In Vitro and In Vivo Anti-Cancer Activity of Lasiokaurin in a Triple-Negative Breast Cancer Model
Abstract
:1. Introduction
2. Results
2.1. LAS Inhibits the Proliferation of TNBC Cells
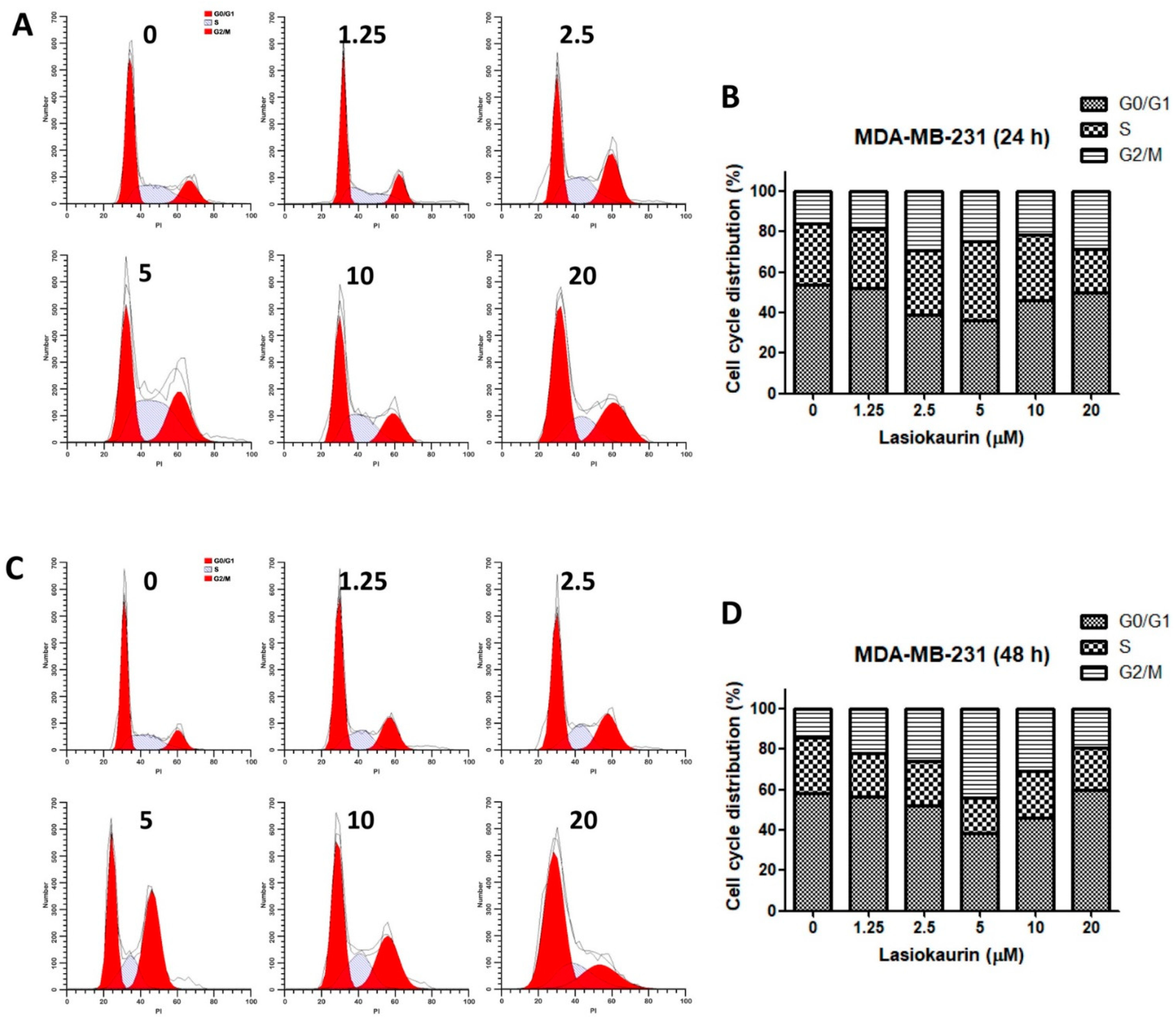
2.2. LAS Modulates Cell Cycle Progression in TNBC Cells
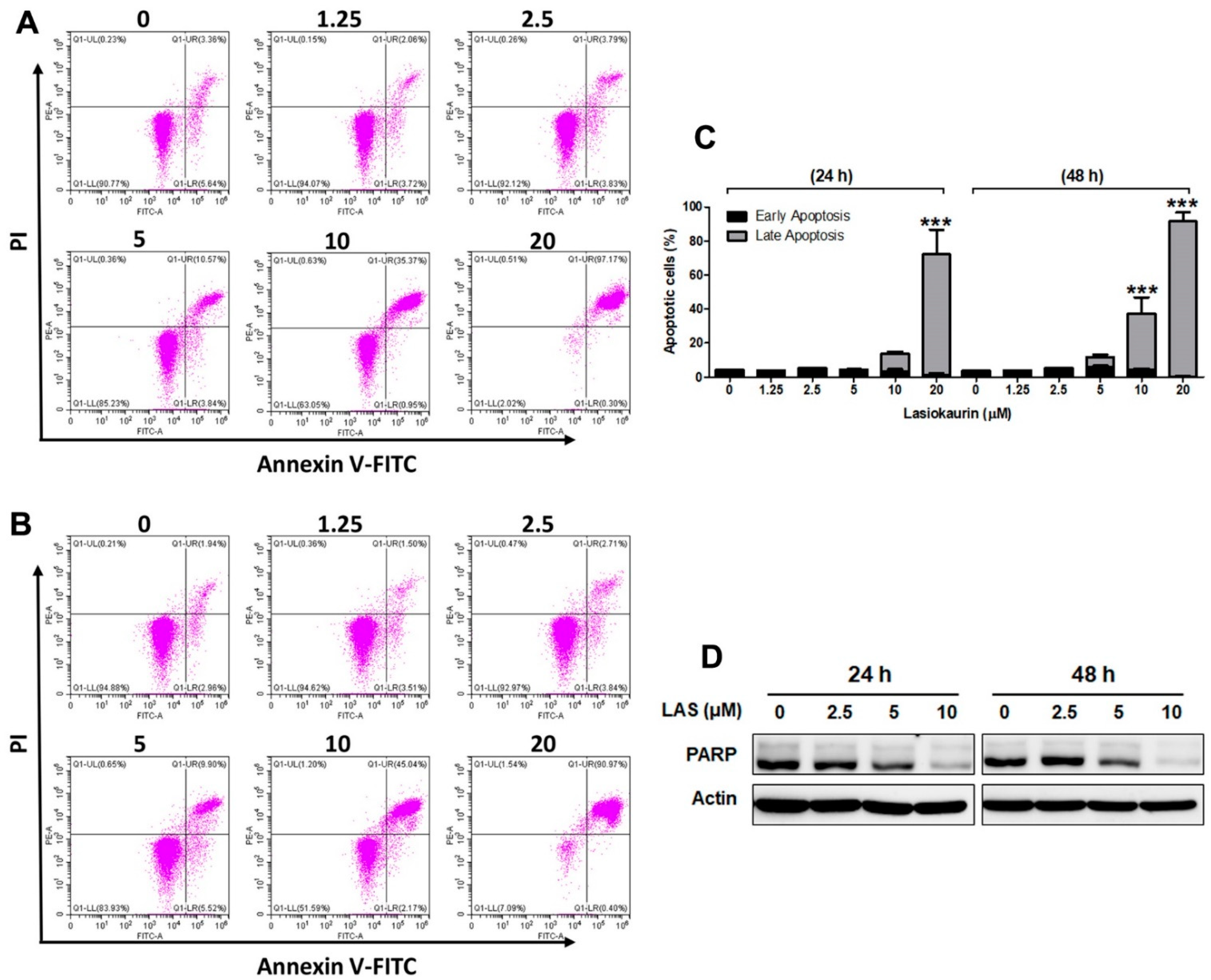
2.3. LAS Induces Apoptosis and DNA Damage in TNBC Cells
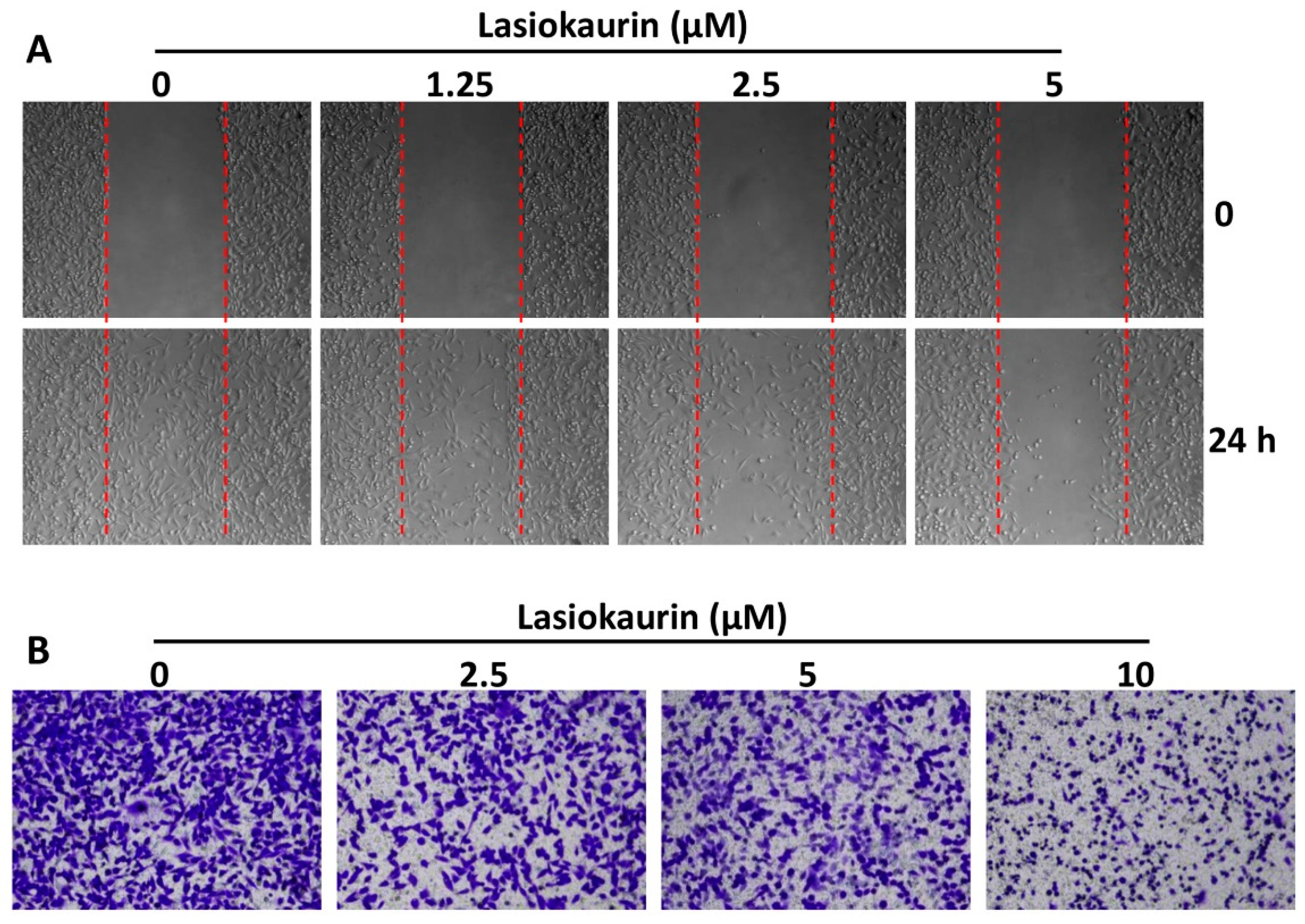
2.4. LAS Inhibits the Migration and Invasion of TNBC Cells
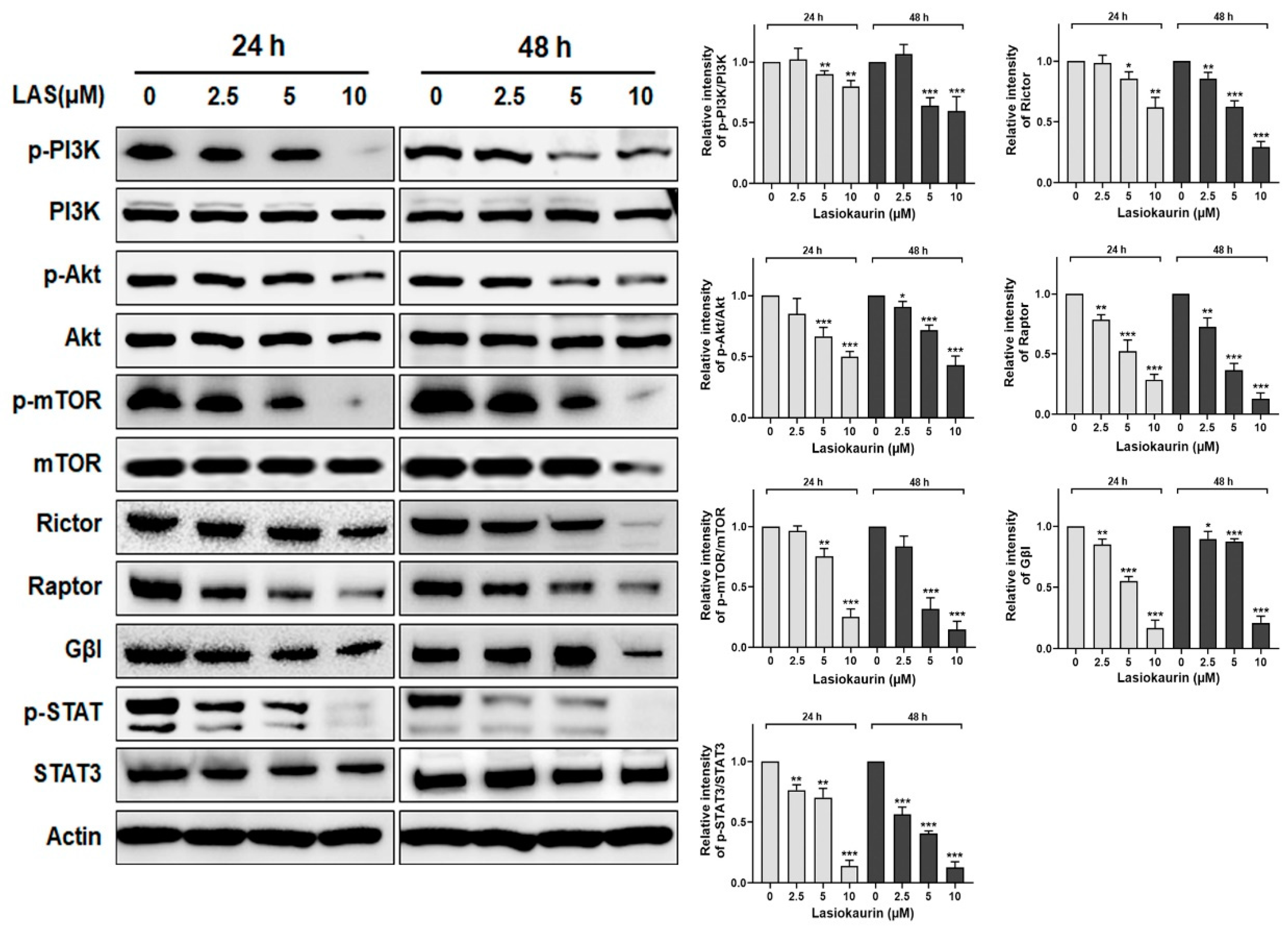
2.5. LAS Inhibits PI3K/Akt/mTOR and STAT3 Signalling in TNBC Cells
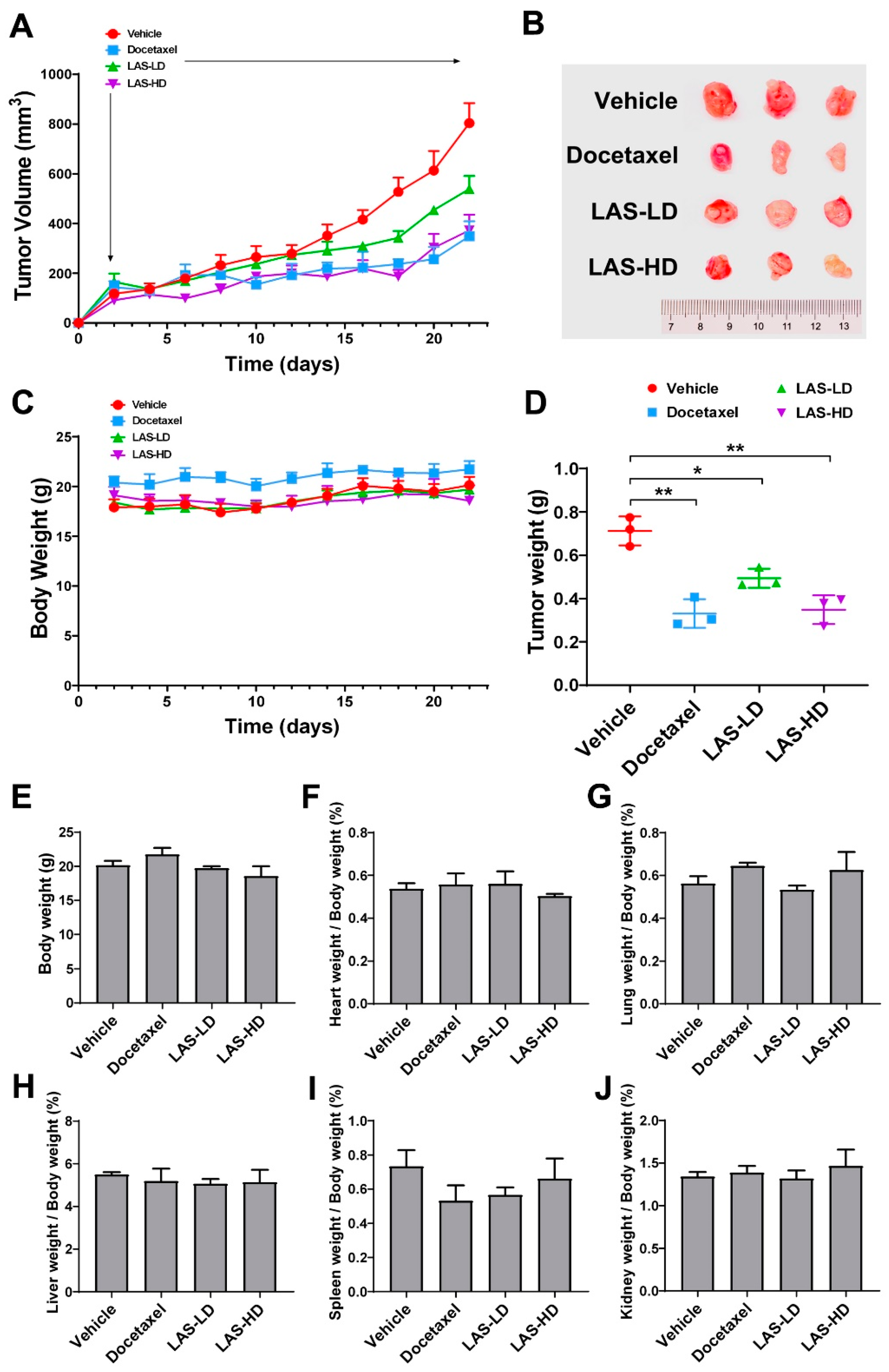
2.6. LAS Inhibits Tumor Growth in a Xenograft Nude Mouse Model
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Cell Cycle Assay
4.6. Annexin V-FITC Apoptosis Assay
4.7. Wound-Healing Assay
4.8. Transwell Invasion Assay
4.9. Western Blot Analysis
4.10. Establishment of Tumor Xenograft Model
4.11. Haematoxylin and Eosin (H&E) Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Kuo, Y.C.; Ho, Y.S.; Huang, Y.H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Shastry, M.; Yardley, D.A. Updates in the treatment of basal/triple-negative breast cancer. Curr. Opin. Obstet. Gynecol. 2013, 25, 40–48. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Shi, C.; Yang, E.J.; Tao, S.; Ren, G.; Mou, P.K.; Shim, J.S. Natural products targeting cancer cell dependency. J. Antibiot. 2021, 74, 677–686. [Google Scholar] [CrossRef]
