One-Pot Synthesis of Semiconducting Quantum Dots–Organic Linker–Carbon Nanotubes for Potential Applications in Bulk Heterojunction Solar Cells
Abstract
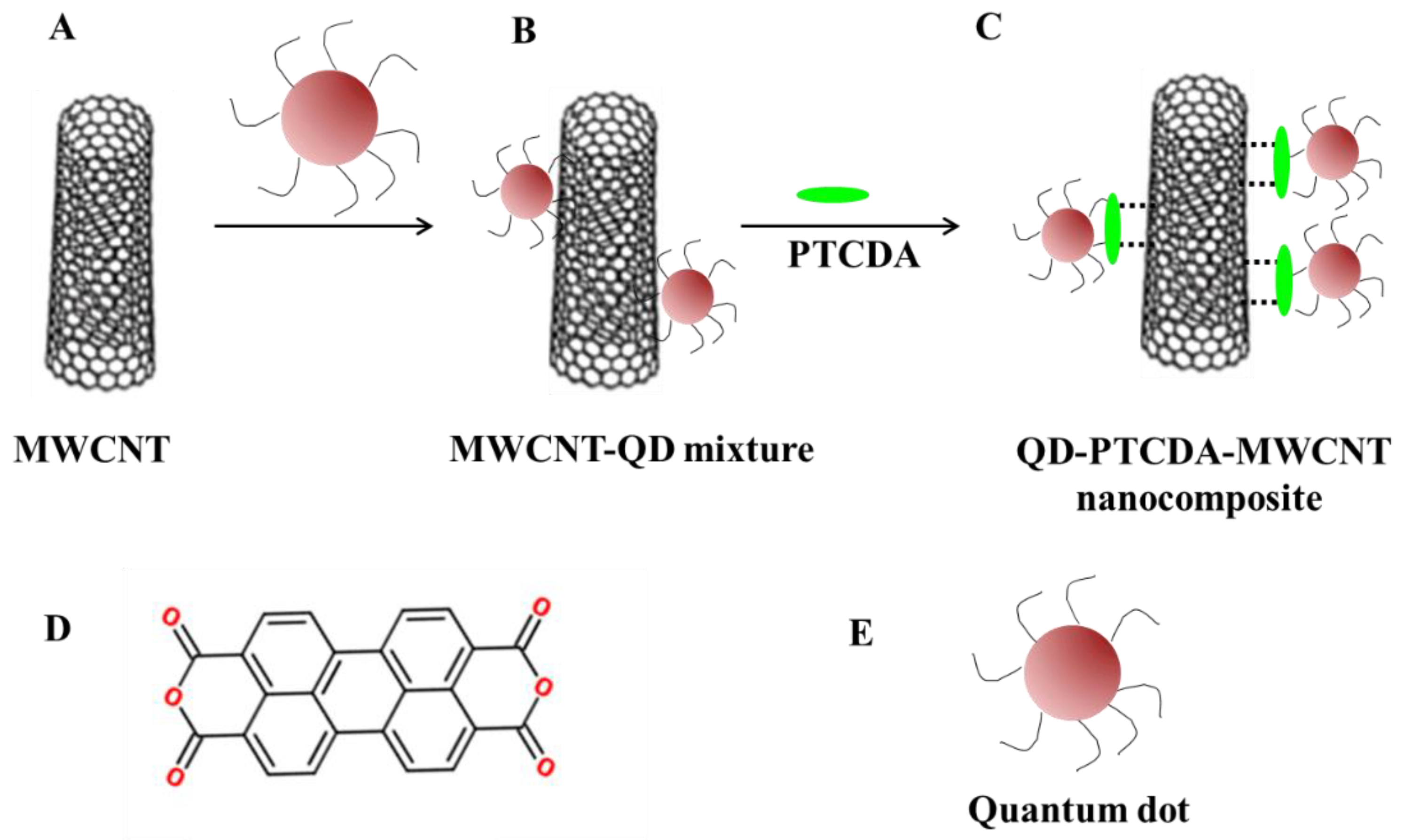
:1. Introduction
2. Results and Discussion
2.1. Infrared Spectroscopy Characterization
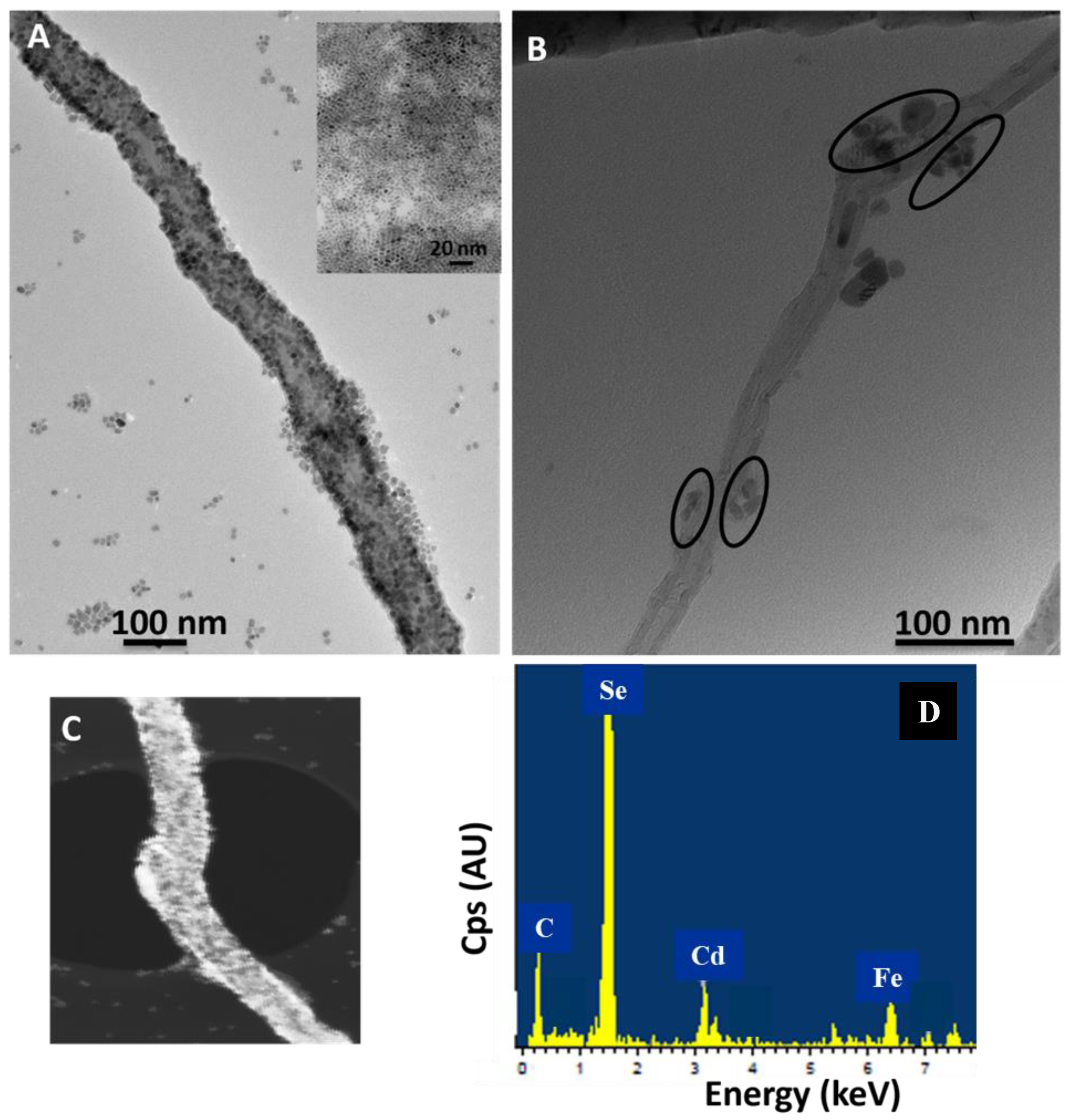
2.2. Transmission Electron Microscopy (TEM) Analysis of QD–PTCDA–MWCNTs
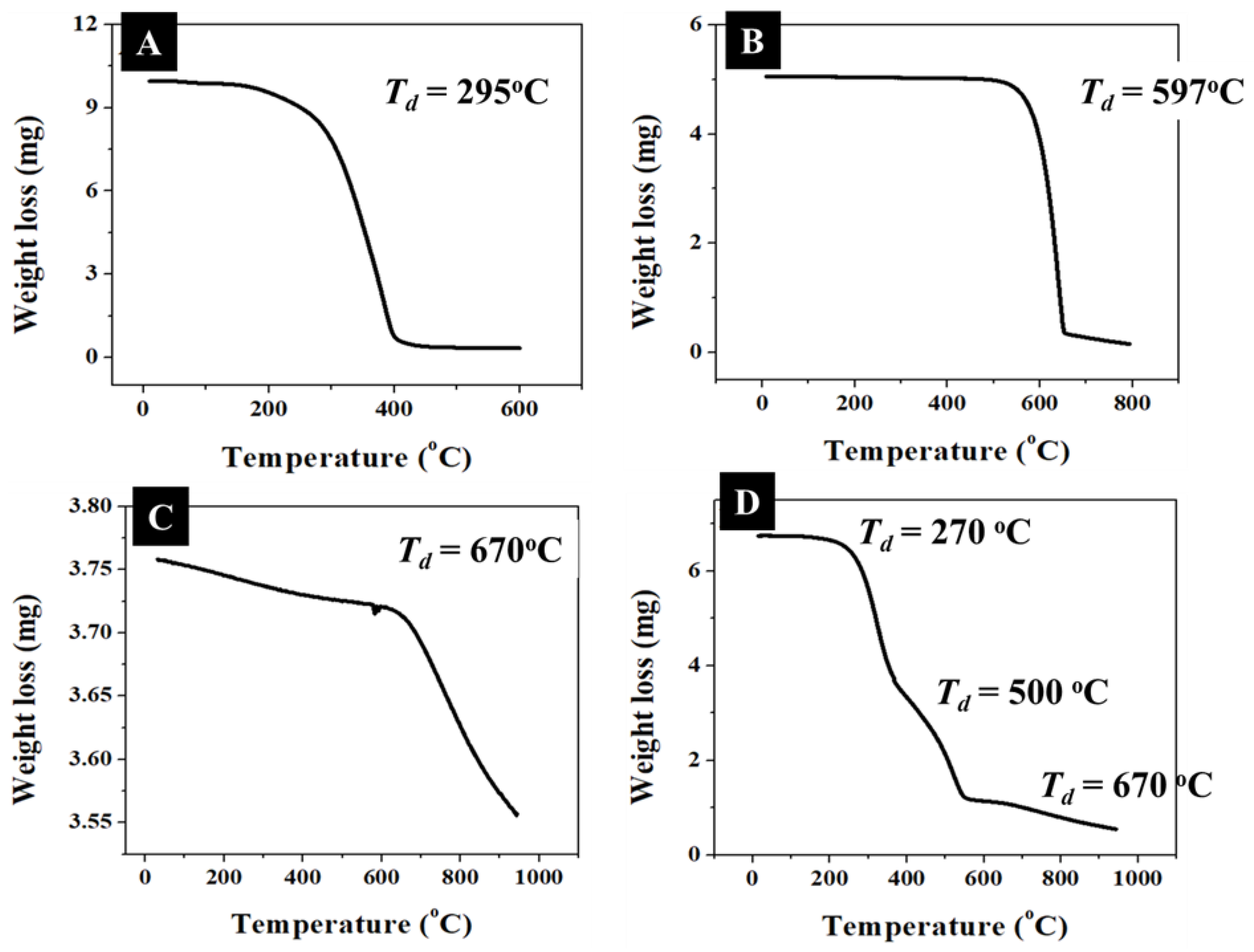
2.3. Thermogravimetric Analysis (TGA)
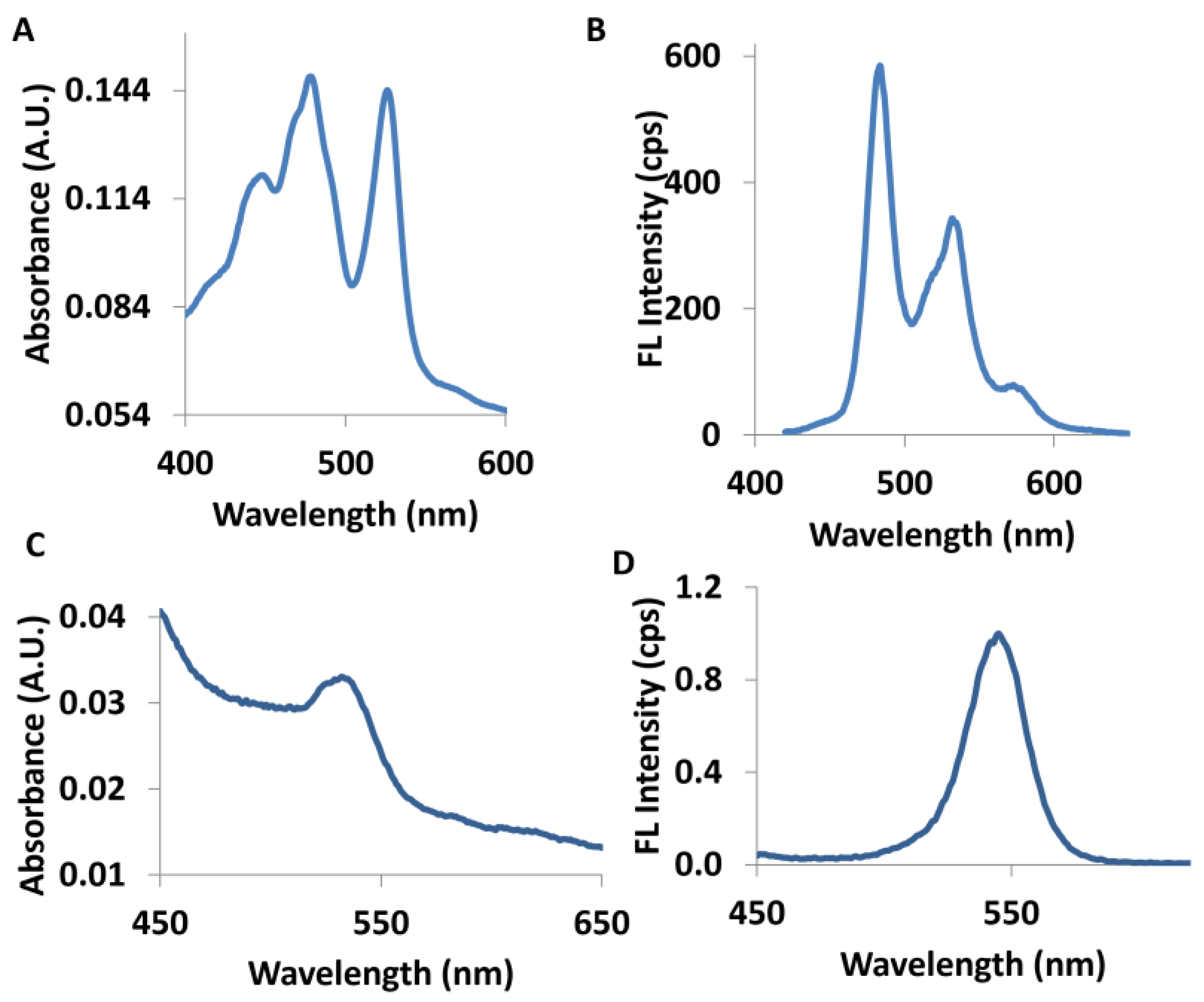
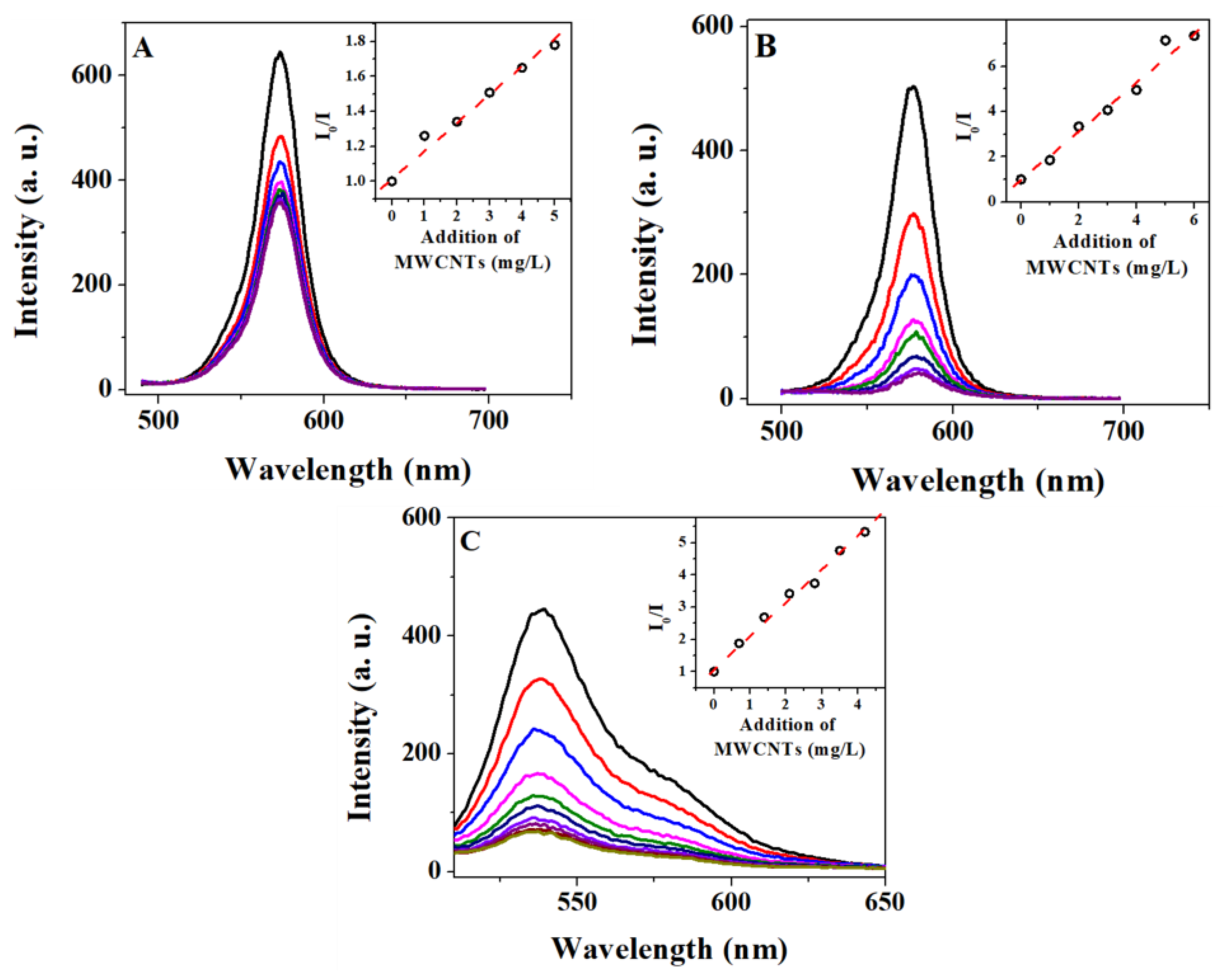
2.4. Photophysical Quenching Studies of QD–PTCDA–MWCNTs Nanocomposites
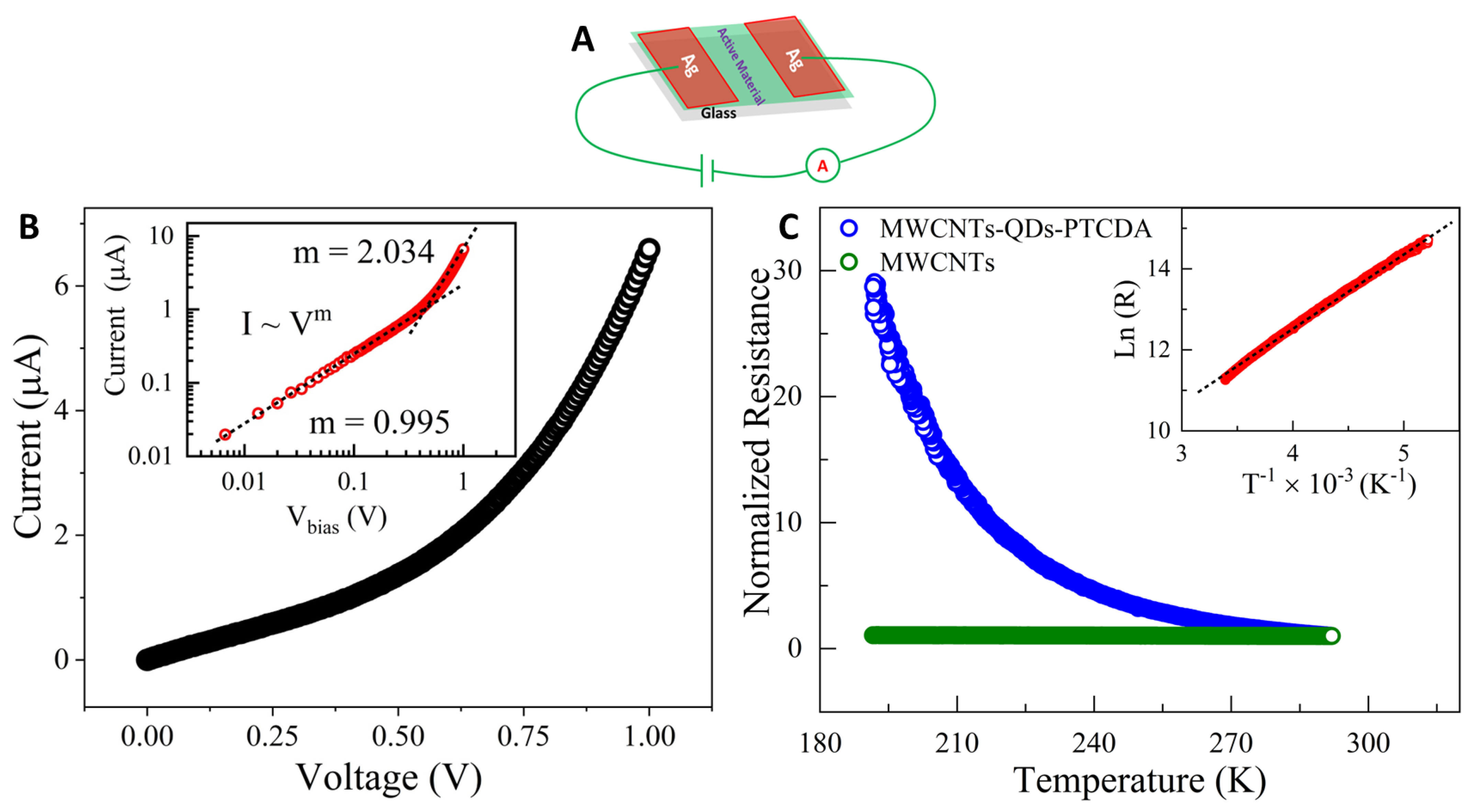
2.5. Electrical Transport Characterization of the QD–PTCDA–MWCNTs Nanocomposites
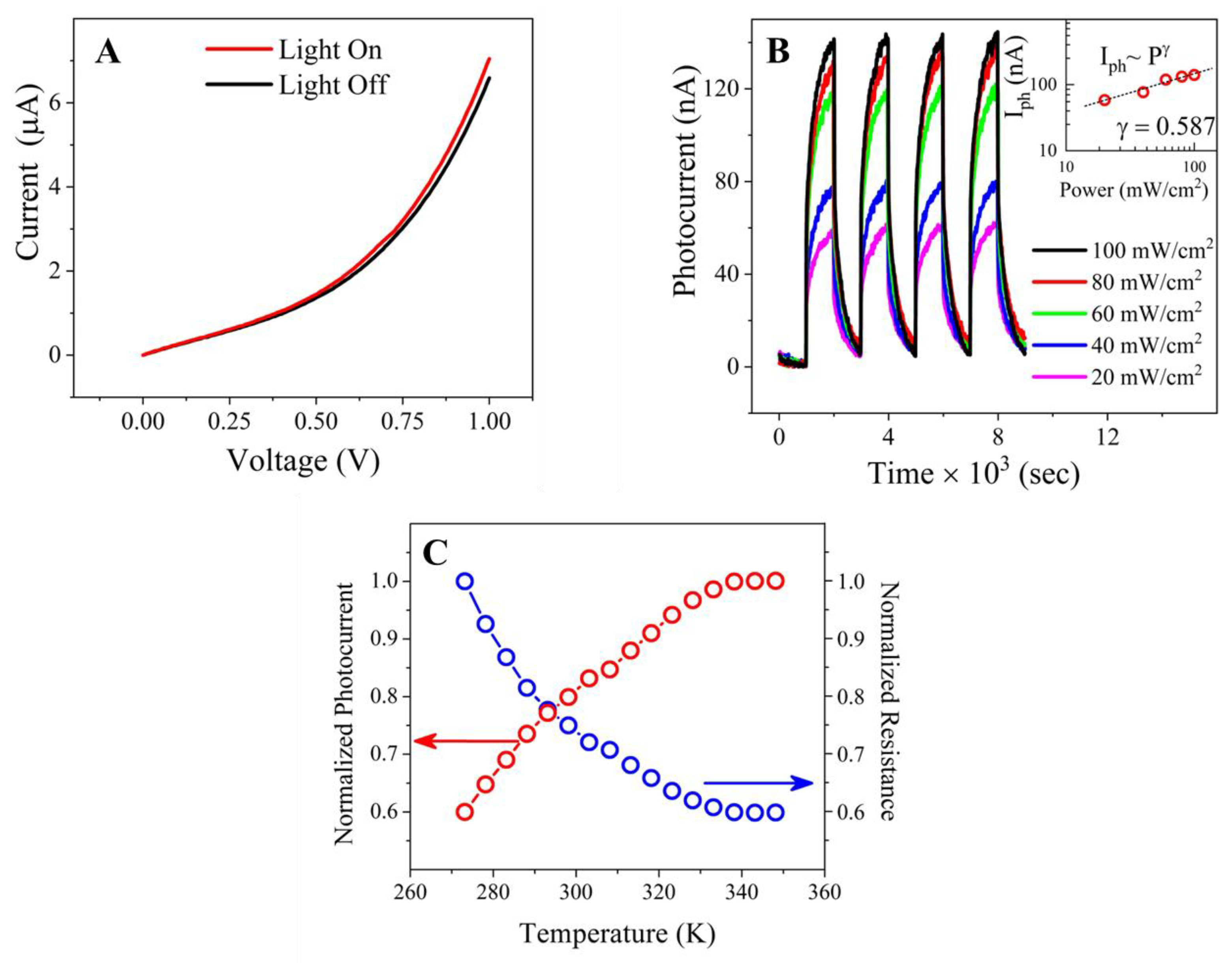
2.6. Photoinduced Current Characterization
2.7. Temperature-Dependent Photocurrent Characteristics
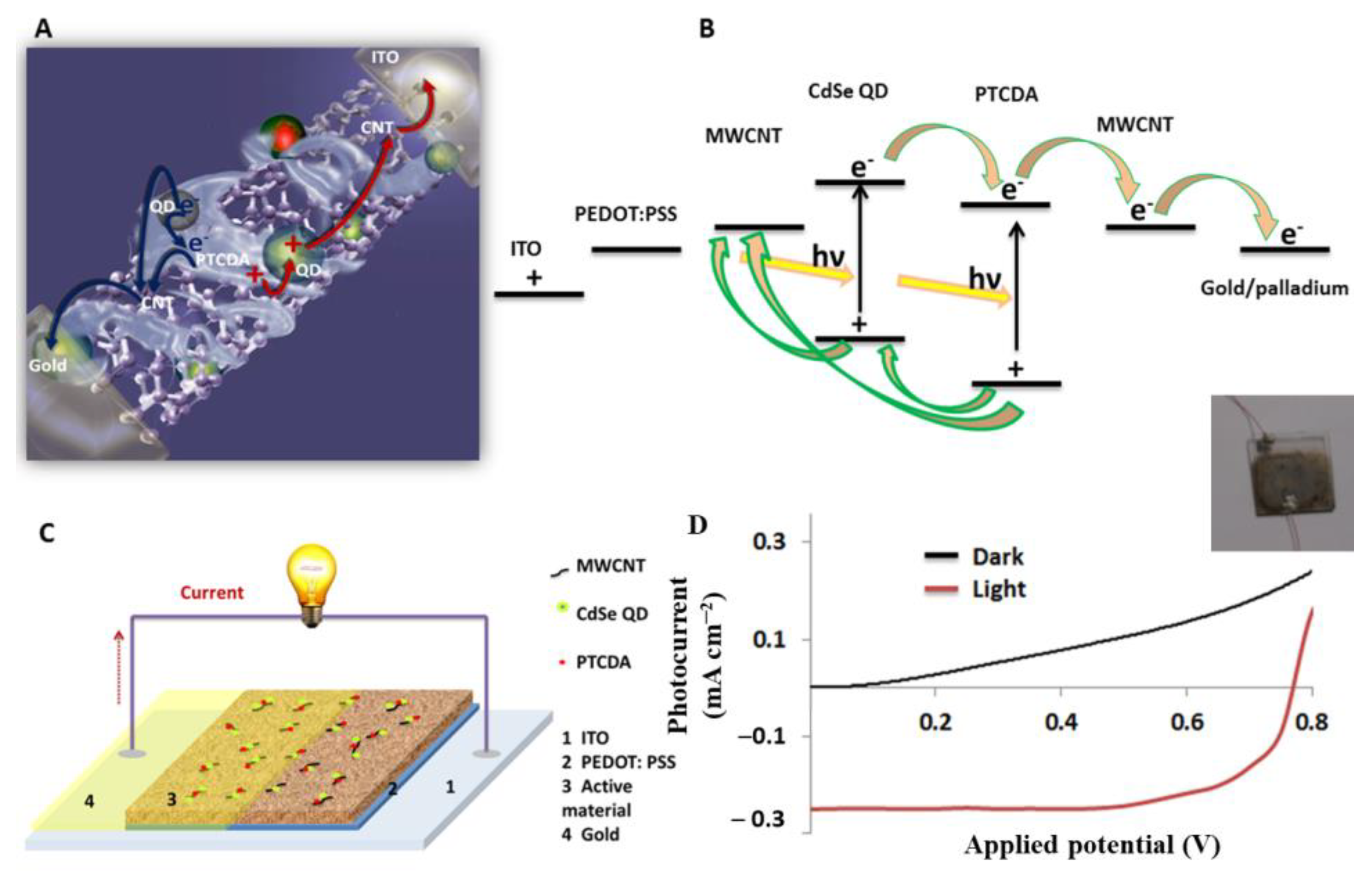
2.8. Fabrication and Characterization of BHJSC That Comprise the QD–PTCDA–MWCNTs Nanocomposite
3. Experimental Section
3.1. Materials
3.2. Methods
3.3. MWCNT Growth Procedure
3.4. Synthesis of Photoactive Nanocomposites
4. Summary
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal Quantum Dot Solar Cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Troparevsky, M.C.; Kronik, L.; Chelikowsky, J.R. Optical properties of CdSe quantum dots. J. Chem. Phys. 2003, 119, 2284–2287. [Google Scholar] [CrossRef]
- Hsueh-Shih, C.; Cheng-Kuo, H.; Hsin-Yen, H. InGaN-CdSe-ZnSe quantum dots white LEDs. IEEE Photon. Technol. Lett. 2006, 18, 193–195. [Google Scholar] [CrossRef]
- Klein, D.L.; Roth, R.; Lim, A.K.L.; Alivisatos, A.P.; McEuen, P.L. A single-electron transistor made from a cadmium selenide nanocrystal. Nature 1997, 389, 699–701. [Google Scholar] [CrossRef]
