Insight into Organization of Gliadin and Glutenin Extracted from Gluten Modified by Phenolic Acids
Abstract
:1. Introduction
2. Results
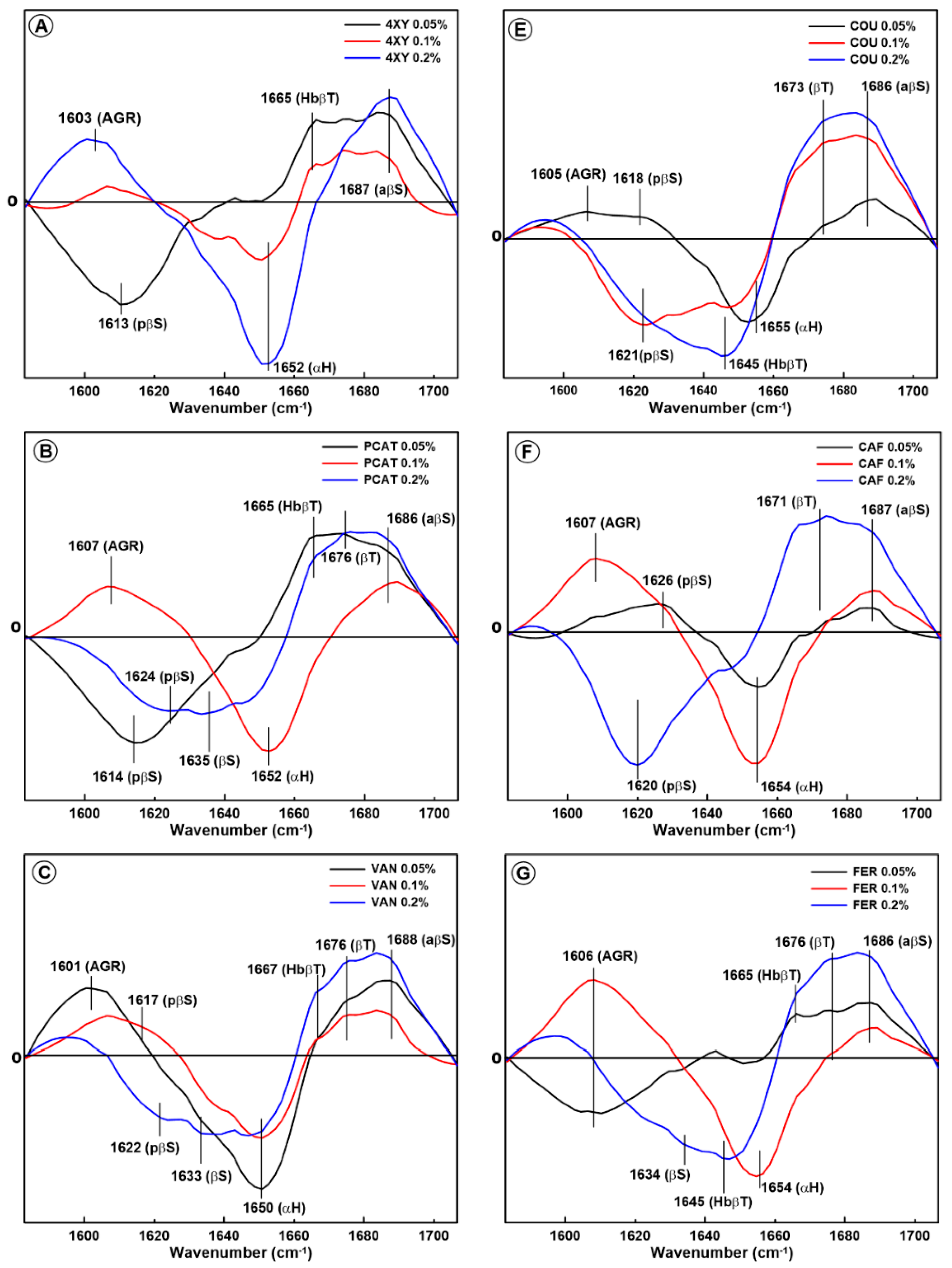
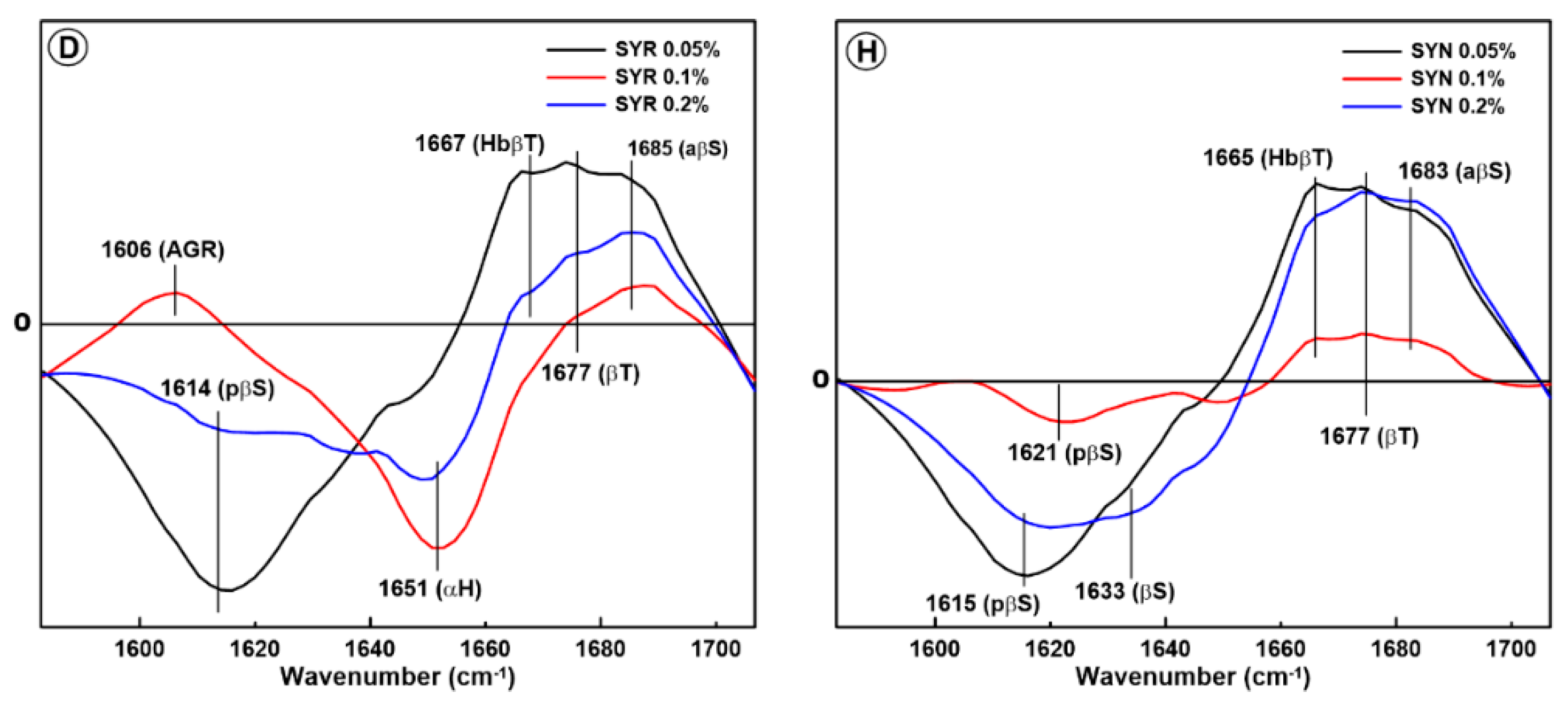
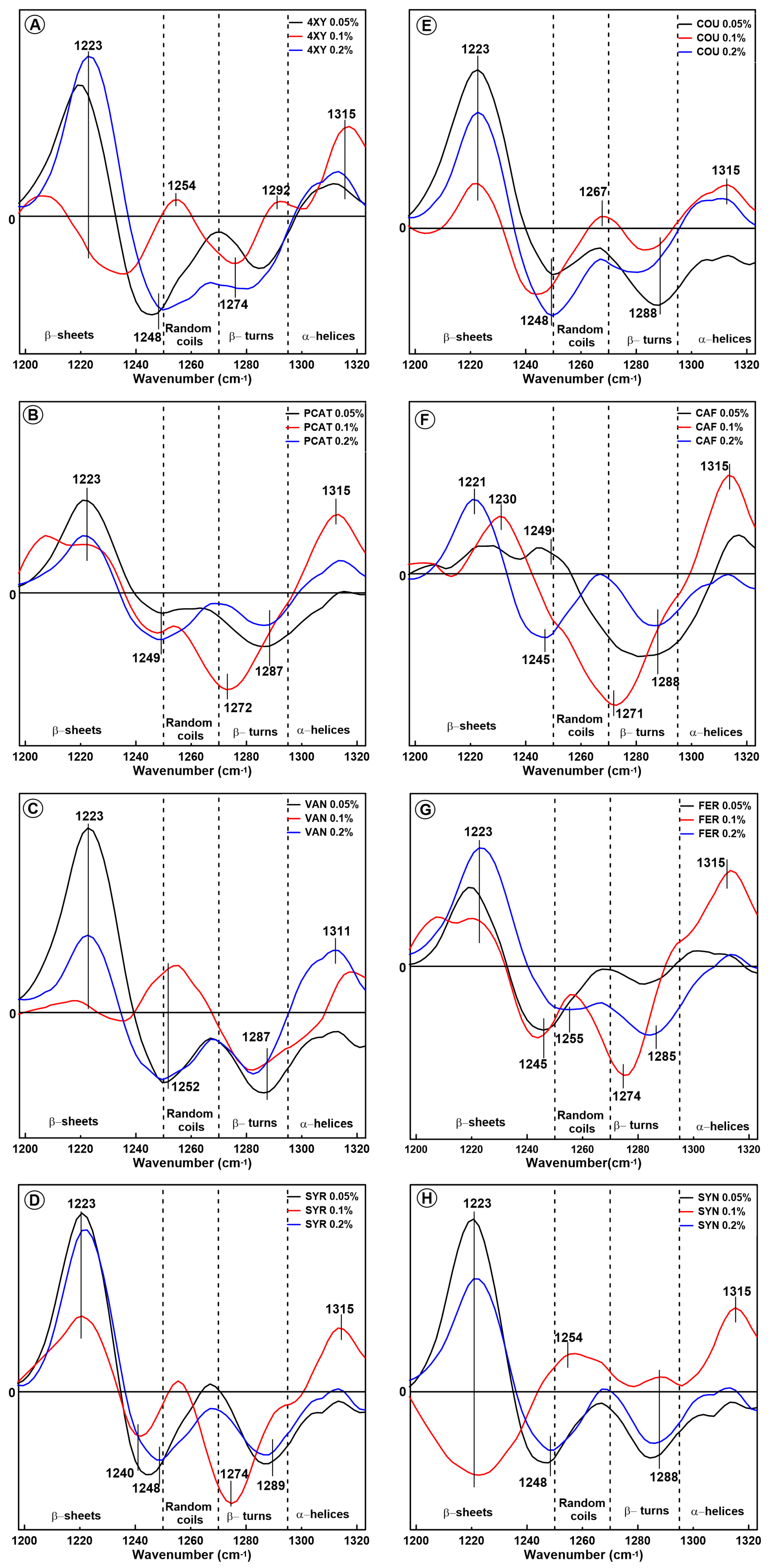
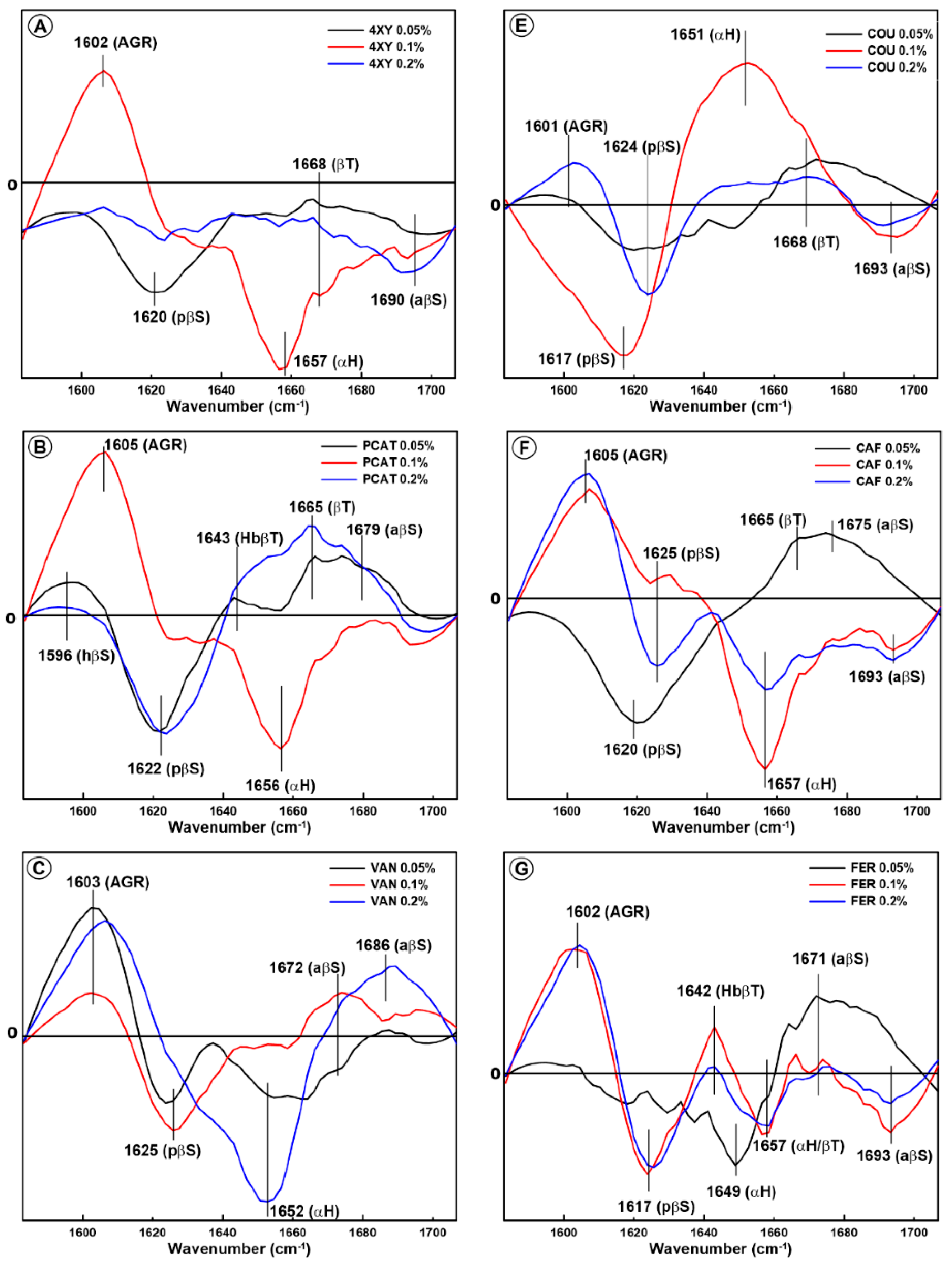
2.1. Changes in the Secondary Structure of Gliadin
2.2. Changes in the Secondary Structure of Glutenin
3. Materials and Methods
3.1. Materials
3.2. Dough Preparation
3.3. Fourier Transform Infrared Spectra (FT-IR)
3.4. Gliadins and Glutenins Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barak, S.; Mudgil, D.; Khatkar, B.S. Biochemical and Functional Properties of Wheat Gliadins: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of Wheat Gluten Proteins: Qualitative Composition. Cereal Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effects of Physical and Chemical Factors on the Structure of Gluten, Gliadins and Glutenins as Studied with Spectroscopic Methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Lafiandra, D. Wheat Glutenin Polymers 1. Structure, Assembly and Properties. J. Cereal Sci. 2022, 106, 103486. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The Structure and Properties of Gluten: An Elastic Protein from Wheat Grain. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Taddei, P.; Zanna, N.; Tozzi, S. Raman Characterization of the Interactions between Gliadins and Anthocyanins. J. Raman Spectrosc. 2013, 44, 1435–1439. [Google Scholar] [CrossRef]