- Gairola, K.; Gururani, S.; Bahuguna, A.; Garia, V.; Pujari, R.; Dubey, S.K. Natural products targeting cancer stem cells: Implications for cancer chemoprevention and therapeutics. J. Food Biochem. 2021, 45, e13772. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Yang, Y.; Zhang, J.; Xu, Y.; Lu, X. The Natural Products and Extracts: Anti-Triple-Negative Breast Cancer in Vitro. Chem. Biodivers. 2021, 18, e2001047. [Google Scholar] [CrossRef]
- Yap, K.M.; Sekar, M.; Seow, L.J.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Lum, P.T.; Subramaniyan, V.; Wu, Y.S.; Fuloria, N.K.; et al. Mangifera indica (Mango): A Promising Medicinal Plant for Breast Cancer Therapy and Understanding Its Potential Mechanisms of Action. Breast Cancer 2021, 13, 471–503. [Google Scholar] [CrossRef]
- Yap, K.M.; Sekar, M.; Wu, Y.S.; Gan, S.H.; Rani, N.; Seow, L.J.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Lum, P.T. Hesperidin and its aglycone hesperetin in breast cancer therapy: A review of recent developments and future prospects. Saudi J. Biol. Sci. 2021, 28, 6730–6747. [Google Scholar] [CrossRef] [PubMed]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; de Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In Flowering Plants Dicotyledons: Lamiales (Except Acanthaceae Including Avicenniaceae); Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–275. [Google Scholar]
- Sun, H.D.; Huang, S.X.; Han, Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, W.G.; Sun, H.D.; Pu, J.X. Diterpenoids from Isodon species: An update. Nat. Prod. Rep. 2017, 34, 1090–1140. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.T.; Ma, W.; Xie, X.; Huang, Q. Oridonin: A Review of Its Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 2021, 12, 645824. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, T.; Ma, Z.; Qiao, C.; Yuan, F.; Guo, X.; Liu, J.; Shen, Y.; Yu, L.; Xiang, A. Oridonin inhibits 4T1 tumor growth by suppressing Treg differentiation via TGF-beta receptor. Int. Immunopharmacol. 2020, 88, 106831. [Google Scholar] [CrossRef]
- Sun, B.; Wang, G.; Liu, H.; Liu, P.; Twal, W.O.; Cheung, H.; Carroll, S.L.; Ethier, S.P.; Mevers, E.E.; Clardy, J.; et al. Oridonin inhibits aberrant AKT activation in breast cancer. Oncotarget 2018, 9, 23878–23889. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Shen, S.; Wei, X.; Li, G. Oridonin inhibits VEGF-A-associated angiogenesis and epithelial-mesenchymal transition of breast cancer in vitro and in vivo. Oncol. Lett. 2018, 16, 2289–2298. [Google Scholar] [CrossRef]
- Bu, H.Q.; Shen, F.; Cui, J. The inhibitory effect of oridonin on colon cancer was mediated by deactivation of TGF-beta1/Smads-PAI-1 signaling pathway in vitro and vivo. OncoTargets Ther. 2019, 12, 7467–7476. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Shi, X.; Hao, S.; Zhang, F.; Guo, Z.; Gao, Y.; Guo, H.; Liu, L. Oridonin inhibits tumor angiogenesis and induces vessel normalization in experimental colon cancer. J. Cancer 2021, 12, 3257–3264. [Google Scholar] [CrossRef]
- Xu, L.; Bi, Y.; Xu, Y.; Zhang, Z.; Xu, W.; Zhang, S.; Chen, J. Oridonin inhibits the migration and epithelial-to-mesenchymal transition of small cell lung cancer cells by suppressing FAK-ERK1/2 signalling pathway. J. Cell. Mol. Med. 2020, 24, 4480–4493. [Google Scholar] [CrossRef]
