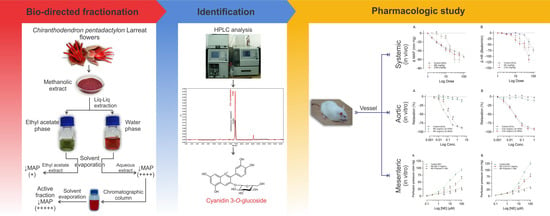
The Hypotensive and Vasodilatory Effects Observed in Rats Exposed to Chiranthodendron pentadactylon Larreat Flowers Can Be Attributed to Cyanidin 3-O-Glucoside
Abstract
:1. Introduction
2. Results
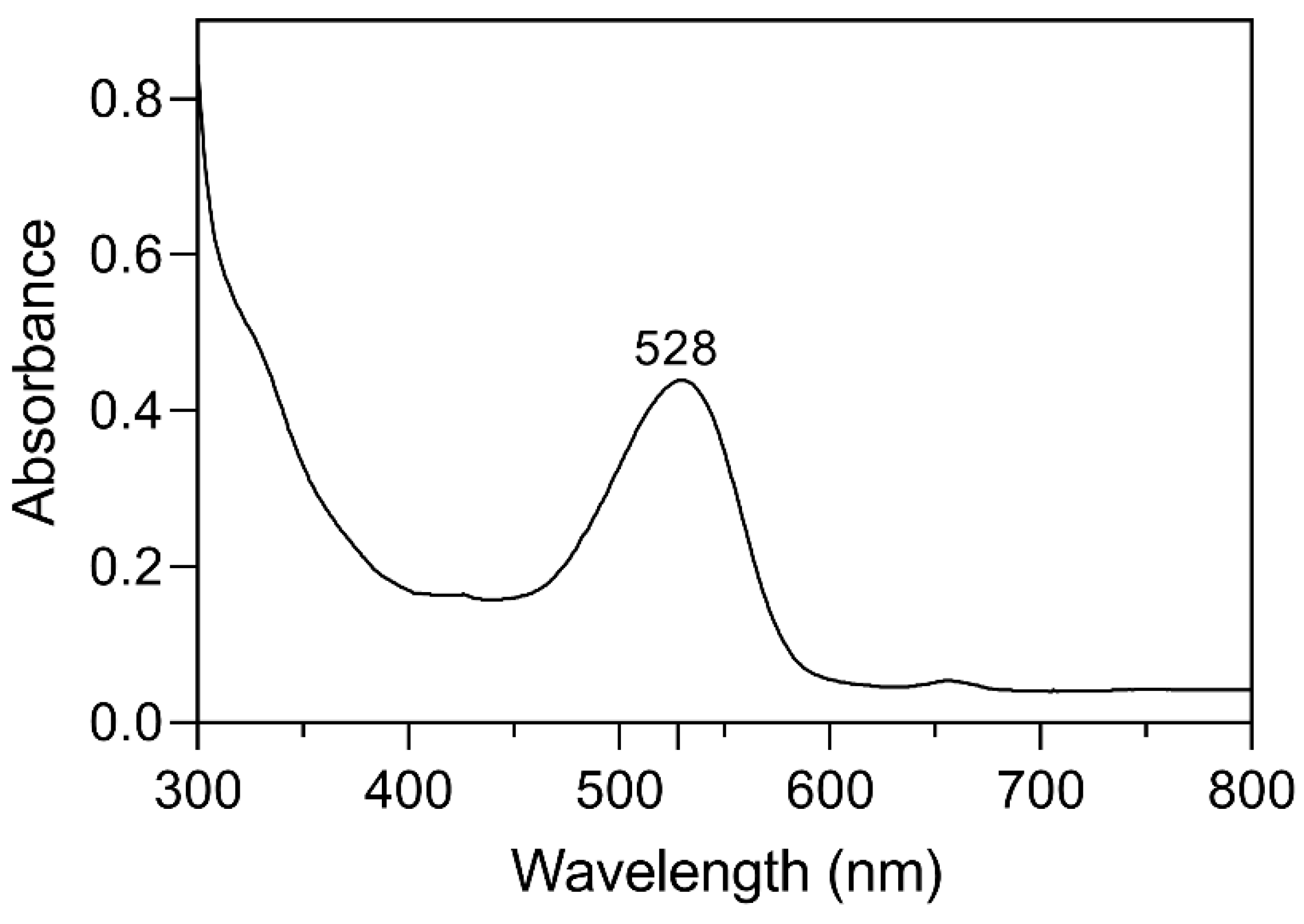
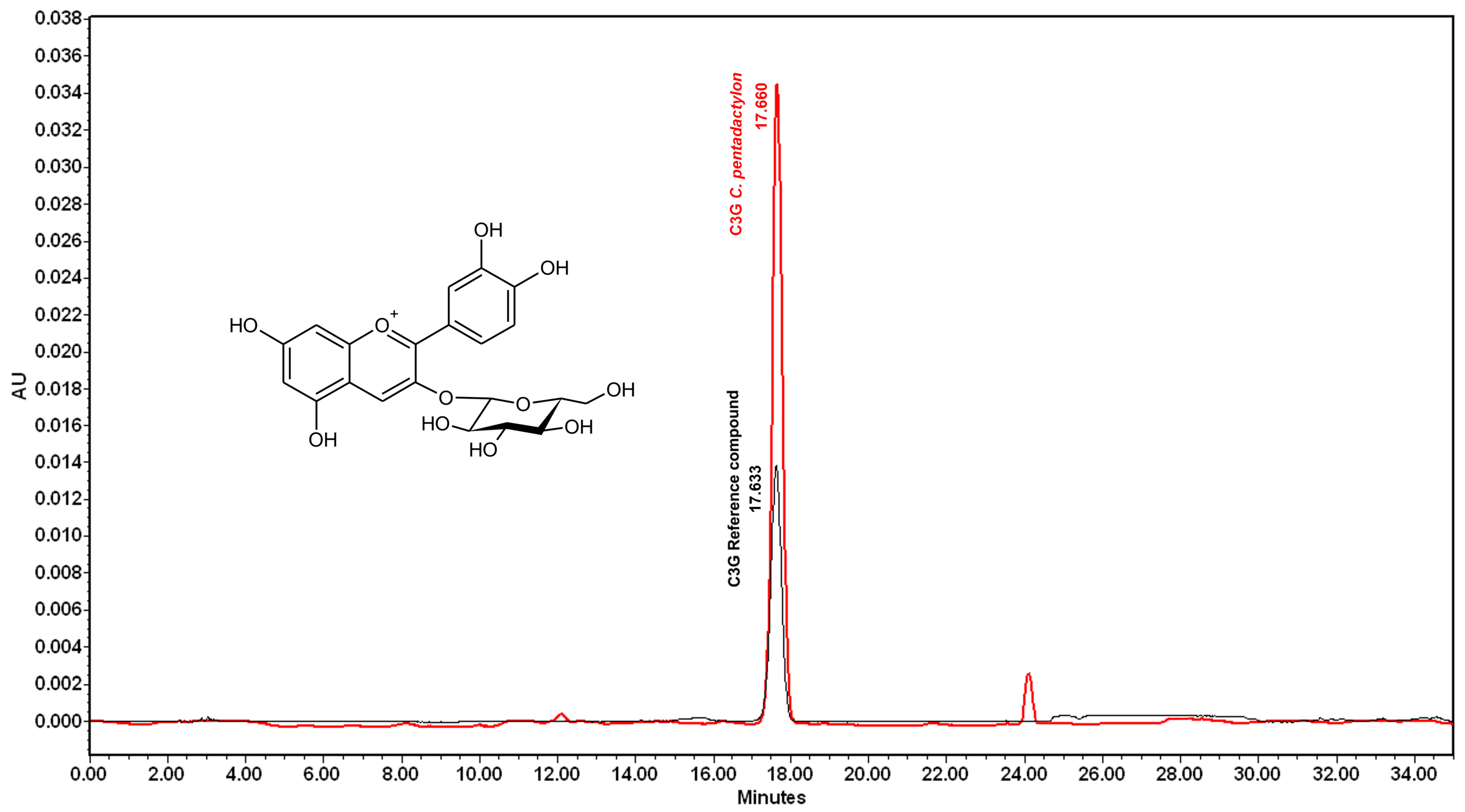
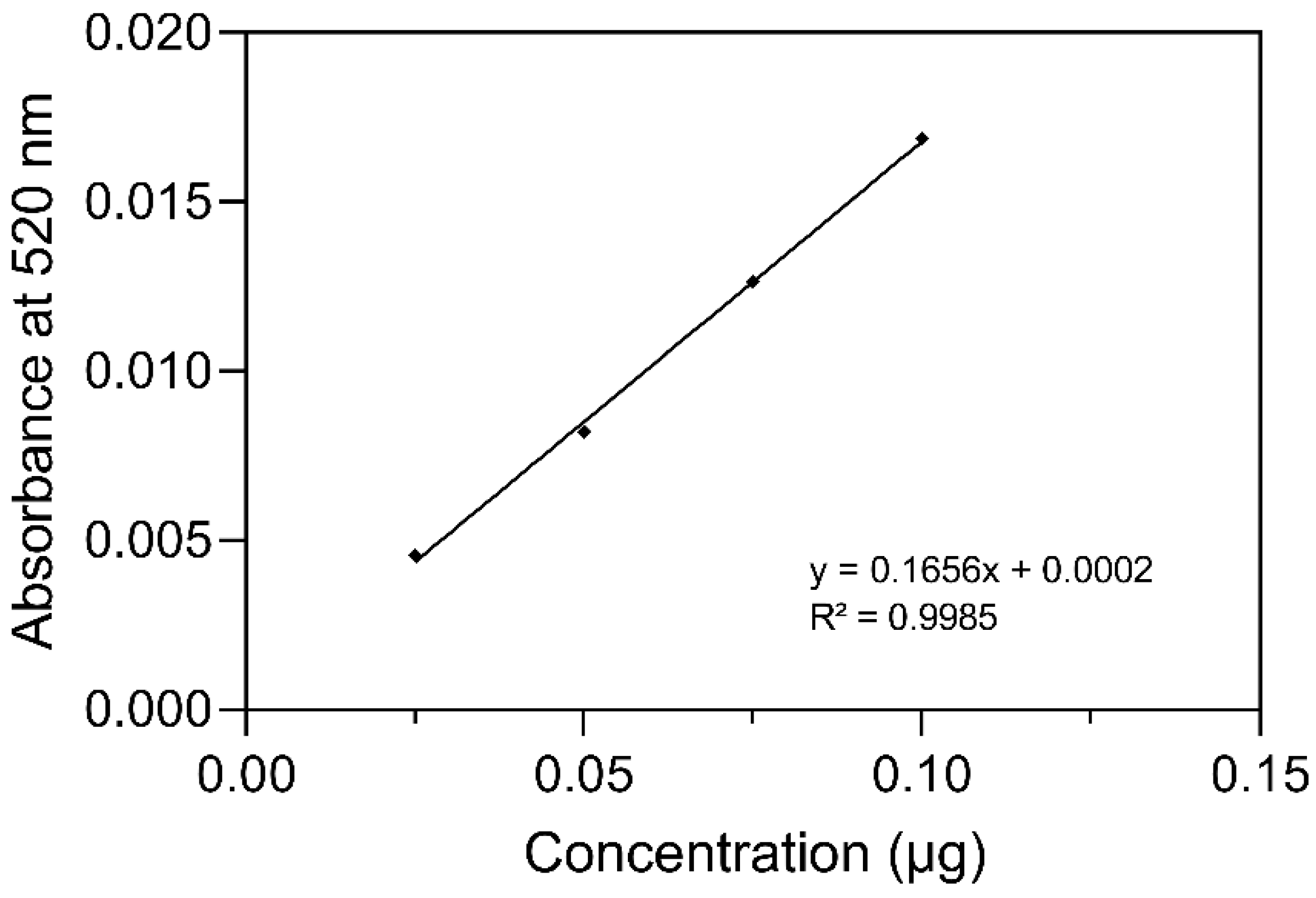
2.1. Identification and Quantification of Anthocyanin
2.2. Effects of Increasing Doses of ACh, ME, or C3G on the MAPHR
2.3. Effects of a Single Dose of ME or C3G on the MAPHR
2.4. Selection of the Aqueous Extract
2.5. Influence of L-NAME, Atropine, and Indomethacin on the Effects of C3G in the MAPHR
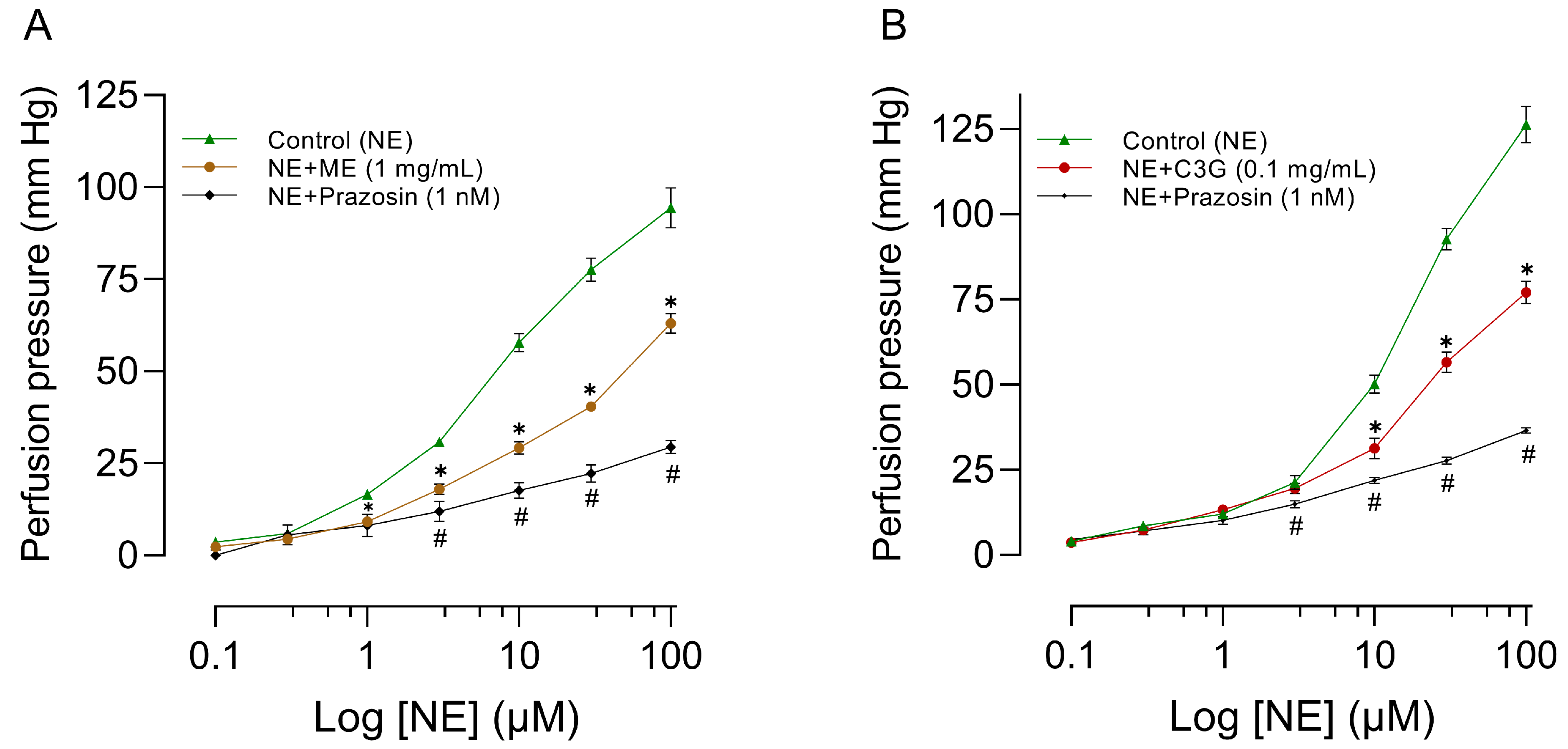
2.6. Effects of Increasing Doses of ME or C3G on RIAR
2.7. Influence of L-NAME, Atropine, and Indomethacin on the Effects of ME or C3G of the Flowers on RIAR
2.8. Effects of a Single Dose of ME or C3G on the Flowers on the MABR
2.9. Influence of L-NAME on the Effects of C3G of the Flowers on the MABR
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Plant Material
4.3. Extraction and Fractionation
4.4. Anthocyanin Extraction
4.5. Identification and Quantification of Anthocyanin
4.6. Sample and C3G-Standard Preparation
4.7. Animals
4.8. Pharmacological Experiments
4.8.1. Effects of Increasing Doses of ME from the Flowers on the MAPHR
4.8.2. Effects of One Dose of ME on the MAPHR
4.8.3. Selection of the Active Extract and Their Division
4.8.4. Effects of Increasing Doses of C3G of the Flowers on the MAPHR
4.8.5. Effects of a Single Dose of C3G of the Flowers on the MAPHR
4.8.6. Influence of L-NAME, Atropine, and Indomethacin on the Effects of C3G of the Flowers in MAPH
4.8.7. Effects of Increasing Doses of ME or C3G of the Flowers on RIAR
4.8.8. Influence of L-NAME, Atropine, and Indomethacin on the Effects of ME or C3G of the Flowers in RIAR
4.8.9. Effects of the Dose of ME or C3G of the Flowers on the MABR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Thouvenot. Diccionario Náhuatl-Español. Basado en los Diccionarios de Alonso de Molina con el Náhuatl Normalizado y el Español Modernizado, Colaboración de Javier Manríquez, Prólogo de Miguel León-Portilla; Instituto de Investigaciones Históricas/Fideicomiso Felipe Teixidor y Monserrat Alfau de Teixidor: Mexico City, México, 2014; p. 484. [Google Scholar]
- de la Cruz, M.; Badiano, J. Libellus de Medicinalibus Indorum Herbis. Manuscrito Azteca de 1552. Según Traducción Latina de Juan Badiano; Fondo de Cultura Económica/Instituto Mexicano del Seguro Social: México City, México, 1996. [Google Scholar]