- Waga, J. Structure and Allergenicity of Wheat Gluten Proteins—A Review. Pol. J. Food Nutr. Sci. 2004, 54, 327–338. [Google Scholar]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Richard, T.; Lefeuvre, D.; Descendit, A.; Quideau, S.; Monti, J.P. Recognition Characters in Peptide–Polyphenol Complex Formation. Biochim. Biophys. Acta BBA Gen. Subj. 2006, 1760, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Ptaszyńska, A.A.; Kowalski, R.; Waśko, P.; Gruszecki, W.I. Influence of Dietary Fibre on Gluten Proteins Structure—A Study on Model Flour with Application of FT-Raman Spectroscopy. J. Raman Spectrosc. 2015, 46, 309–316. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc-Stanowska, R.; Nawrocka, A. Changes in the Conformation and Biochemical Properties of Gluten Network after Phenolic Acid Supplementation. J. Cereal Sci. 2023, 110, 103651. [Google Scholar] [CrossRef]
- Krekora, M.; Szymańska-Chargot, M.; Niewiadomski, Z.; Miś, A.; Nawrocka, A. Effect of Cinnamic Acid and Its Derivatives on Structure of Gluten Proteins—A Study on Model Dough with Application of FT-Raman Spectroscopy. Food Hydrocoll. 2020, 107, 105935. [Google Scholar] [CrossRef]
- Welc, R.; Kłosok, K.; Szymańska-Chargot, M.; Nawrocka, A. Effect of Chemical Structure of Selected Phenolic Acids on the Structure of Gluten Proteins. Food Chem. 2022, 389, 133109. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Exploring the Interactions between Blackcurrant Polyphenols, Pectin and Wheat Biopolymers in Model Breads; a FTIR and HPLC Investigation. Food Chem. 2012, 131, 802–810. [Google Scholar] [CrossRef]