- Liu, W.; Huang, G.; Yang, Y.; Gao, R.; Zhang, S.; Kou, B. Oridonin inhibits epithelial-mesenchymal transition of human nasopharyngeal carcinoma cells by negatively regulating AKT/STAT3 signaling pathway. Int. J. Med. Sci. 2021, 18, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, X.; Zhang, L.; Li, Y.; Cheng, B.; Xia, J. Oridonin inhibits oral cancer growth and PI3K/Akt signaling pathway. Biomed. Pharmacother. 2018, 100, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Chen, K.; Ye, Q.; Jiang, X.H.; Sun, Y.W. Oridonin inhibits pancreatic cancer cell migration and epithelial-mesenchymal transition by suppressing Wnt/beta-catenin signaling pathway. Cancer Cell Int. 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Zhan, J.; Zhao, F.; Zhong, Z.; Chen, M.; Han, R.; Wang, Y. Oridonin Promotes Apoptosis and Restrains the Viability and Migration of Bladder Cancer by Impeding TRPM7 Expression via the ERK and AKT Signaling Pathways. Biomed. Res. Int. 2021, 2021, 4340950. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Zhang, C.; Guo, Z.Y.; Yang, J.M.; Guo, J.H.; Chen, C.; Yao, Q.H.; Liu, F.; Zhang, Q.W.; Gao, F.H. Oridonin induces Mdm2-p60 to promote p53-mediated apoptosis and cell cycle arrest in neuroblastoma. Cancer Med. 2019, 8, 5313–5326. [Google Scholar] [CrossRef]
- Li, D.H.; Hu, P.; Xu, S.T.; Fang, C.Y.; Tang, S.; Wang, X.Y.; Sun, X.Y.; Li, H.; Xu, Y.; Gu, X.K.; et al. Lasiokaurin derivatives: Synthesis, antimicrobial and antitumor biological evaluation, and apoptosis-inducing effects. Arch. Pharm. Res. 2017, 40, 796–806. [Google Scholar] [CrossRef]