- Linares, E.; Flores, B.; Bye, R. Selección de Plantas Medicinales de México; Limusa: Mexico City, México, 1988. [Google Scholar]
- Pulido-Salas, M.T. Atlas de las Plantas de la Medicina Tradicional Mexicana (Atlas of Plants from the Traditional Mexican Medicine); Instituto Indigenista Mexicano: Mexico City, México, 1994; Volume I–III. [Google Scholar]
- Martínez, M. Las Plantas Medicinales de México; Ediciones Botas: Mexico City, México, 1933. [Google Scholar]
- Espinoza Salas, A.J. Plantas Medicinales de la Huasteca Hidalguense México; Universidad Nacional Autónoma de México: Mexico City, México, 1985. [Google Scholar]
- Berenzon, S.; Saavedra, N. Presencia de la herbolaria en el tratamiento de los problemas emocionales: Entrevista a los curanderos urbanos. Salud Mental 2002, 25, 55–66. [Google Scholar]
- Tello-Ortega, K.E.; Hernández-Santiago, E.; Rodríguez-Ortíz, G. Medicina alternativa complementaria en el tratamiento de enfermedades crónicas en el sur de Oaxaca, México. CIENCIA Ergo-Sum 2020, 27, e87. [Google Scholar] [CrossRef]
- Ruiz Martínez, A.; Carmona López, A.M.; Ramírez Sánchez, P. Manejo forestal y saberes de los ejidatarios de San Sebastián Río Hondo, Oaxaca. Textual 2022, 79, 229–256. [Google Scholar] [CrossRef]
- Rosenheim, O. Observations on Anthocyanins. I: The Anthocyanins of the Young Leaves of the Grape Vine. Biochem. J. 1920, 14, 178–188. [Google Scholar] [CrossRef]
- Harborne, J.B.; Smith, D.M. Notizen: Flavonoid Pigments and Plant Phylogeny: The Case of the Hand-flower tree Chiranthodendron pentadactylon. Zeitschrift Für Naturforschung B 1972, 27, 210. [Google Scholar] [CrossRef]
- Chandra Singh, M.; Price, W.E.; Kelso, C.; Charlton, K.; Probst, Y. Impact of molar absorbance on anthocyanin content of the foods. Food Chem. 2022, 386, 132855. [Google Scholar] [CrossRef]
- Domínguez, X.A.; Quevedo, J.; Gutierrez, A.M.A. Estudio químico de la Flor de Manita (Macpaxochitl) Chiranihodendron pentadactylon. Cienc. Mex. 1970, XXVII, 87–89. [Google Scholar]
- Velázquez, C.; Calzada, F.; Esquivel, B.; Barbosa, E.; Calzada, S. Antisecretory activity from the flowers of Chiranthodendron pentadactylon and its flavonoids on intestinal fluid accumulation induced by Vibrio cholerae toxin in rats. J. Ethnopharmacol. 2009, 126, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez Vizcaíno, F.; Utrilla, P.; Jiménez, J.; Tamargo, J.; Zarzuelo, A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen. Pharmacol. 1993, 24, 857–862. [Google Scholar] [CrossRef]
