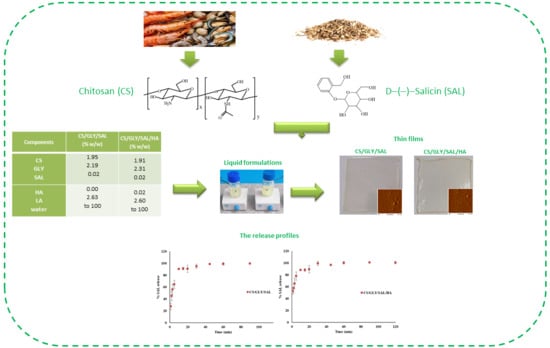
Physical Properties and Release Profiles of Chitosan Mixture Films Containing Salicin, Glycerin and Hyaluronic Acid
Abstract
:1. Introduction
2. Results and Discussion
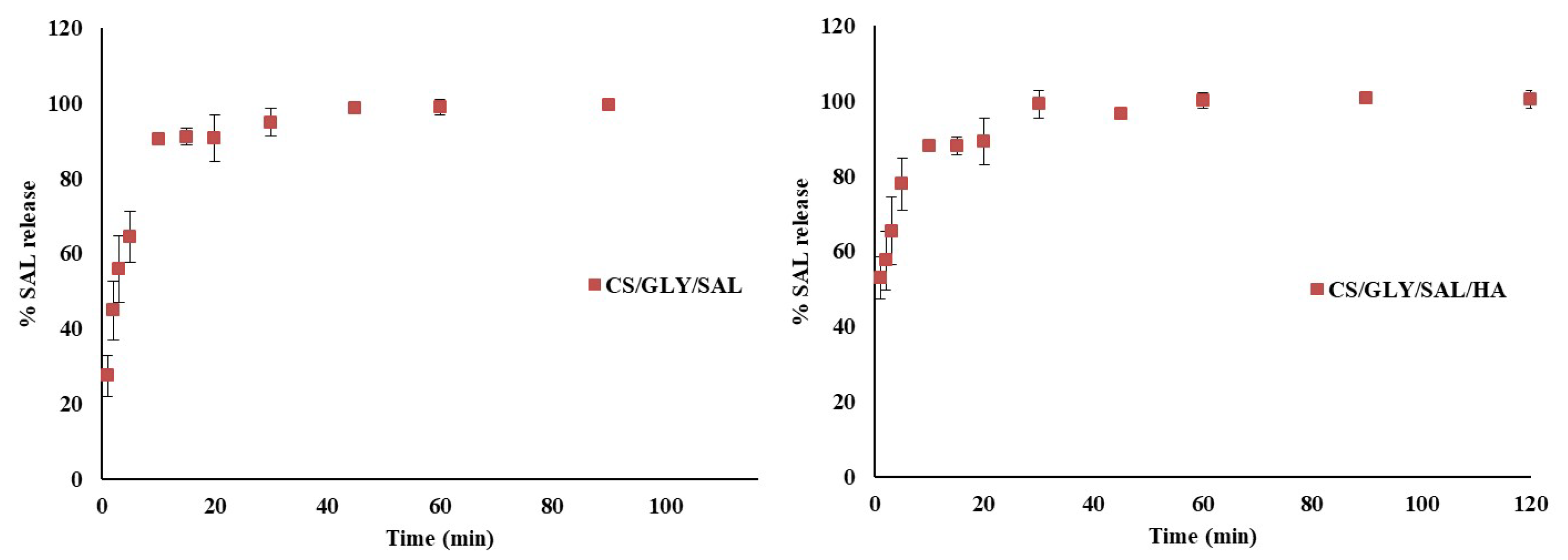
2.1. Release Process of Salicin
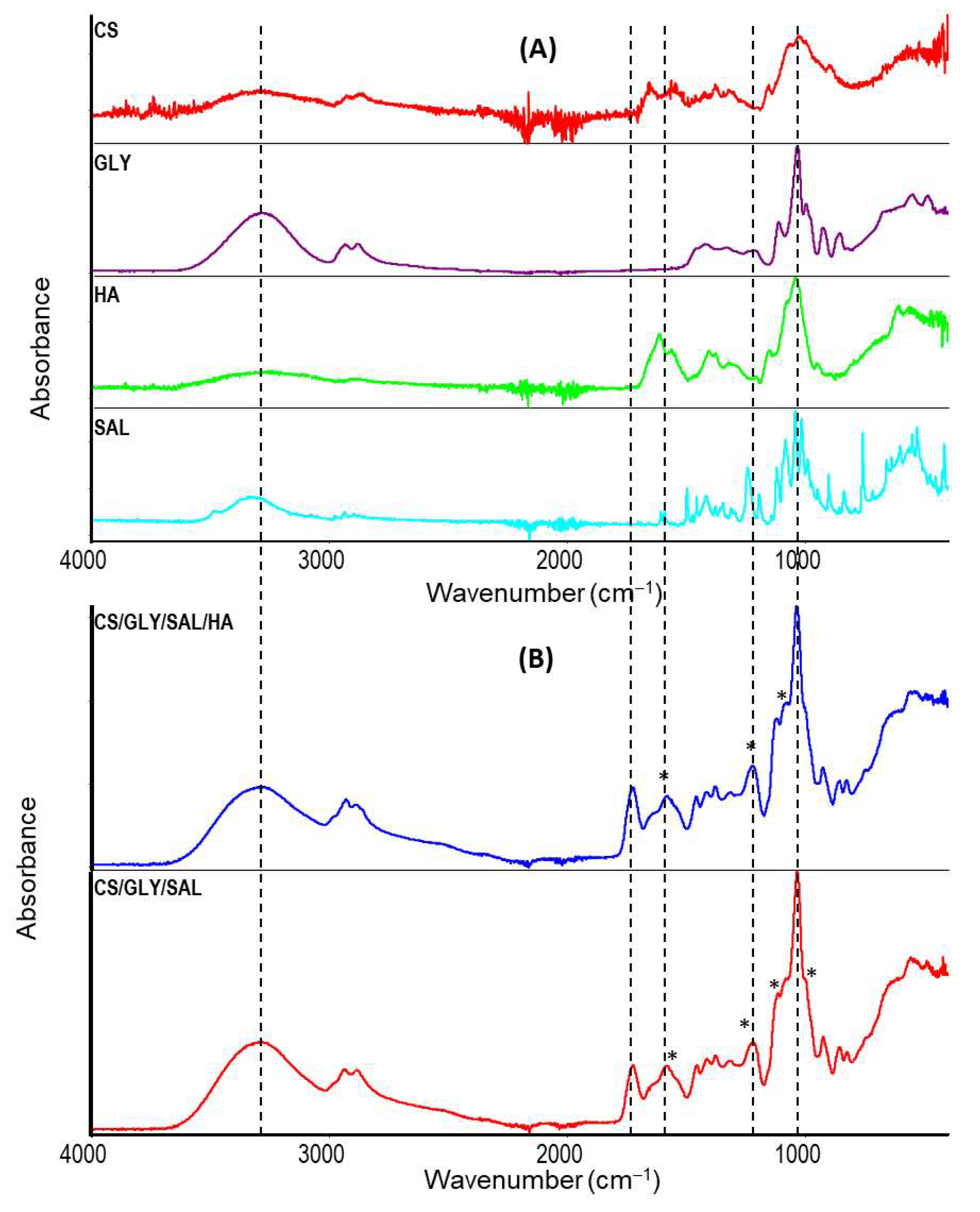
2.2. Infrared Spectroscopy
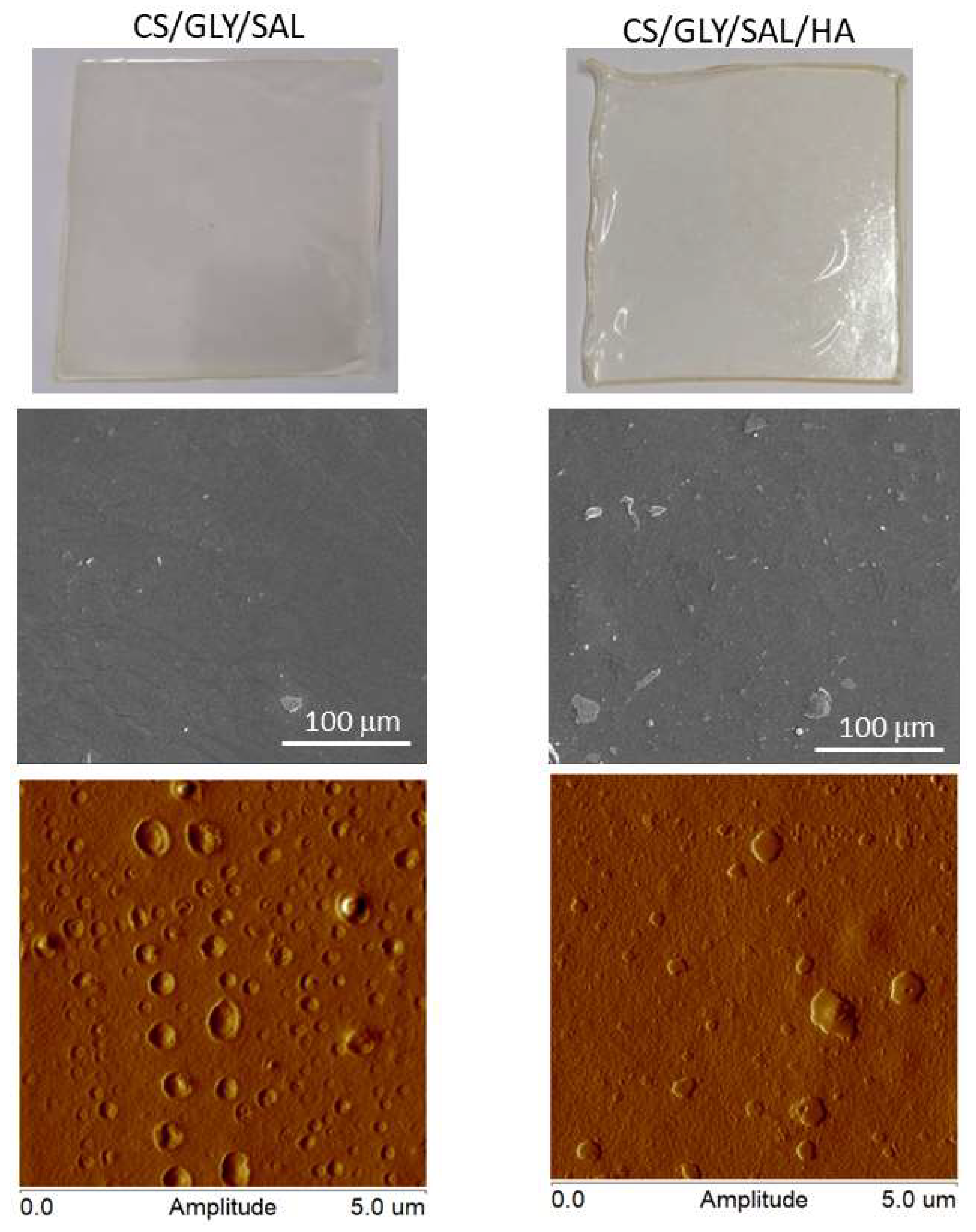
2.3. Morphology
2.4. Thermogravimetric Analysis
3. Materials and Methods
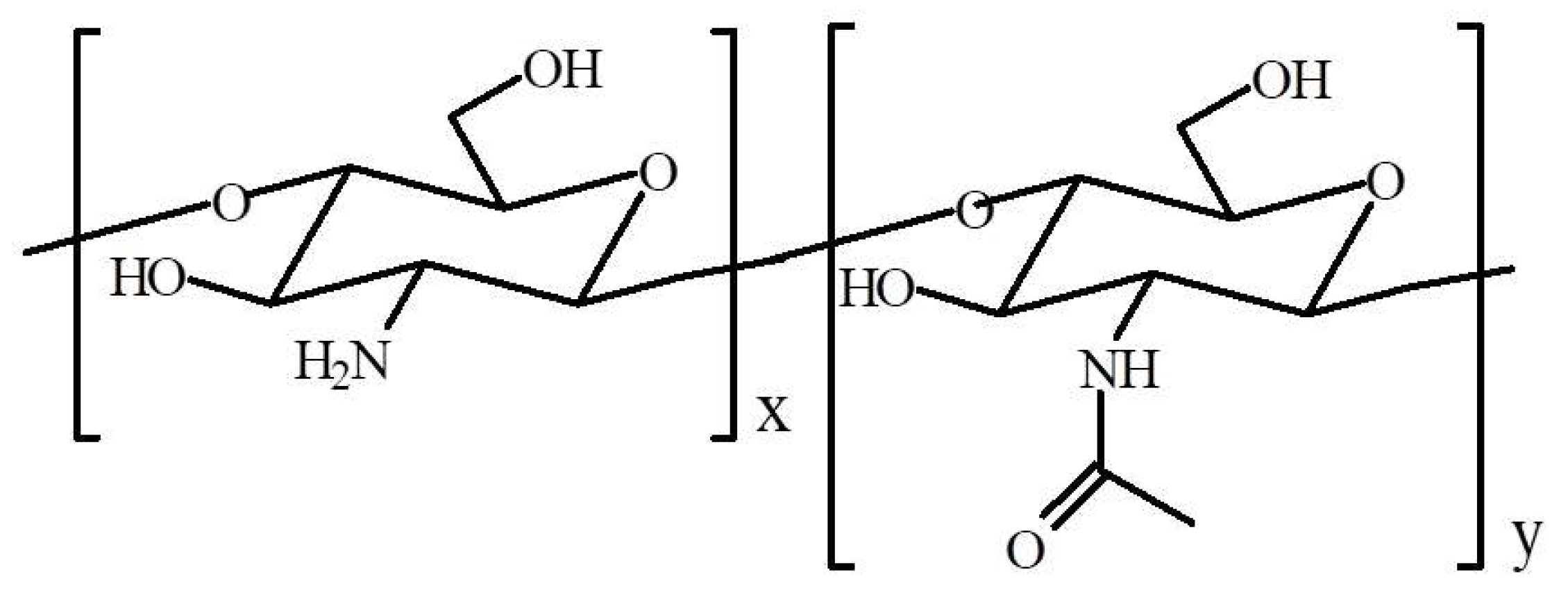
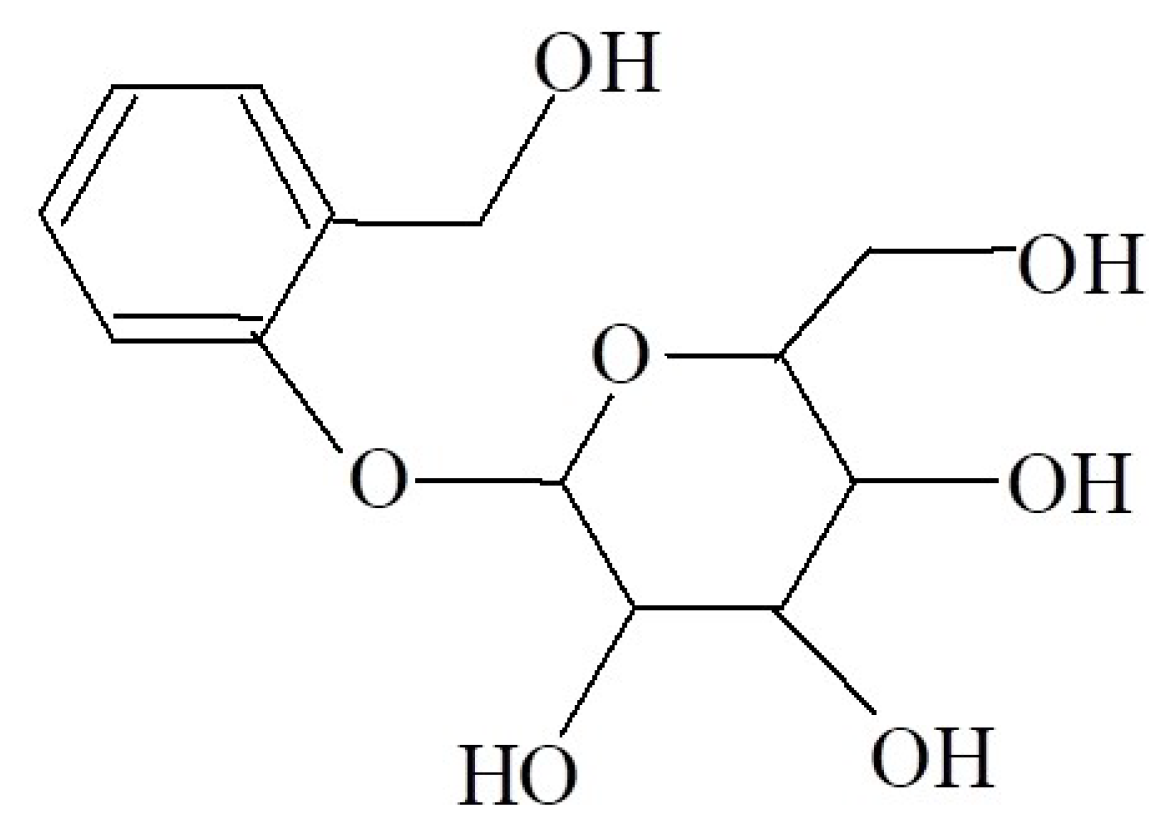
3.1. Materials
3.2. Preparation of CS Films
3.3. SAL Release Behavior
3.4. Infrared Spectroscopy
3.5. Morphological Properties
3.6. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurakula, M.; Raghavendra, N.N. Review Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; El Seoud, O.A. Sustainable biomaterials based on cellulose, chitin and chitosan composites—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Barbosa de Lima, M.A.; de Oliveira Franco, L.; de Campos-Takaki, G.M. Review: Seafood waste as attractive source of chitin and chitosan production and applications. Int. J. Mol. Sci. 2020, 21, 42902. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films. Effect of the type of solvent acid. LWT Food Sci. Technol. 2021, 135, 109984. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Valachova, K.; Soltes, L. Review: Versatile use of chitosan and hyaluronan in medicine. Molecules 2021, 26, 1195. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Luo, Y. A review on the preparation and characterization of chitosan-clay nanocomposite films and coatings for food packaging applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100102. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Górak, A. New ionic liquids for modification of chitin particles. Res. Chem. Intermed. 2018, 44, 4841–4854. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Szulc, M.; Lewandowska, K. Biomaterials based on chitosan and its derivatives and their potential in tissue engineering and other biomedical applications—A review. Molecules 2023, 28, 247. [Google Scholar] [CrossRef] [PubMed]
- Hiorth, M.; Nilsen, S.; Tho, I. Bioadhesive Mini-Tablets for Vaginal Drug Delivery. Pharmaceutics 2014, 6, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kaur, N.; Kaur, R.; Kaur, R. A review on valorization of chitinous waste. J. Polym. Res. 2021, 28, 406. [Google Scholar] [CrossRef]
- Sari, M.; Tamrin; Kaban, J.; Alfian, Z. A novel composite membrane pectin from Cyclea Barbata Miers blend with chitosan for accelerated wound healing. Polym. Test. 2021, 99, 107207. [Google Scholar] [CrossRef]
- Barbosa, R.; Villarreal, A.; Rodrigues, C.; De Leon, H.; Gilkerson, R.; Lozano, K. Aloe Vera extract-based composite nanofibers for wound dressing applications. Mater. Sci. Eng. C 2021, 124, 112061. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruszka, J.; Wiśniewska-Wrona, M. Chitin and chitosan as polymers of the future—Obtaining, modification, life cycle assessment and main directions of application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Jummaat, F.; Yahya, E.B.; Khalil, A.; Adnan, A.S.; Alqadhi, A.M.; Abdullah, C.K.; Sofea, A.; Olaiya, N.G.; Abdat, M. The role of biopolymer-based materials in obstetrics and gynecology applications: A review. Polymers 2021, 13, 633. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Polymers 2022, 27, 94. [Google Scholar] [CrossRef] [PubMed]
- Rudeekulthamrong, P.; Kaulpiboon, J. Application of amylomaltase for the synthesis of salicin-α-glucosides as efficient anticoagulant and anti-inflammatory agents. Carbohydr. Res. 2016, 432, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Peng, X.; Zheng, Y.; Cheng, Z.; Sun, S.; Ding, Q.; Liu, W.; Ding, C. A poloxamer/hyaluronic acid/chitosan-based thermosensitive hydrogel that releases dihydromyricetin to promote wound healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Guo, C.; Liao, W.; Liu, X.; Wang, Q. Development and characterization of chitosan/bacterial cellulose/pullulan bilayer film with sustained release curcumin. Int. J. Biol. Macromol. 