- Pattantyus-Abraham, A.G.; Kramer, I.J.; Barkhouse, A.R.; Wang, X.; Konstantatos, G.; Debnath, R.; Levina, L.; Raabe, I.; Nazeeruddin, M.K.; Grätzel, M.; et al. Depleted-Heterojunction Colloidal Quantum Dot Solar Cells. ACS Nano 2010, 4, 3374–3380. [Google Scholar] [CrossRef] [PubMed]
- Barea, E.M.; Shalom, M.; Giménez, S.; Hod, I.; Mora-Seró, I.; Zaban, A.; Bisquert, J. Design of Injection and Recombination in Quantum Dot Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 6834–6839. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design Rules for Donors in Bulk-Heterojunction Solar Cells—Towards 10 % Energy-Conversion Efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photon. 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer–Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Emery, K.; King, D.L.; Igari, S.; Warta, W. Solar cell efficiency tables (version 19). Prog. Photovolt Res. Appl. 2002, 10, 55–61. [Google Scholar] [CrossRef]
- Chang, J.A.; Rhee, J.H.; Im, S.H.; Lee, Y.H.; Kim, H.-J.; Seok, S.I.; Nazeeruddin, M.K.; Gratzel, M. High-Performance Nanostructured Inorganic–Organic Heterojunction Solar Cells. Nano Lett. 2010, 10, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yun, D.; Zhang, X.; Feng, W. Solution-processed bulk heterojunction photovoltaic devices based on poly(2-methoxy,5-octoxy)-1,4-phenylenevinylene-multiwalled carbon nanotubes/PbSe quantum dots bilayer. Appl. Phys. Lett. 2010, 96, 093301. [Google Scholar] [CrossRef]
- Emin, S.; Singh, S.P.; Han, L.; Satoh, N.; Islam, A. Colloidal quantum dot solar cells. Sol. Energy 2011, 85, 1264–1282. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid Nanorod-Polymer Solar Cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef]