- Gasparotto Junior, A.; Gasparotto, F.M.; Lourenço, E.L.; Crestani, S.; Stefanello, M.E.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.; Kassuya, C.A. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef]
- Galleano, M.; Bernatova, I.; Puzserova, A.; Balis, P.; Sestakova, N.; Pechanova, O.; Fraga, C.G. (−)-Epicatechin reduces blood pressure and improves vasorelaxation in spontaneously hypertensive rats by NO-mediated mechanism. IUBMB Life 2013, 65, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.C.; Pereira, A.C.; Rezende, B.A.; da Silva, J.P.; Cruz, J.S.; de Souza Mde, F.; Gomes, R.A.; Teles, Y.C.; Cortes, S.F.; Lemos, V.S. Mechanism of the antihypertensive and vasorelaxant effects of the flavonoid tiliroside in resistance arteries. Planta Med. 2013, 79, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Bondonno, C.P.; Rich, L.; Mas, E.; Shinde, S.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Acute effects of quercetin-3-O-glucoside on endothelial function and blood pressure: A randomized dose-response study. Am. J. Clin. Nutr. 2016, 104, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.; Jones, G.; Ung, A. Catechin Reduces Blood Pressure in Spontaneously Hypertensive Rats through Modulation of Arachidonic Acid Metabolism. Molecules 2022, 27, 8432. [Google Scholar] [CrossRef]
- Calzada, F.; Juárez, T.; García-Hernández, N.; Valdes, M.; Ávila, O.; Mulia, L.Y.; Velázquez, C. Antiprotozoal, Antibacterial and Antidiarrheal Properties from the Flowers of Chiranthodendron pentadactylon and Isolated Flavonoids. Pharmacogn. Mag. 2017, 13, 240–244. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Yang, M.; Abdullah; Ahmad, N.; Hussain, M.; Lu, X.; Xu, J.; Zhong, H.; Guan, R. A review of recent advances on cyanidin-3-glucoside: The biotransformation, absorption, bioactivity, and applications of nano-encapsulation. Food Funct. 2023, 14, 6320–6345. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Osawa, T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999, 449, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Vrhovsek, U.; Tramer, F.; Mattivi, F.; Passamonti, S. Exceptionally fast uptake and metabolism of cyanidin 3-glucoside by rat kidneys and liver. J. Nat. Prod. 2011, 74, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, L.; Tramer, F.; Moze, S.; Vrhovsek, U.; Mattivi, F.; Passamonti, S. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic. Biol. Med. 2012, 52, 1750–1759. [Google Scholar] [CrossRef]