2023, 226, 301–311. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, J.; Zhong, Z. Preparation and characterization of long-term antibacterial and pH-responsive Polylactic acid/Octenyl succinic anhydride-chitosan @ tea tree oil microcapsules. Int. J. Biol. Macromol. 2022, 220, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Shafreen, M.M.; Achudhan, A.B.; Gupta, A.; Saleena, L.M. A review on applications of β-glucosidase in food, brewery, pharmaceutical and cosmetic industries. Carbohydr. Res. 2023, 530, 108855. [Google Scholar] [CrossRef] [PubMed]
- Hedner, T.; Everts, B. The early clinical history of salicylates in rheumatology and pain. Clin. Rheumatol. 1998, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Ludtke, R.; Selbmann, H.K.; Kotter, I.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo-controlled, double blind clinical trial. Phytother. Res. 2001, 15, 344–350. [Google Scholar] [CrossRef]
- Ishikado, A.; Sono, Y.; Matsumoto, M.; Stubbs, S.R.; Okuno, A.; Goto, M.; King, G.L.; Blackwell, T.K.; Makino, T. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radic. Biol. Med. 2013, 65, 1506–1515. [Google Scholar] [CrossRef]
- Willow bark: Salicis cortex. In European Pharmacopoeia 5.2; Conseil d’Europe: Strasbourg, France, 2005; pp. 2702–2703.
- Guo, M.-M.; Xue, W.-T.; Liao, L.-Y.; Ling, X.; Yu, D.; Lan, X.-L.; Zhou, W.-Q.; Li, L. Anti-allergic activity of natural plant products for the treatment of sensitive skin: A review. Pharmacol. Res.-Mod. Chin. Med. 2022, 3, 100117. [Google Scholar] [CrossRef]
- Gopaul, R.; Knaggs, H.E.; Lephart, J.F.; Holley, K.C.; Gibson, E.M. An evaluation of the effect of a topical product containing salicin on the visible signs of human skin aging. J. Cosmet. Dermatol. 2010, 9, 196–201. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Kurzawa, M. Chitosan-based films containing rutin for potential cosmetic applications. Polymers 2023, 15, 3224. [Google Scholar] [CrossRef]
- Alani, S.N.; AlMeani, S.A.L. Inhibition of Streptococcus Agalactiae Biofilm Formation in Response to Purified Phytochemical Antimicrobial Materials. J. Pharm. Negat. Results 2022, 13, 601–607. [Google Scholar] [CrossRef]
- Dou, J.; Ilina, P.; Cruz, C.D.; Nurmi, D.; Zegarra Vidarte, P.; Rissanen, M.; Tammela, P.; Vuorinen, T. Willow bark-derived material with antibacterial and antibiofilm properties for potential wound dressing applications. J. Agric. Food Chem. 2023, 71, 16554–16567. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Khatoon, F.; Ahmed, S. An overview on wounds their issues and natural remedies for wound healing. Biochem. Physiol. 2015, 4, 165. [Google Scholar]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B.; Michalska, M. The miscibility of collagen/hyaluronic acid/chitosan blends investigated in dilute solutions and solids. J. Mol. Liq. 2016, 220, 726–730. [Google Scholar] [CrossRef]
- Ritthidej, G.C.; Phaechamud, T.; Koizumi, T. Moist heat treatment on physicochemical change of chitosan salt films. Int. J. Pharm. 2002, 232, 11–22. [Google Scholar] [CrossRef]
- Melro, E.; Antunes, F.E.; da Silva, G.J.; Cruz, I.; Ramos, P.E.; Carvalho, F.; Alves, I. Chitosan films in food applications. Tuning film properties by changing acidic dissolution conditions. Polymers 2021, 13, 1. [Google Scholar] [CrossRef]
- Kim, K.M.; Son, J.H.S.; Kim, S.-K. Properties of chitosan films as a function of pH and solvent type. J. Food Sci. E 2006, 71, 119–124. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska, S. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Albu, M.G.; Vuluga, Z.; Panaitescu, D.M.; Vuluga, D.M.; Căşărică, A.; Ghiurea, M. Morphology and thermal stability of bacterial cellulose/ collagen composites. Cent. Eur. J. Chem. 2014, 12, 968–975. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Sionkowska, A.; Lewandowska, K.; Brudzyńska, P.; Szulc, M.; Saha, N.; Saha, T.; Saha, P. Chitosan modified by kombucha-derived bacterial cellulose: Rheological behavior and properties of convened biopolymer films. Polymers 2022, 14, 4572. [Google Scholar] [CrossRef]
- Bohdanecký, M.; Kovář, I. Viscosity of Polymer Solution; Jenkins, A.D., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1982; Volume 2, pp. 167–186. [Google Scholar]
- Roberts, G.A.F.; Domoszy, J.G. Determination of viscometric constants for chitosan. Int. J. Biol. Macromol. 1982, 4, 374–377. [Google Scholar] [CrossRef]
- Garcia-Abuin, A.; Gomez-Diaz, D.; Navaza, J.M.; Regueiro, L.; Vidal-Tato, I. Viscosimetric behaviour of hyaluronic acid in different aqueous solutions. Carbohydr. Polym. 2011, 85, 500–505. [Google Scholar] [CrossRef]
- Huang, J.; Jacobsen, J.; Genina, N.; Larsen, S.W.; Nielsen, H.M.; Müllertz, A.; Mu, H. Investigating the effect of graphene oxide in chitosan/alginate-based foams on the release and antifungal activity of clotrimazole in vitro. Eur. J. Pharm. Sci. 2022, 174, 106204. [Google Scholar] [CrossRef] [PubMed]
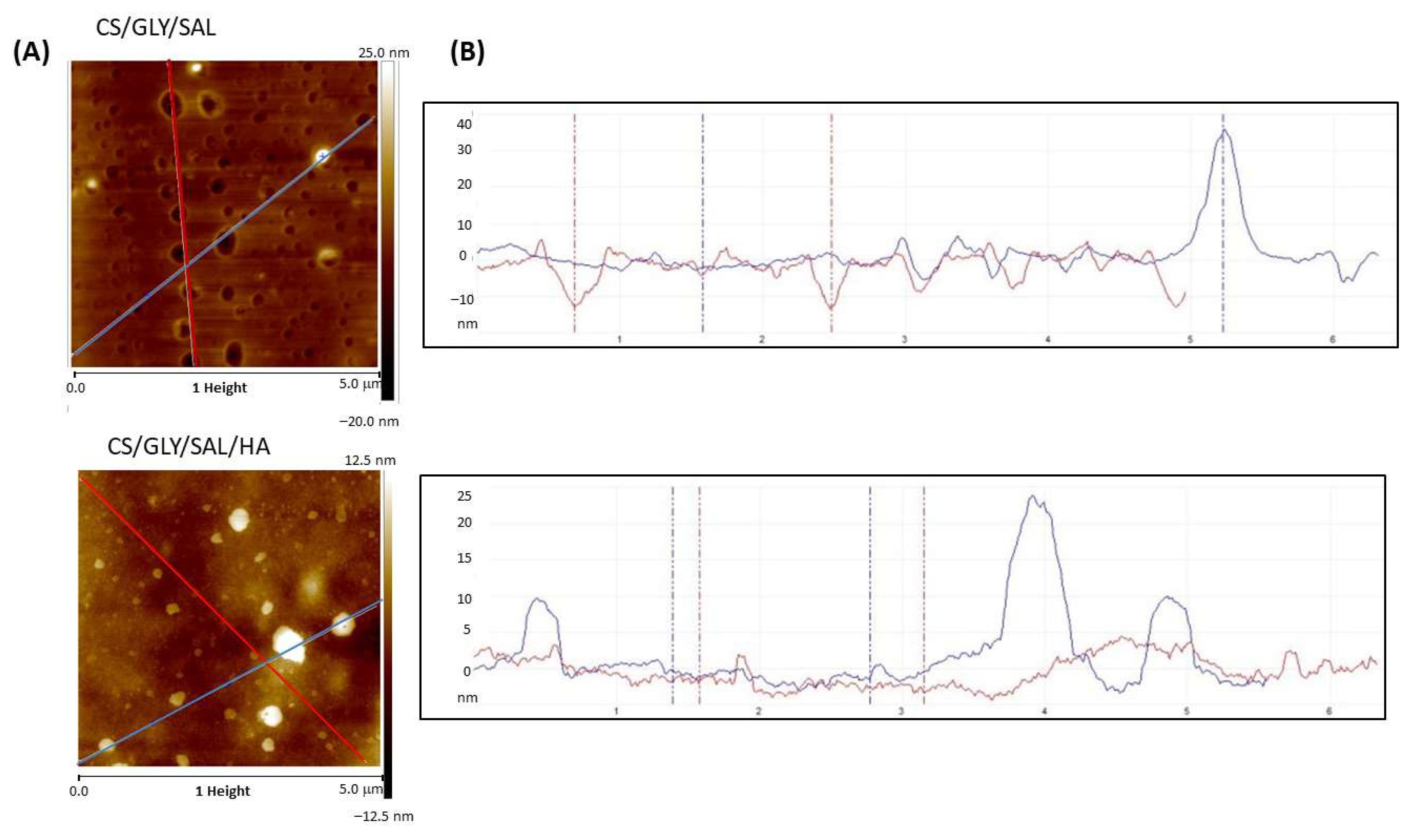

| Sample | Rq (nm) | Ra (nm) | Th (mm) |
|---|---|---|---|
| CS/GLY/SAL | 2.84 ± 0.12 | 1.90 ± 0.10 | 0.063 ± 0.002 |