- Sun, B.; Greenham, N.C. Improved efficiency of photovoltaics based on CdSe nanorods and poly(3-hexylthiophene) nanofibers. Phys. Chem. Chem. Phys. 2006, 8, 3557. [Google Scholar] [CrossRef]
- Gong, X.; Tong, M.; Xia, Y.; Cai, W.; Moon, J.S.; Cao, Y.; Yu, G.; Shieh, C.-L.; Nilsson, B.; Heeger, A.J. High-Detectivity Polymer Photodetectors with Spectral Response from 300 nm to 1450 nm. Science 2009, 325, 1665–1667. [Google Scholar] [CrossRef]
- Yip, H.-L.; Jen, A.K.Y. Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Energy Environ. Sci. 2012, 5, 5994. [Google Scholar] [CrossRef]
- Chamberlain, G.A. Organic solar cells: A review. Sol. Cells 1983, 8, 47–83. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Morphology of polymer/fullerene bulk heterojunction solar cells. J. Mater. Chem. 2006, 16, 45–61. [Google Scholar] [CrossRef]
- de Mello Donegá, C.; Bode, M.; Meijerink, A. Size- and temperature-dependence of exciton lifetimes in CdSe quantum dots. Phys. Rev. B 2006, 74, 085320. [Google Scholar] [CrossRef]
- Kramer, I.J.; Sargent, E.H. The Architecture of Colloidal Quantum Dot Solar Cells: Materials to Devices. Chem. Rev. 2014, 114, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.I.; Hirose, Y.; Sirringhaus, H.; Kahn, A. Electron-hole interaction energy in the organic molecular semiconductor PTCDA. Chem. Phys. Lett. 1997, 272, 43–47. [Google Scholar] [CrossRef]
- Hirose, Y.; Kahn, A.; Aristov, V.; Soukiassian, P. Chemistry, diffusion, and electronic properties of a metal/organic semiconductor contact: In/perylenetetracarboxylic dianhydride. Appl. Phys. Lett. 1996, 68, 217–219. [Google Scholar] [CrossRef]