- Perusquía, M.; Mendoza, S.; Bye, R.; Linares, E.; Mata, R. Vasoactive effects of aqueous extracts from five Mexican medicinal plants on isolated rat aorta. J. Ethnopharmacol. 1995, 46, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Alvarado, C.; Rojas, A.; Mendoza, S.; Bah, M.; Gutiérrez, D.M.; Hernández-Sandoval, L.; Martínez, M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010, 48, 732–739. [Google Scholar] [CrossRef]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 2004, 44, 217–222. [Google Scholar] [CrossRef]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Cyanidin-3-glucoside regulates phosphorylation of endothelial nitric oxide synthase. FEBS Lett. 2004, 574, 176–180. [Google Scholar] [CrossRef]
- Fushimi, T.; Oyama, S.; Koizumi, R.; Fujii, Y.; Osakabe, N. Impact of cyanidin 3-O-glucoside on rat micro-and systemic circulation, possibly through angiogenesis. J. Clin. Biochem. Nutr. 2023, 72, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, E.A. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995, 9, 1319–1330. [Google Scholar] [CrossRef]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, M.; Liu, C.; Guo, H.; Ye, Q.; Hu, Y.; Zhang, Y.; Hou, M.; Zhu, H.; Ma, J.; et al. Cyanidin-3-O-beta-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci. 2008, 83, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Alshihabi, S.N.; Chang, Y.S.; Frangos, J.A.; Tarbell, J.M. Shear stress-induced release of PGE2 and PGI2 by vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1996, 224, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.; Schulz, R.; Hodson, H.F.; Moncada, S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990, 101, 746–752. [Google Scholar] [CrossRef]
- Gardiner, S.M.; Compton, A.M.; Kemp, P.A.; Bennett, T. Regional and cardiac hemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: Effects of NG-nitro-L-arginine methyl ester. Br. J. Pharmacol. 1990, 101, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.A.; Liles, J.T.; Baber, S.R.; Golwala, N.H.; Murthy, S.N.; Kadowitz, P.J. Analysis of L-NAME-dependent and -resistant responses to acetylcholine in the rat. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H688–H698. [Google Scholar] [CrossRef]