- Tozzi, S.; Zanna, N.; Taddei, P. Study on the Interaction between Gliadins and a Coumarin as Molecular Model System of the Gliadins–Anthocyanidins Complexes. Food Chem. 2013, 141, 3586–3597. [Google Scholar] [CrossRef]
- Welc-Stanowska, R.; Kłosok, K.; Nawrocka, A. Effects of Gluten-Phenolic Acids Interaction on the Gluten Structure and Functional Properties of Gluten and Phenolic Acids. J. Cereal Sci. 2023, 111, 103682. [Google Scholar] [CrossRef]
- Anderle, G.; Mendelsohn, R. Thermal Denaturation of Globular Proteins. Fourier Transform-Infrared Studies of the Amide III Spectral Region. Biophys. J. 1987, 52, 69–74. [Google Scholar] [CrossRef]
- Georget, D.M.R.; Belton, P.S. Effects of Temperature and Water Content on the Secondary Structure of Wheat Gluten Studied by FTIR Spectroscopy. Biomacromolecules 2006, 7, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F.; Guerrieri, N. ATR-FT/IR Study on the Interactions between Gliadins and Dextrin and Their Effects on Protein Secondary Structure. J. Agric. Food Chem. 2005, 53, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Surewicz, W.K.; Mantsch, H.H. New Insight into Protein Secondary Structure from Resolution-Enhanced Infrared Spectra. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1988, 952, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. FTIR Studies of Gluten Matrix Dehydration after Fibre Polysaccharide Addition. Food Chem. 2018, 252, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Wellner, N.; Belton, P.S.; Tatham, A.S. Fourier