules28237830
Milosheska D, Roškar R. Simple HPLC-UV Method for Therapeutic Drug Monitoring of 12 Antiepileptic Drugs and Their Main Metabolites in Human Plasma. Molecules. 2023; 28(23):7830. https://doi.org/10.3390/molecules28237830
Chicago/Turabian StyleMilosheska, Daniela, and Robert Roškar. 2023. "Simple HPLC-UV Method for Therapeutic Drug Monitoring of 12 Antiepileptic Drugs and Their Main Metabolites in Human Plasma" Molecules 28, no. 23: 7830. https://doi.org/10.3390/molecules28237830
APA StyleMilosheska, D., & Roškar, R. (2023). Simple HPLC-UV Method for Therapeutic Drug Monitoring of 12 Antiepileptic Drugs and Their Main Metabolites in Human Plasma. Molecules, 28(23), 7830. https://doi.org/10.3390/molecules28237830