The Physicochemical Characteristics and Heavy Metal Retention Capability of Black Liquor Lignin-Based Biochars
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of LGBCs
2.1.1. Morphological and Textural Properties
2.1.2. Surface Functional Groups
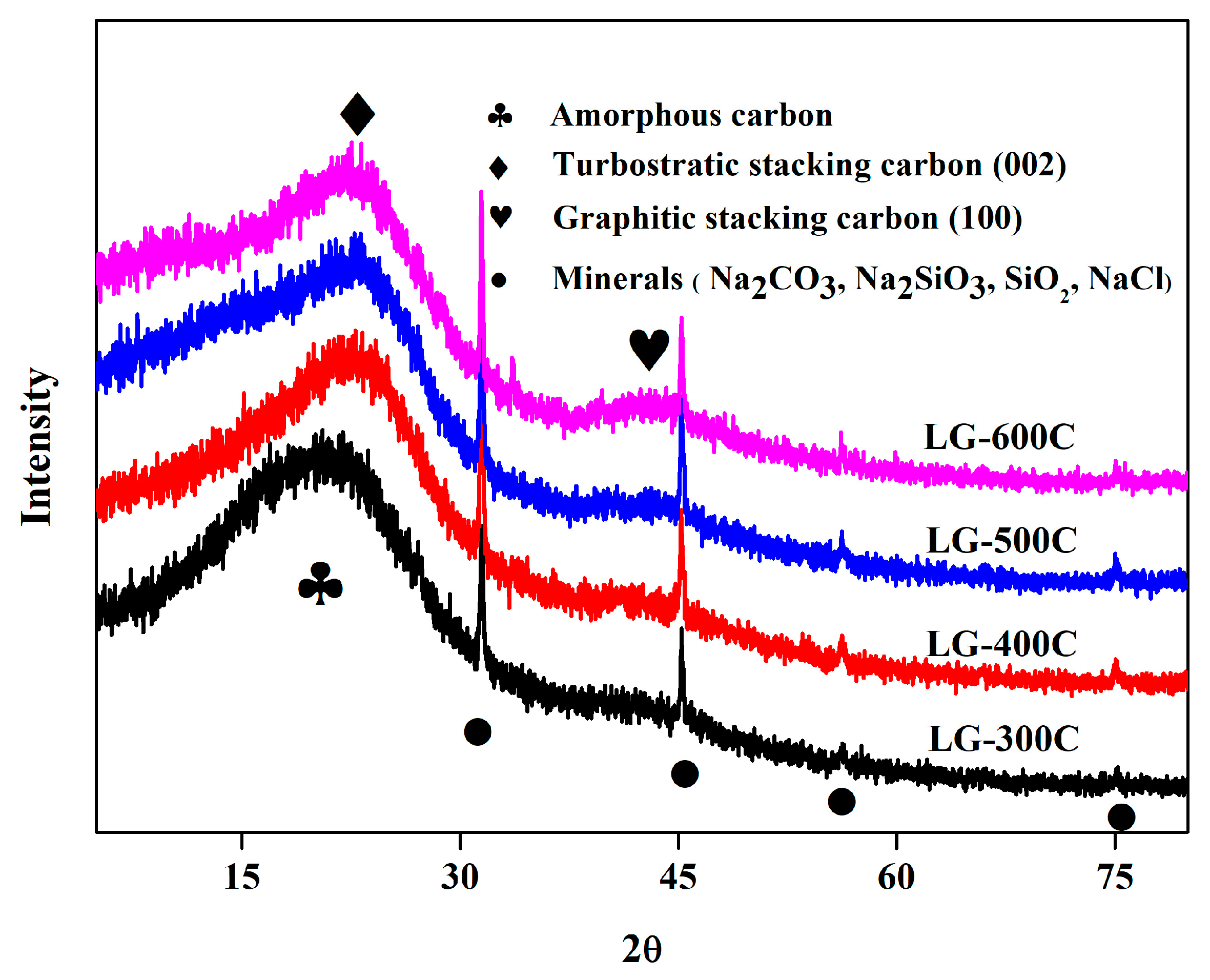
2.1.3. Minerals
2.2. Adsorption Behavior of LGBCs towards Cd(II)
2.3. Adsorption Mechanism of LGBCs towards Cd(II)
2.3.1. Adsorption Isotherm
2.3.2. Adsorption Kinetics
2.3.3. Thermodynamic Analysis
2.3.4. Potential Mechanisms
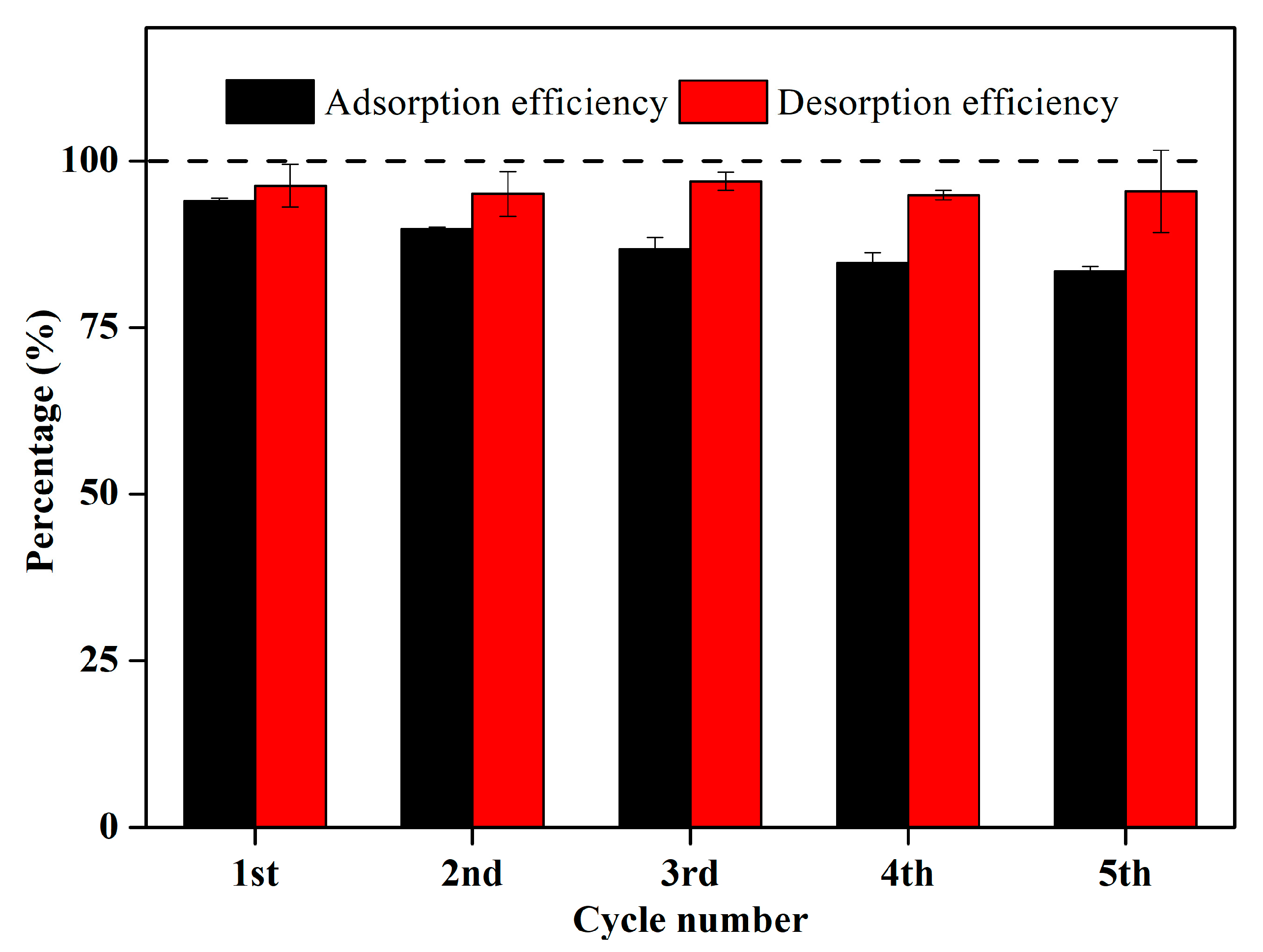
2.3.5. Reusability of LGBCs
3. Materials and Methods
3.1. Materials
3.2. Preparation of LGBCs
3.3. Characterization of LGBCs
3.4. Adsorption Tests of LGBCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Qin, S.Y.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and beneficial impact of biochar for environmental application: A comprehensive review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef]
- Wu, F.; Chen, L.; Hu, P.; Zhou, X.; Zhou, H.; Wang, D.; Lu, X.; Mi, B. Comparison of properties, adsorption performance and mechanisms to Cd(II) on lignin-derived biochars under different pyrolysis temperatures by microwave heating. Environ. Technol. Innov. 2022, 25, 102196. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Moulefera, I.; Rosas, J.M.; Benyoucef, A.; Rodríguez-Mirasol, J.; Cordero, T. Alcohol dehydrogenation on kraft lignin-derived chars with surface basicity. Catalysts 2017, 7, 308. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Wang, H.; Zhao, X.; Kong, F.; Liu, Y. Novel nitrogen-doped porous carbon with high surface areas prepared from industrial alkali lignin for supercapacitors. ChemElectroChem 2022, 9, 24. [Google Scholar] [CrossRef]
- Wang, D.; Li, G.; Zhang, C.; Wang, Z.; Li, X. Nickel nanoparticles inlaid in lignin-derived carbon as high effective catalyst for lignin depolymerization. Bioresour. Technol. 2019, 289, 121629. [Google Scholar] [CrossRef]