transform IR spectroscopic study of hydration-induced structure changes in the solid state of ω-gliadins. Biochem. J. 1996, 3, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Pelton, J.P.; McLean, L.R. Spectroscopic Methods for Analysis of Protein Secondary Structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, H.; Fu, B.; Iftikhar, M.; Liu, G.; Wang, J. Interactions between Dietary Fiber and Ferulic Acid Change the Aggregation of Glutenin, Gliadin and Glutenin Macropolymer in Wheat Flour System. J. Sci. Food Agric. 2021, 101, 1979–1988. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid. Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from Wheat Grain: An Overview, from Primary Structure to Nanostructures of Aggregates. Biophys. Rev. 2017, 10, 435–443. [Google Scholar] [CrossRef]
- Welc, R.; Luchowski, R.; Kłosok, K.; Gruszecki, W.I.; Nawrocka, A. How Do Phenolic Acids Change the Secondary and Tertiary Structure of Gliadin? Studies with an Application of Spectroscopic Techniques. Int. J. Mol. Sci. 2022, 23, 6053. [Google Scholar] [CrossRef]
- Mangavel, C.; Barbot, J.; Popineau, Y.; Guéguen, J. Evolution of Wheat Gliadins Conformation during Film Formation: A Fourier Transform Infrared Study. J. Agric. Food Chem. 2001, 49, 867–872. [Google Scholar] [CrossRef]
- Juszczyk, P.; Kołodziejczyk, A.S.; Grzonka, Z. FTIR Spectroscopic Studies on Aggregation Process of the Beta-Amyloid 11-28 Fragment and Its Variants. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 23–29. [Google Scholar] [CrossRef]