- Fujita, E.; Nagao, Y.; Node, M.; Kaneko, K.; Nakazawa, S.; Kuroda, H. Antitumor activity of the Isodon diterpenoids: Structural requirements for the activity. Experientia 1976, 32, 203–206. [Google Scholar] [CrossRef]
- Nik Nabil, W.N.; Xi, Z.; Liu, M.; Li, Y.; Yao, M.; Liu, T.; Dong, Q.; Xu, H. Advances in therapeutic agents targeting quiescent cancer cells. Acta Mater. Medica 2022, 1, 56–71. [Google Scholar] [CrossRef]
- Gong, R.H.; Yang, D.J.; Kwan, H.Y.; Lyu, A.P.; Chen, G.Q.; Bian, Z.X. Cell death mechanisms induced by synergistic effects of halofuginone and artemisinin in colorectal cancer cells. Int. J. Med. Sci. 2022, 19, 175–185. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Yap, T.A.; Plummer, R.; Azad, N.S.; Helleday, T. The DNA Damaging Revolution: PARP Inhibitors and Beyond. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, S.B.; Terawaki, H.; Gogineni, K. A Review of Systemic Treatment in Metastatic Triple-Negative Breast Cancer. Breast Cancer 2016, 10, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Han, H.S.; Gradishar, W.J. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res. Treat. 2018, 169, 397–406. [Google Scholar] [CrossRef]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Retraction Note to: Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2021, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Jain, V.K.; Rizwanullah, M.; Ahmad, J.; Jain, K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: A review on drug discovery and future challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sabatini, D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005, 17, 596–603. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62. [Google Scholar] [CrossRef]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. 2019, 38, 195. [Google Scholar] [CrossRef]