- Li, Y.Z.; Gupta, R.; Zhang, Q.Z.; You, S.M. Review of biochar production via crop residue pyrolysis: Development and perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 7. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Wu, C.; Gu, S. Ginkgo biloba L. shells-based adsorbent for the removal of Cu2+ and Cd2+ from aqueous solution: Kinetics, isotherm, thermodynamics and mechanisms. J. Mol. Liq. 2017, 241, 603–611. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, F.; Shen, D.; Jiang, Y.; Xiao, R. Immobilization of Cu(2+) and Cd(2+) by earthworm manure derived biochar in acidic circumstance. J. Environ. Sci. (China) 2017, 53, 293–300. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Shen, D.; Wu, C.; Gu, S. Co-pyrolysis of lignin and polyethylene with the addition of transition metals—Part I: Thermal behavior and kinetics analysis. J. Energy Inst. 2020, 93, 281–291. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Geoffrey Chan, W.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Su, D.S.; Chen, X.W. Natural lavas as catalysts for efficient production of carbon nanotubes and nanofibers. Angew Chem. Int. Ed. Engl. 2007, 46, 1823–1824. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.; Gao, C.; Sun, Q.; Liu, J.; She, D. Synthesis of honeycomb lignin-based biochar and its high-efficiency adsorption of norfloxacin. Bioresour. Technol. 2023, 369, 128402. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wu, Y.; Zheng, M. A comparison of biochars from lignin, cellulose and wood as the sorbent to an aromatic pollutant. J. Hazard Mater. 2014, 280, 450–457. [Google Scholar] [CrossRef]
- Wu, P.; Cui, P.; Fang, G.; Gao, J.; Zhou, D.; Wang, Y. Sorption mechanism of zinc on reed, lignin, and reed- and lignin-derived biochars: Kinetics, equilibrium, and spectroscopic studies. J. Soils Sediments 2018, 18, 2535–2543. [Google Scholar] [CrossRef]
- Cha, J.S.; Jang, S.H.; Lam, S.S.; Kim, H.; Kim, Y.M.; Jeon, B.H.; Park, Y.K. Performance of CO2 and Fe-modified lignin char on arsenic (V) removal from water. Chemosphere 2021, 279, 130521. [Google Scholar] [CrossRef]
- Li, C.; Hayashi, J.-i.; Sun, Y.; Zhang, L.; Zhang, S.; Wang, S.; Hu, X. Impact of heating rates on the evolution of function groups of the biochar from lignin pyrolysis. J. Anal. Appl. Pyrolysis 2021, 155, 105031. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, H.; Wang, X.; Kong, F.; Liu, Y.; Zhang, F. Fabrication of N-doped micro-mesoporous carbons from industrial alkali lignin with urea assistance for high-efficiency adsorption of methylene blue. Ind. Crops Prod. 2023, 203, 117146. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Han, Q.; Xie, A.; Zhou, Z.; Yang, J.; Tang, Q.; Mi, B.; Wu, F. Application of distributed activation energy model and Coats-Redfern integration method in the study of industrial lignin pyrolysis kinetics. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Wang, D.; Dong, S.; Fu, S.; Shen, Y.; Zeng, T.; Yu, W.; Lu, X.; Wang, L.; Song, S.; Ma, J. Catalytic ozonation for imazapic degradation over kelp-derived biochar: Promotional role of N- and S-based active sites. Sci. Total Environ. 2023, 860, 160473. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Zhu, K.; An, Q.; Hao, J.; Xiao, Z.; Dong, X.; Zhai, S. CoSx produced in Porphyra biochar by exogenous Co and endogenous S doping to enhance peroxymonosulfate activation for carbamazepine degradation. J. Environ. Chem. Eng. 2023, 11, 110988. [Google Scholar] [CrossRef]
- Zhang, Y.; He, R.; Zhao, J. Removal mechanism of tetracycline-Cr(Ⅵ) combined pollutants by different S-doped sludge biochars: Role of environmentally persistent free radicals. Chemosphere 2023, 317, 137856. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Nishchak, O.Y.; Zavidovskiy, I.A.; Maslakov, K.I.; Pavlikov, A.V. Sp-based thin films synthesized by magnetron sputtering of dehydrohalogenated Polyvinylidenchloride. Thin Solid Film. 2021, 739, 138993. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Balabanyan, V.Y.; Tsiskarashvili, A.V. Antibacterial properties of modified aC and ta-C coatings: The effects of the sp2/sp3 ratio, oxidation, nitridation, and silver incorporation. Appl. Phys. A 2022, 128, 929. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Wang, Z.; Ogata, H.; Morimoto, S.; Ortiz-Medina, J.; Fujishige, M.; Takeuchi, K.; Muramatsu, H.; Hayashi, T.; Terrones, M.; Hashimoto, Y.; et al. Nanocarbons from rice husk by microwave plasma irradiation: From graphene and carbon nanotubes to graphenated carbon nanotube hybrids. Carbon 2015, 94, 479–484. [Google Scholar] [CrossRef]
- Zhang, L.; You, T.; Zhou, T.; Zhou, X.; Xu, F. Interconnected hierarchical porous carbon from lignin-derived byproducts of bioethanol production for ultra-high performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 13918–13925. [Google Scholar] [CrossRef]