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally Stable, Efficient Polymer Solar Cells with Nanoscale Control of the Interpenetrating Network Morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Machui, F.; Brabec, C.J. Solubility, Miscibility, and the Impact on Solid-State Morphology. In Semiconducting Polymer Composites, 1st ed.; Yang, X., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 1–38. [Google Scholar]
- Berger, S.; Heimer, K.; Mack, H.G.; Ziegler, C. IR and SFM study of PTCDA thin films on different substrates. Appl. Surf. Sci. 2005, 252, 81–84. [Google Scholar] [CrossRef]
- Cooper, J.K.; Franco, A.M.; Gul, S.; Corrado, C.; Zhang, J.Z. Characterization of Primary Amine Capped CdSe, ZnSe, and ZnS Quantum Dots by FT-IR: Determination of Surface Bonding Interaction and Identification of Selective Desorption. Langmuir 2011, 27, 8486–8493. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.-Y.; Liu, Y.; Jiang, F.-L. Thermodynamic Implications of the Ligand Exchange with Alkylamines on the Surface of CdSe Quantum Dots: The Importance of Ligand–Ligand Interactions. J. Phys. Chem. C 2020, 124, 4613–4625. [Google Scholar] [CrossRef]
- La Rosa, M.; Payne, E.H.; Credi, A. Semiconductor Quantum Dots as Components of Photoactive Supramolecular Architectures. ChemistryOpen 2020, 9, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Landi, B.J.; Castro, S.L.; Ruf, H.J.; Evans, C.M.; Bailey, S.G.; Raffaelle, R.P. CdSe quantum dot-single wall carbon nanotube complexes for polymeric solar cells. Sol. Energy Mater. Sol. Cells 2005, 87, 733–746. [Google Scholar] [CrossRef]
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-Functionalized Carbon Nanotubes for Binding to Polymers and Biological Systems. Chem. Mater. 2005, 17, 1290–1295. [Google Scholar] [CrossRef]