- López, R.M.; Pérez, T.; Castillo, C.; Castillo, M.C.; Castillo, E.F. Acute intravenous injection and short-term oral administration of N(G)-nitro-L-arginine methyl ester to the rat provoke increased pressor responses to agonists and hypertension, but not inhibition of acetylcholine-induced hypotensive responses. Fundam. Clin. Pharmacol. 2011, 25, 333–342. [Google Scholar] [CrossRef]
- Wenceslau, C.F.; McCarthy, C.G.; Earley, S.; England, S.K.; Filosa, J.A.; Goulopoulou, S.; Gutterman, D.D.; Isakson, B.E.; Kanagy, N.L.; Martinez-Lemus, L.A.; et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H77–H111. [Google Scholar] [CrossRef]
- Árbol de Manitas. Chiranthodendron Pentadactylon Larreat. Available online: https://enciclovida.mx/especies/172327-chiranthodendron-pentadactylon (accessed on 15 May 2023).
- White, R. Elsevier’s Dictionary of Plant Names of North America Including Mexico; Elsevier Science: Amsterdam, The Netherlands; Boston, MA, USA, 2003. [Google Scholar]
- NOM-059-SEMARNAT-2010; Official Mexican Standards. Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo. Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, México. 2010. Available online: https://www.profepa.gob.mx/innovaportal/file/435/1/NOM_059_SEMARNAT_2010.pdf (accessed on 15 May 2023).
- Chiranthodendron Pentadactylon Larreat. Available online: http://www.theplantlist.org/tpl1.1/record/kew-2718398 (accessed on 15 May 2023).
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001, F1.1.1–F1.1.11. [Google Scholar] [CrossRef]
- Jhan, J.K.; Chung, Y.C.; Chen, G.H.; Chang, C.H.; Lu, Y.C.; Hsu, C.K. Anthocyanin contents in the seed coat of black soya bean and their anti-human tyrosinase activity and antioxidative activity. Int. J. Cosmet. Sci. 2016, 38, 319–324. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Official Mexican Standards. Especificaciones Técnicas para la Producción. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: Mexico City, México. 1999. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 15 May 2023).
- NOM-087-SEMARNAT-SSA1-2002; Official Mexican Standards. Protección Ambiental-Salud Ambiental Residuos Peligrosos Biológico-Infecciosos-Clasificación y Especificaciones de Manejo. Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, México. 2002. Available online: https://www.cndh.org.mx/sites/default/files/doc/Programas/VIH/Leyes%20y%20normas%20y%20reglamentos/Norma%20Oficial%20Mexicana/NOM-087-SEMARNAT-SSA1-2002%20Proteccion%20ambiental-salud.pdf (accessed on 15 May 2023).