| CS/GLY/SAL/HA | 2.86 ± 0.30 | 1.93 ± 0.28 | 0.070 ± 0.004 |
| Components | CS/GLY/SAL (% w/w) | CS/GLY/SAL/HA (% w/w) |
|---|---|---|
| CS | 1.95 | 1.91 |
| GLY | 2.19 | 2.31 |
| SAL | 0.02 | 0.02 |
| HA | 0.00 | 0.02 |
| LA | 2.63 | 2.60 |
| water | to 100 | to 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, K.; Sionkowska, A.; Kurzawa, M. Physical Properties and Release Profiles of Chitosan Mixture Films Containing Salicin, Glycerin and Hyaluronic Acid. Molecules 2023, 28, 7827. https://doi.org/10.3390/molecules28237827
Lewandowska K, Sionkowska A, Kurzawa M. Physical Properties and Release Profiles of Chitosan Mixture Films Containing Salicin, Glycerin and Hyaluronic Acid. Molecules. 2023; 28(23):7827. https://doi.org/10.3390/molecules28237827
Chicago/Turabian StyleLewandowska, Katarzyna, Alina Sionkowska, and Marzanna Kurzawa. 2023. "Physical Properties and Release Profiles of Chitosan Mixture Films Containing Salicin, Glycerin and Hyaluronic Acid" Molecules 28, no. 23: 7827. https://doi.org/10.3390/molecules28237827
APA StyleLewandowska, K., Sionkowska, A., & Kurzawa, M. (2023). Physical Properties and Release Profiles of Chitosan Mixture Films Containing Salicin, Glycerin and Hyaluronic Acid. Molecules, 28(23), 7827. https://doi.org/10.3390/molecules28237827