- Weaver, J.E.; Dasari, M.R.; Datar, A.; Talapatra, S.; Kohli, P. Investigating Photoinduced Charge Transfer in Carbon Nanotube–Perylene–Quantum Dot Hybrid Nanocomposites. ACS Nano 2010, 4, 6883–6893. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Dasari, M.; Rajasekaran, P.R.; Iyer, R.; Kohli, P. Calligraphic solar cells: Acknowledging paper and pencil. J. Mater. Res. 2016, 31, 2578–2589. [Google Scholar] [CrossRef]
- Pan, B.; Cui, D.; Ozkan, C.S.; Ozkan, M.; Xu, P.; Huang, T.; Liu, F.; Chen, H.; Li, Q.; He, R.; et al. Effects of Carbon Nanotubes on Photoluminescence Properties of Quantum Dots. J. Phys. Chem. C 2008, 112, 939–944. [Google Scholar] [CrossRef]
- Murgatroyd, P.N. Theory of space-charge-limited current enhanced by Frenkel effect. J. Phys. D Appl. Phys. 1970, 3, 151–156. [Google Scholar] [CrossRef]
- Chandra, W.; Ang, L.K.; Pey, K.L.; Ng, C.M. Two-dimensional analytical Mott-Gurney law for a trap-filled solid. Appl. Phys. Lett. 2007, 90, 153505. [Google Scholar] [CrossRef]
- Muchharla, B.; Narayanan, T.N.; Balakrishnan, K.; Ajayan, P.M.; Talapatra, S. Temperature dependent electrical transport of disordered reduced graphene oxide. 2D Mater. 2014, 1, 011008. [Google Scholar] [CrossRef]
- Wei, B.; Spolenak, R.; Kohler-Redlich, P.; Rühle, M.; Arzt, E. Electrical transport in pure and boron-doped carbon nanotubes. Appl. Phys. Lett. 1999, 74, 3149–3151. [Google Scholar] [CrossRef]
- Johnston, D.E.; Islam, M.F.; Yodh, A.G.; Johnson, A.T. Electronic devices based on purified carbon nanotubes grown by high-pressure decomposition of carbon monoxide. Nat. Mater. 2005, 4, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. Recombination Processes in Insulators and Semiconductors. Phys. Rev. 1955, 97, 322–333. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, C.; Wu, J.; Zhou, H.; Pan, T.; Mao, J.; Yin, X.; Liu, R.; Gao, W.; Fang, Z.; et al. Broadband photodetection in a microfiber-graphene device. Opt. Express 2015, 23, 25209. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Li, C.; Yin, Y.; Liu, Y. Large-area synthesis of monolayer WS2 and its ambient-sensitive photo-detecting performance. Nanoscale 2015, 7, 5974–5980. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Patil, P.D.; Wasala, M.; Lei, S.; Nolander, A.; Sivakumar, P.; Vajtai, R.; Ajayan, P.; Talapatra, S. Fast photoresponse and high detectivity in copper indium selenide (CuIn7 Se11) phototransistors. 2D Mater. 2017, 5, 015001. [Google Scholar] [CrossRef]
- Marcus, M.S.; Simmons, J.M.; Castellini, O.M.; Hamers, R.J.; Eriksson, M.A. Photogating carbon nanotube transistors. J. Appl. Phys. 2006, 100, 084306. [Google Scholar] [CrossRef]
- Ghosh, S.; Winchester, A.; Muchharla, B.; Wasala, M.; Feng, S.; Elias, A.L.; Krishna, M.B.M.; Harada, T.; Chin, C.; Dani, K.; et al. Ultrafast Intrinsic Photoresponse and Direct Evidence of Sub-gap States in Liquid Phase Exfoliated MoS2Thin Films. Sci. Rep. 2015, 5, 11272. [Google Scholar] [CrossRef]
- Bozano, L.; Carter, S.A.; Scott, J.C.; Malliaras, G.G.; Brock, P.J. Temperature- and field-dependent electron and hole mobilities in polymer light-emitting diodes. Appl. Phys. Lett. 1999, 74, 1132–1134. [Google Scholar] [CrossRef]
- Joshi, R.P. Temperature-dependent electron mobility in GaN: Effects of space charge and interface roughness scattering. Appl. Phys. Lett. 1994, 64, 223–225. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Liu, T.; Meng, B.; Li, X.; Liang, G.; Hu, X.; Wang, Q.J. Broadband high photoresponse from pure monolayer graphene photodetector. Nat. Commun. 2013, 4, 1811. [Google Scholar] [CrossRef]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Sariciftci, N.S.; Rispens, M.T.; Sanchez, L.; Hummelen, J.C.; Fromherz, T. The influence of materials work function on the open circuit voltage of plastic solar cells. Thin Solid Films 2002, 403–404, 368–372. [Google Scholar] [CrossRef]