- Council, N.R. Guide for the Care and Use of Laboratory Animals: Eighth Edition; The National Academies Press: Washington, DC, USA, 2011; p. 246. [Google Scholar]
- Magos, G.A.; Vidrio, H.; Enríquez, R. Pharmacology of Casimiroa edulis; III. Relaxant and contractile effects in rat aortic rings. J. Ethnopharmacol. 1995, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Magos, G.A.; Vidrio, H.; Reynolds, W.F.; Enríquez, R.G. Pharmacology of Casimiroa edulis IV. Hypotensive effects of compounds isolated from methanolic extracts in rats and guinea pigs. J. Ethnopharmacol. 1999, 64, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, P.A.; Stitzel, R.E.; Head, R.J. Perfusion of the intact and partially isolated rat mesenteric vascular bed: Application to vessels from hypertensive and normotensive rats. Blood Vessels 1986, 23, 288–296. [Google Scholar] [CrossRef] [PubMed]

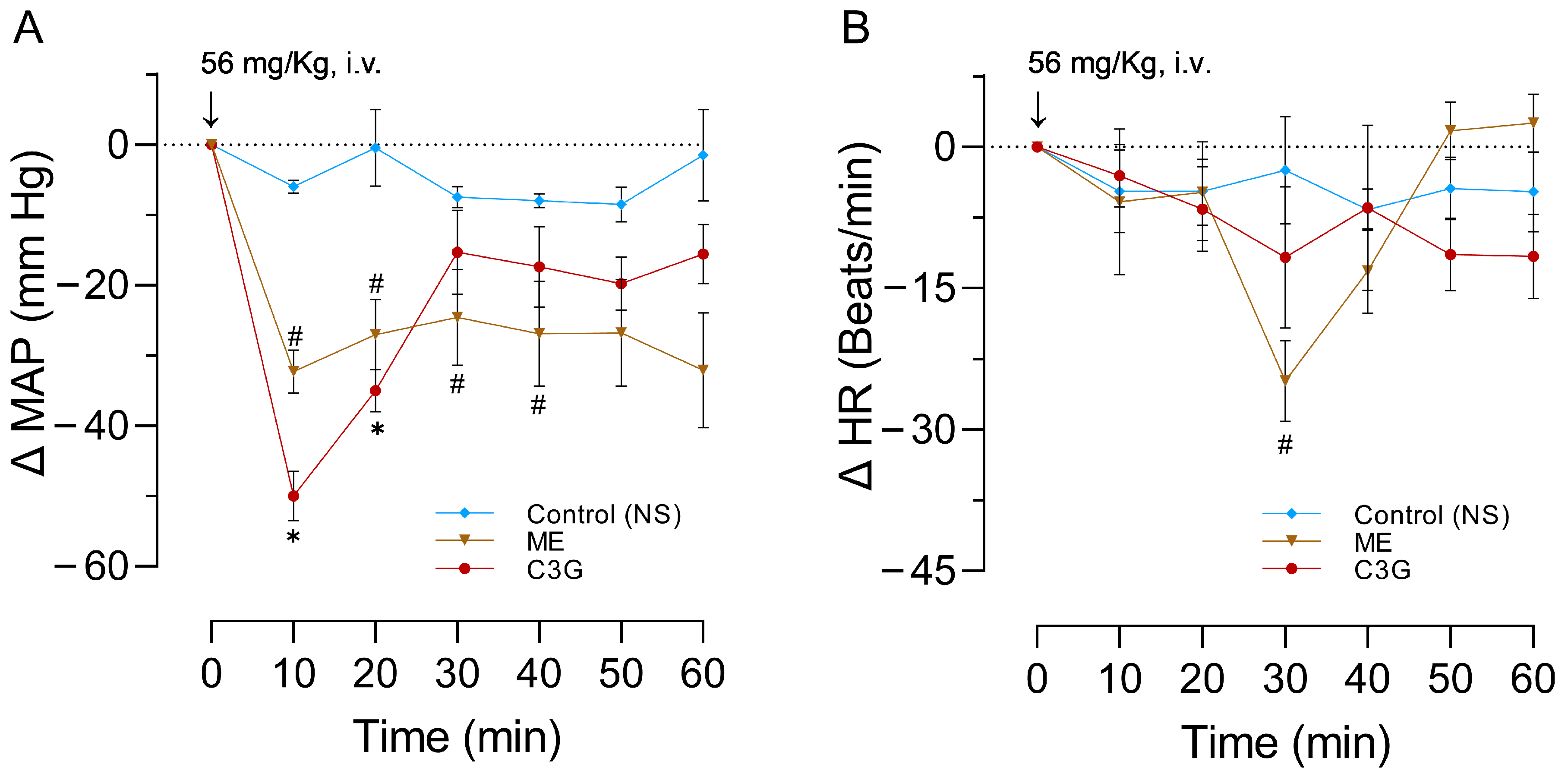
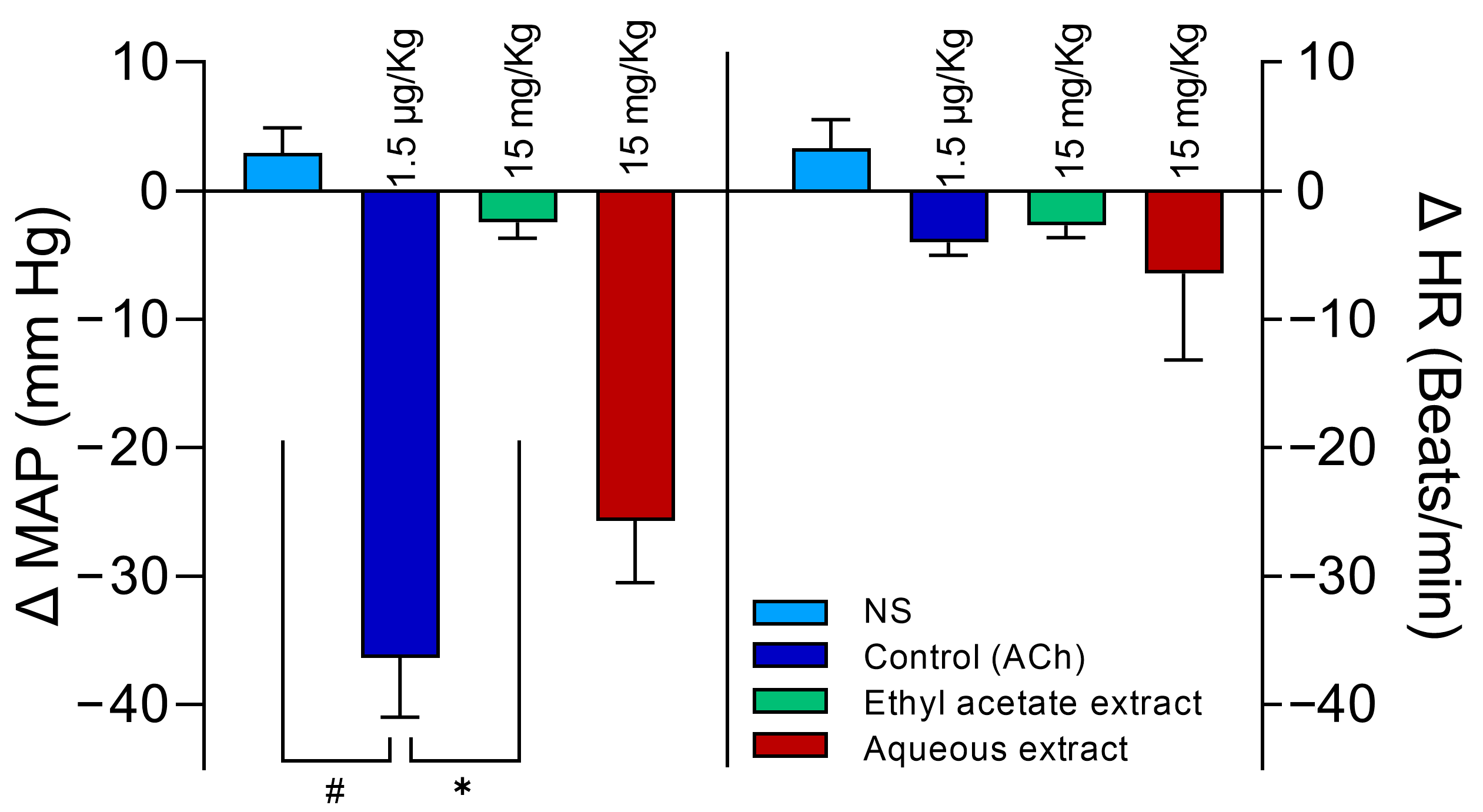
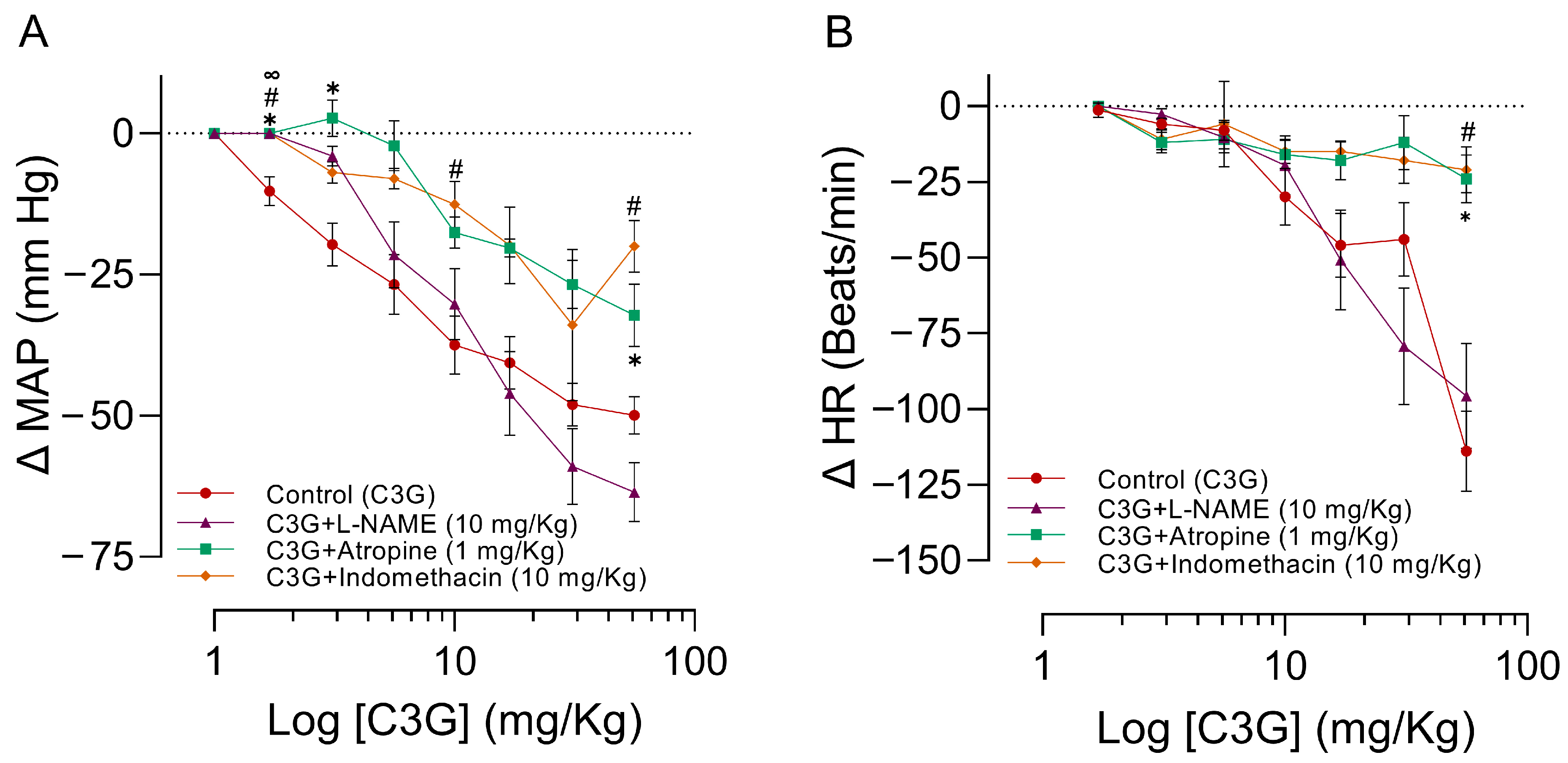
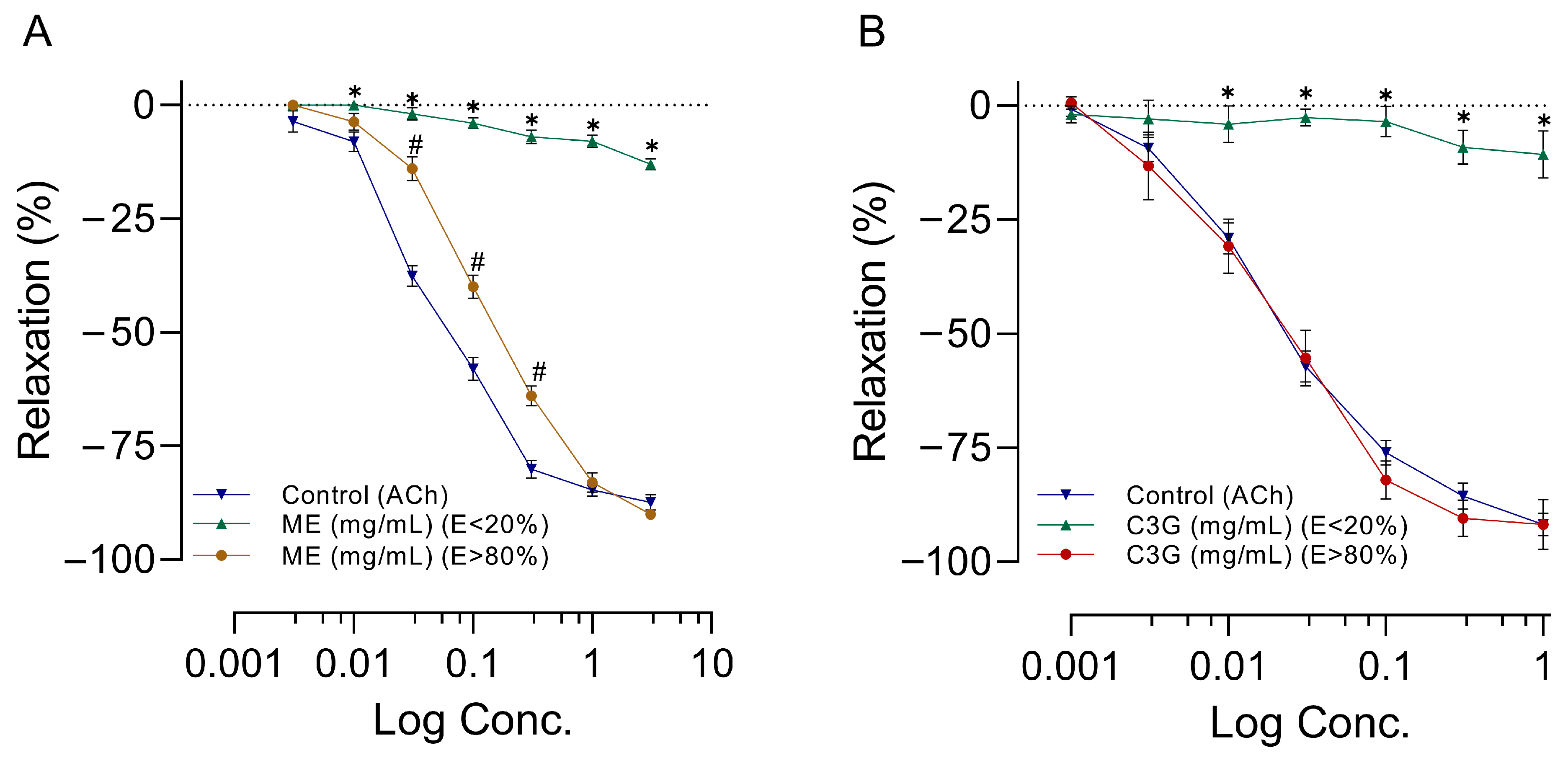


| Treatment | MAP (mm Hg) | HR (Beats/min) |
|---|---|---|
| Control (ACh) | 138.8 ± 11.7 | 318.7 ± 9.3 |