- Siersbaek, R.; Scabia, V.; Nagarajan, S.; Chernukhin, I.; Papachristou, E.K.; Broome, R.; Johnston, S.J.; Joosten, S.E.P.; Green, A.R.; Kumar, S.; et al. IL6/STAT3 Signaling Hijacks Estrogen Receptor alpha Enhancers to Drive Breast Cancer Metastasis. Cancer Cell 2020, 38, 412–423.e9. [Google Scholar] [CrossRef]
- Williams, K.; Sobol, R.W. Mutation research/fundamental and molecular mechanisms of mutagenesis: Special issue: DNA repair and genetic instability. Mutat. Res. 2013, 743, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Geenen, J.J.J.; Linn, S.C.; Beijnen, J.H.; Schellens, J.H.M. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin. Pharmacokinet. 2018, 57, 427–437. [Google Scholar] [CrossRef]
- Singh, D.D.; Yadav, D.K. TNBC: Potential Targeting of Multiple Receptors for a Therapeutic Breakthrough, Nanomedicine, and Immunotherapy. Biomedicines 2021, 9, 876. [Google Scholar] [CrossRef] [PubMed]
- Kalimutho, M.; Parsons, K.; Mittal, D.; Lopez, J.A.; Srihari, S.; Khanna, K.K. Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol. Sci. 2015, 36, 822–846. [Google Scholar] [CrossRef] [PubMed]
- Ueng, S.H.; Chen, S.C.; Chang, Y.S.; Hsueh, S.; Lin, Y.C.; Chien, H.P.; Lo, Y.F.; Shen, S.C.; Hsueh, C. Phosphorylated mTOR expression correlates with poor outcome in early-stage triple negative breast carcinomas. Int. J. Clin. Exp. Pathol. 2012, 5, 806–813. [Google Scholar]