- Krekora, M.; Miś, A.; Nawrocka, A. Molecular Interactions between Gluten Network and Phenolic Acids Studied during Overmixing Process with Application of FT-IR Spectroscopy. J. Cereal Sci. 2021, 99, 103203. [Google Scholar] [CrossRef]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Assaf, A.; Thouand, G.; Le-Bail, A. Study of Structural Changes of Gluten Proteins during Bread Dough Mixing by Raman Spectroscopy. Food Chem. 2021, 358, 129916. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Singh, B.R. Identification of Beta-Turn and Random Coil Amide III Infrared Bands for Secondary Structure Estimation of Proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Wellner, N.; Mills, E.N.C.; Brownsey, G.; Wilson, R.H.; Brown, N.; Freeman, J.; Halford, N.G.; Shewry, P.R.; Belton, P.S. Changes in Protein Secondary Structure during Gluten Deformation Studied by Dynamic Fourier Transform Infrared Spectroscopy. Biomacromolecules 2005, 6, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ren, Z.; Yin, Z.; Qian, H.; Ma, D. Amide II and Amide III Bands in Polyurethane Model Soft and Hard Segments. Polym. Bull. 2008, 60, 97–101. [Google Scholar] [CrossRef]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. Characteristics of the Chemical Processes Induced by Celluloses in the Model and Gluten Dough Studied with Application of FTIR Spectroscopy. Food Hydrocoll. 2018, 85, 176–184. [Google Scholar] [CrossRef]