- Brus, V.V.; Maryanchuk, P.D.; Ilashchuk, M.I.; Rappich, J.; Babichuk, I.S.; Kovalyuk, Z.D. Graphitic carbon/n-CdTe Schottky-type heterojunction solar cells prepared by electron-beam evaporation. Sol. Energy 2015, 112, 78–84. [Google Scholar] [CrossRef]
- Ye, Y.; Gan, L.; Dai, L.; Dai, Y.; Guo, X.; Meng, H.; Yu, B.; Shi, Z.; Shang, K.; Qin, G. A simple and scalable graphene patterning method and its application in CdSe nanobelt/graphene Schottky junction solar cells. Nanoscale 2011, 3, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Perea-López, N.; Rebollo-Plata, B.; Briones-León, J.A.; Morelos-Gómez, A.; Hernández-Cruz, D.; Hirata, G.A.; Meunier, V.; Botello-Méndez, A.R.; Charlier, J.-C.; Maruyama, B.; et al. Millimeter-Long Carbon Nanotubes: Outstanding Electron-Emitting Sources. ACS Nano 2011, 5, 5072–5077. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, S.; Kar, S.; Pal, S.K.; Vajtai, R.; Ci, L.; Victor, P.; Shaijumon, M.M.; Kaur, S.; Nalamasu, O.; Ajayan, P.M. Direct growth of aligned carbon nanotubes on bulk metals. Nat. Nanotech 2006, 1, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Ci, L.; Shah, R.; Wolfe, C.; Kar, S.; Talapatra, S.; Ajayan, P.M. Air-assisted growth of ultra-long carbon nanotube bundles. Nanotechnology 2008, 19, 455609. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Formation of High-Quality CdTe, CdSe, and CdS Nanocrystals Using CdO as Precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef]
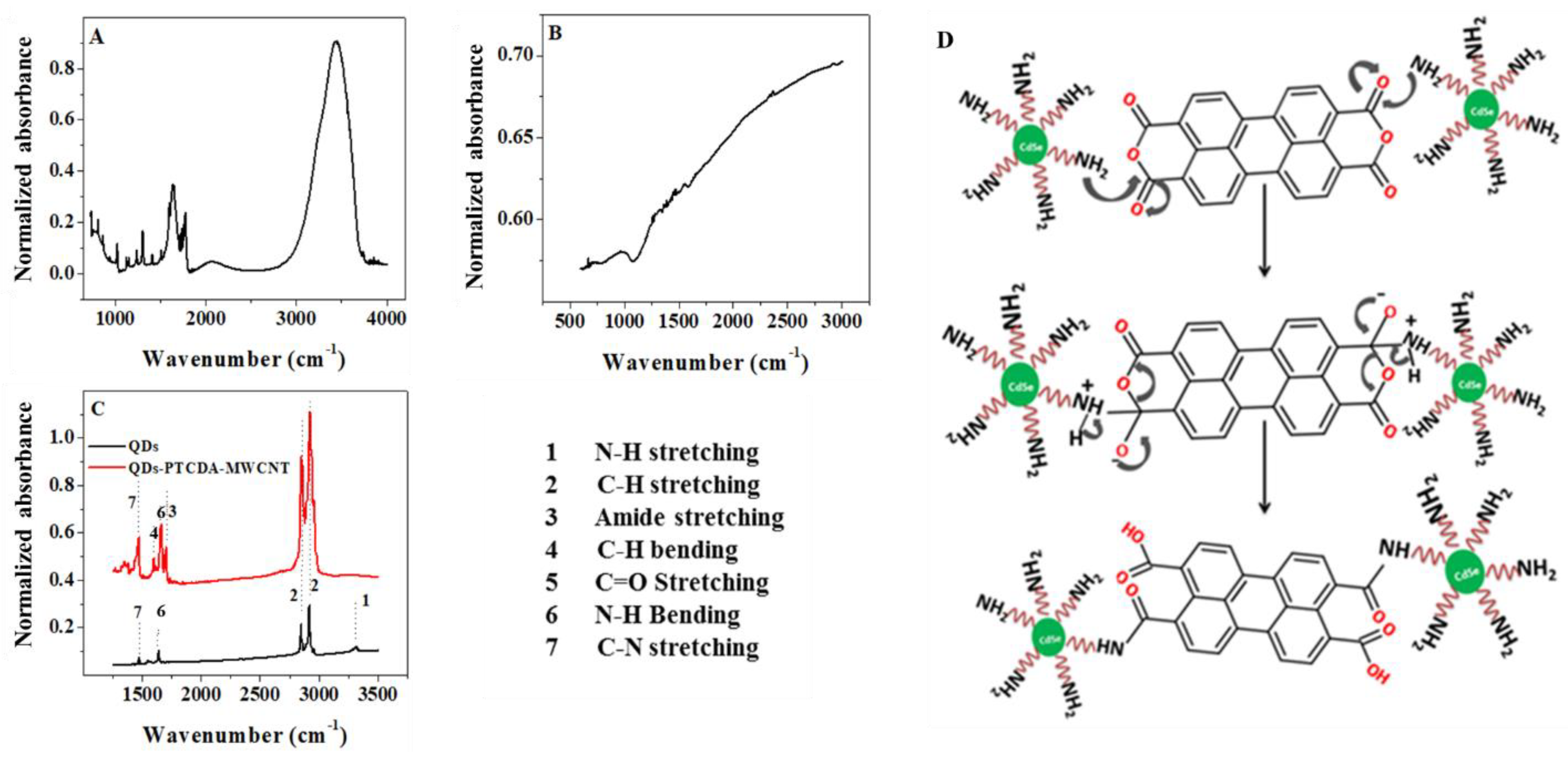
| Material | Peak Position (cm−1) | Peak Assignment | References |