- Vitali, F.; Cohen, L.D.; Demartini, A.; Amato, A.; Eterno, V.; Zambelli, A.; Bellazzi, R. A Network-Based Data Integration Approach to Support Drug Repurposing and Multi-Target Therapies in Triple Negative Breast Cancer. PLoS ONE 2016, 11, e0162407. [Google Scholar] [CrossRef]
- Vundavilli, H.; Datta, A.; Sima, C.; Hua, J.; Lopes, R.; Bittner, M. Bayesian Inference Identifies Combination Therapeutic Targets in Breast Cancer. IEEE Trans. Biomed. Eng. 2019, 66, 2684–2692. [Google Scholar] [CrossRef]
- Tada, H.; Shiho, O.; Kuroshima, K.; Koyama, M.; Tsukamoto, K. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 1986, 93, 157–165. [Google Scholar] [CrossRef]
- Qu, Z.; Lin, Y.; Mok, D.K.; Bian, Q.; Tai, W.C.; Chen, S. Arnicolide D Inhibits Triple Negative Breast Cancer Cell Proliferation by Suppression of Akt/mTOR and STAT3 Signaling Pathways. Int. J. Med. Sci. 2020, 17, 1482–1490. [Google Scholar] [CrossRef]
- Chen, G.; Gong, R.; Shi, X.; Yang, D.; Zhang, G.; Lu, A.; Yue, J.; Bian, Z. Halofuginone and artemisinin synergistically arrest cancer cells at the G1/G0 phase by upregulating p21Cip1 and p27Kip1. Oncotarget 2016, 7, 50302–50314. [Google Scholar] [CrossRef]
- Qu, Z.; Lin, Y.; Mok, D.K.; Bian, Q.; Tai, W.C.; Chen, S. Brevilin A, a Natural Sesquiterpene Lactone Inhibited the Growth of Triple-Negative Breast Cancer Cells via Akt/mTOR and STAT3 Signaling Pathways. OncoTargets Ther. 2020, 13, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Chandrasekaran, B.; Tyagi, A.; Shukla, V.; Singh, A.; Sharma, A.K.; Damodaran, C. A small molecule inhibitor of Notch1 modulates stemness and suppresses breast cancer cell growth. Front. Pharmacol. 2023, 14, 1150774. [Google Scholar] [CrossRef] [PubMed]