- Ferrer, E.G.; Bosch, A.; Yantorno, O.; Baran, E.J. A Spectroscopy Approach for the Study of the Interactions of Bioactive Vanadium Species with Bovine Serum Albumin. Bioorg. Med. Chem. 2008, 16, 3878–3886. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, H.; Wang, J.; Chen, H. Dynamic Changes in Glutenin Macropolymer during Different Dough Mixing and Resting Processes. Molecules 2021, 26, 541. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Mohanad, B.; Xu, L.; Ning, Y.; Xu, J.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of Frozen Storage on Physico-Chemistry of Wheat Gluten Proteins: Studies on Gluten-, Glutenin- and Gliadin-Rich Fractions. Food Hydrocoll. 2014, 39, 187–194. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welc-Stanowska, R.; Kłosok, K.; Nawrocka, A. Insight into Organization of Gliadin and Glutenin Extracted from Gluten Modified by Phenolic Acids. Molecules 2023, 28, 7790. https://doi.org/10.3390/molecules28237790
Welc-Stanowska R, Kłosok K, Nawrocka A. Insight into Organization of Gliadin and Glutenin Extracted from Gluten Modified by Phenolic Acids. Molecules. 2023; 28(23):7790. https://doi.org/10.3390/molecules28237790
Chicago/Turabian StyleWelc-Stanowska, Renata, Konrad Kłosok, and Agnieszka Nawrocka. 2023. "Insight into Organization of Gliadin and Glutenin Extracted from Gluten Modified by Phenolic Acids" Molecules 28, no. 23: 7790. https://doi.org/10.3390/molecules28237790
APA StyleWelc-Stanowska, R., Kłosok, K., & Nawrocka, A. (2023). Insight into Organization of Gliadin and Glutenin Extracted from Gluten Modified by Phenolic Acids. Molecules, 28(23), 7790. https://doi.org/10.3390/molecules28237790