| ME | 152.4 ± 25.1 | 426.6 * ± 5.5 |
| C3G | 136.4 ± 2.9 | 331.8 ± 8.5 |
| Treatment | MAP (mm Hg) | HR (Beats/min) |
|---|---|---|
| Control (NS) | 123.8 ± 29.2 | 344.8 ± 34.8 |
| ME | 129.4 ± 11.0 | 358.2 ± 25.5 |
| C3G | 148.8 ± 32.9 | 361.6 ± 14.1 |
| Treatment | MAP (mm Hg) | HR (Beats/min) |
|---|---|---|
| NS | 151.1 ± 22.0 | 456.6 ± 6.0 |
| Control (ACh) | 138.5 ± 15.6 | 450.2 ± 8.4 |
| Ethyl acetate extract | 141.4 ± 11.3 | 456.3 ± 6.9 |
| Aqueous extract | 142.5 ± 10.9 | 473.1 ± 24.2 |
| Pretreatment | MAP (mm Hg) | HR (Beats/min) |
|---|---|---|
| Control (C3G) | 136.4 ± 2.9 | 331.8 ± 8.5 |
| C3G + L-NAME | 181.6 * ± 13.1 | 321.5 ± 7.4 |
| C3G + Atropine | 134.3 ± 6.3 | 310.1 ± 11.4 |
| C3G + Indomethacin | 145.8 ± 16.8 | 323.9 ± 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Ramírez, J.L.; Santiago-Mejía, J.; Soto-Núñez, M.; Barrera-Vázquez, O.S.; Vargas-Querea, R.; Magos-Guerrero, G.A. The Hypotensive and Vasodilatory Effects Observed in Rats Exposed to Chiranthodendron pentadactylon Larreat Flowers Can Be Attributed to Cyanidin 3-O-Glucoside. Molecules 2023, 28, 7698. https://doi.org/10.3390/molecules28237698