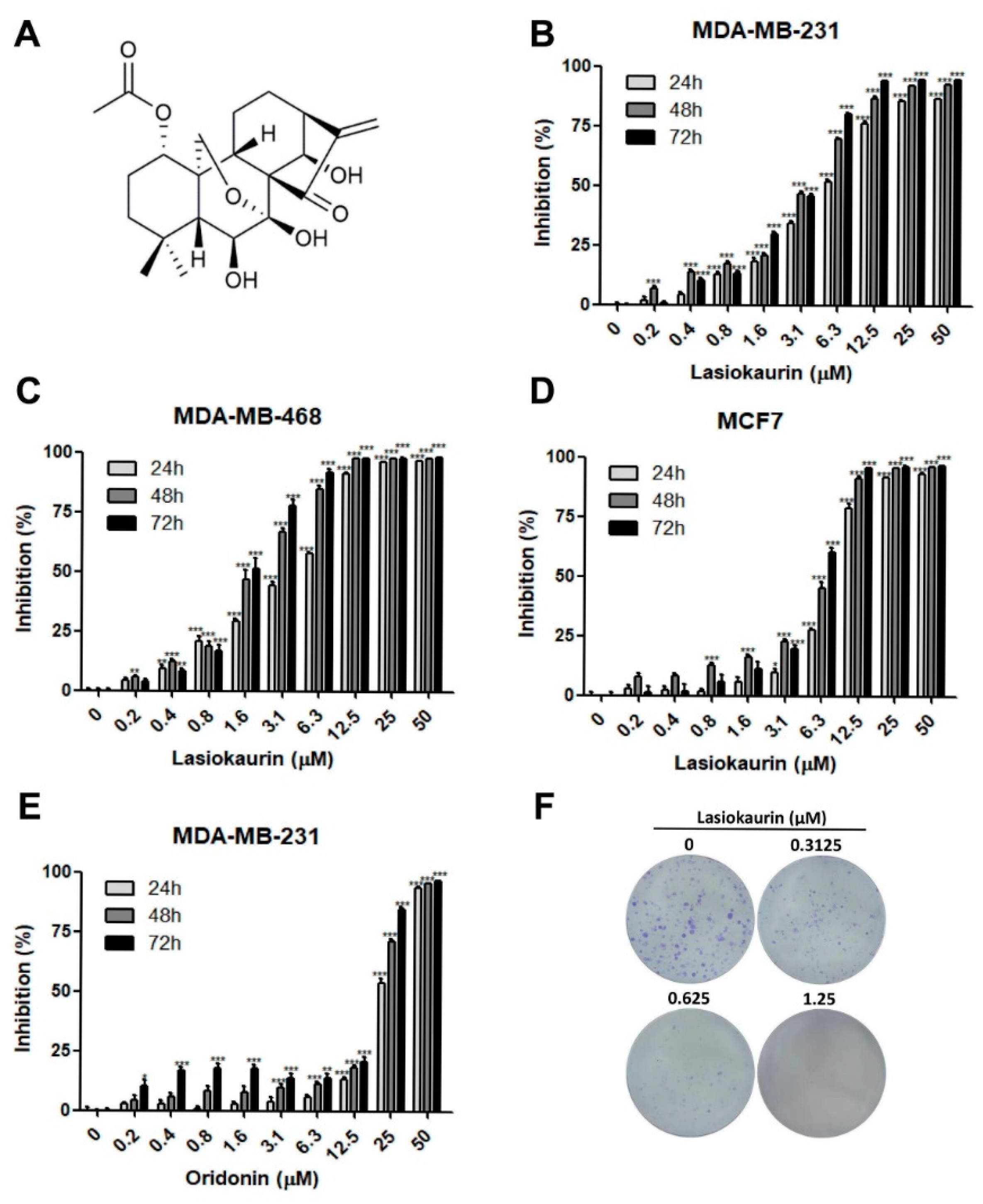

| Compound | Cell Line | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| LAS | MDA-MB-231 | 5.43 | 3.37 | 2.9 |
| MDA-MB-468 | 3.42 | 1.84 | 1.6 | |
| MCF7 | 8.35 | 5.69 | 5.16 | |
| MCF-10A | 25.84 | 6.69 | 5.95 | |
| Oridonin | MDA-MB-231 | 23.38 | 18.96 | 16.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Qu, Z.; Pu, H.; Shen, L.-S.; Yi, X.; Lin, Y.-S.; Gong, R.-H.; Chen, G.-Q.; Chen, S. In Vitro and In Vivo Anti-Cancer Activity of Lasiokaurin in a Triple-Negative Breast Cancer Model. Molecules 2023, 28, 7701. https://doi.org/10.3390/molecules28237701
Lin J, Qu Z, Pu H, Shen L-S, Yi X, Lin Y-S, Gong R-H, Chen G-Q, Chen S. In Vitro and In Vivo Anti-Cancer Activity of Lasiokaurin in a Triple-Negative Breast Cancer Model. Molecules. 2023; 28(23):7701. https://doi.org/10.3390/molecules28237701
Chicago/Turabian StyleLin, Jinrong, Zhao Qu, Huanhuan Pu, Li-Sha Shen, Xianguo Yi, Yu-Shan Lin, Rui-Hong Gong, Guo-Qing Chen, and Sibao Chen. 2023. "In Vitro and In Vivo Anti-Cancer Activity of Lasiokaurin in a Triple-Negative Breast Cancer Model" Molecules 28, no. 23: 7701. https://doi.org/10.3390/molecules28237701
APA StyleLin, J., Qu, Z., Pu, H., Shen, L.-S., Yi, X., Lin, Y.-S., Gong, R.-H., Chen, G.-Q., & Chen, S. (2023). In Vitro and In Vivo Anti-Cancer Activity of Lasiokaurin in a Triple-Negative Breast Cancer Model. Molecules, 28(23), 7701. https://doi.org/10.3390/molecules28237701