|---|---|---|---|
| QD | 2917–2848 | C–H stretching | [33] |
| 1590 | N–H bending | ||
| 1452 | C–H bending | ||
| PTCDA | 3700–2800 (broad) | O–H and C–H stretching | [29] |
| 1770 (sharp) | C=O from cyclic anhydride | ||
| 1400–1600 (sharp) | C=C stretching | ||
| QD–PTCDA–MWCNT | 2911–2848 (sharp) | C–H stretching | [34,35] |
| 1664 (sharp) | C=O amide stretching | ||
| 1592 (sharp) | N–H bending | ||
| 1460 (sharp) | C–H bending |
| Donor | Acceptor | Ksv (L mg−1) |
|---|---|---|
| QD | MWCNT | 0.16 |
| QD–PTCDA | MWCNT | 1.09 |
| PTCDA | MWCNT | 1.05 |
| QD | PTCDA | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasari, M.; Muchharla, B.; Talapatra, S.; Kohli, P. One-Pot Synthesis of Semiconducting Quantum Dots–Organic Linker–Carbon Nanotubes for Potential Applications in Bulk Heterojunction Solar Cells. Molecules 2023, 28, 7702. https://doi.org/10.3390/molecules28237702
Dasari M, Muchharla B, Talapatra S, Kohli P. One-Pot Synthesis of Semiconducting Quantum Dots–Organic Linker–Carbon Nanotubes for Potential Applications in Bulk Heterojunction Solar Cells. Molecules. 2023; 28(23):7702. https://doi.org/10.3390/molecules28237702
Chicago/Turabian StyleDasari, Mallika, Baleeswaraiah Muchharla, Saikat Talapatra, and Punit Kohli. 2023. "One-Pot Synthesis of Semiconducting Quantum Dots–Organic Linker–Carbon Nanotubes for Potential Applications in Bulk Heterojunction Solar Cells" Molecules 28, no. 23: 7702. https://doi.org/10.3390/molecules28237702
APA StyleDasari, M., Muchharla, B., Talapatra, S., & Kohli, P. (2023). One-Pot Synthesis of Semiconducting Quantum Dots–Organic Linker–Carbon Nanotubes for Potential Applications in Bulk Heterojunction Solar Cells. Molecules, 28(23), 7702. https://doi.org/10.3390/molecules28237702