Escobar-Ramírez JL, Santiago-Mejía J, Soto-Núñez M, Barrera-Vázquez OS, Vargas-Querea R, Magos-Guerrero GA. The Hypotensive and Vasodilatory Effects Observed in Rats Exposed to Chiranthodendron pentadactylon Larreat Flowers Can Be Attributed to Cyanidin 3-O-Glucoside. Molecules. 2023; 28(23):7698. https://doi.org/10.3390/molecules28237698
Chicago/Turabian StyleEscobar-Ramírez, Juan Luis, Jacinto Santiago-Mejía, Maribel Soto-Núñez, Oscar Salvador Barrera-Vázquez, Roberto Vargas-Querea, and Gil Alfonso Magos-Guerrero. 2023. "The Hypotensive and Vasodilatory Effects Observed in Rats Exposed to Chiranthodendron pentadactylon Larreat Flowers Can Be Attributed to Cyanidin 3-O-Glucoside" Molecules 28, no. 23: 7698. https://doi.org/10.3390/molecules28237698
APA StyleEscobar-Ramírez, J. L., Santiago-Mejía, J., Soto-Núñez, M., Barrera-Vázquez, O. S., Vargas-Querea, R., & Magos-Guerrero, G. A. (2023). The Hypotensive and Vasodilatory Effects Observed in Rats Exposed to Chiranthodendron pentadactylon Larreat Flowers Can Be Attributed to Cyanidin 3-O-Glucoside. Molecules, 28(23), 7698. https://doi.org/10.3390/molecules28237698