- Qin, K.; Li, J.; Yang, W.; Wang, Z.; Zhang, H. Role of minerals in mushroom residue on its adsorption capability to Cd(II) from aqueous solution. Chemosphere 2023, 324, 138290. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Wu, C.; Gu, S. Equilibrium, kinetics and thermodynamics of cadmium ions (Cd2+ ) removal from aqueous solution using earthworm manure-derived carbon materials. J. Mol. Liq. 2017, 241, 612–621. [Google Scholar] [CrossRef]
- Gao, Z.; Shan, D.; He, J.; Huang, T.; Mao, Y.; Tan, H.; Shi, H.; Li, T.; Xie, T. Effects and mechanism on cadmium adsorption removal by CaCl2-modified biochar from selenium-rich straw. Bioresour. Technol. 2023, 370, 128563. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, J.; Du, G.; Khan, K.Y.; Song, Y.; Cui, X.; Cheng, Z.; Yan, B.; Chen, G. Comparison of cadmium adsorption by hydrochar and pyrochar derived from Napier grass. Chemosphere 2022, 308, 136389. [Google Scholar] [CrossRef]
- Yan, Y.; Qi, F.; Zhang, L.; Zhang, P.; Li, Q. Enhanced Cd adsorption by red mud modified bean-worm skin biochars in weakly alkali environment. Sep. Purif. Technol. 2022, 297, 121533. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Xiao, R.; Wang, M.; Lee, Y.H.; Kang, S.-W.; Seo, D.-C. Characteristics of adsorption behavior of potentially toxic metals by biochar derived from fallen leaves (Platanus) and its mechanism. Sustain. Chem. Pharm. 2022, 29, 100776. [Google Scholar] [CrossRef]
- Tan, W.-T.; Zhou, H.; Tang, S.-F.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Enhancing Cd(II) adsorption on rice straw biochar by modification of iron and manganese oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef]
- Li, Z.; Su, Q.; Xiang, L.; Yuan, Y.; Tu, S. Effect of pyrolysis temperature on the sorption of Cd(II) and Se(IV) by rice husk biochar. Plants 2022, 11, 3234. [Google Scholar] [CrossRef]
- Kılıç, M.; Kırbıyık, Ç.; Çepelioğullar, Ö.; Pütün, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, K.; Wang, Z.; Shen, D.; Wu, C. Carbon nanotubes/Al2O3 composite derived from catalytic reforming of the pyrolysis volatiles of the mixture of polyethylene and lignin for highly-efficient removal of Pb(II). RSC Adv. 2021, 11, 37851–37865. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Wu, Z.; Zhang, Y.; Meng, Q.; Yan, L. Sulfur-modified Pleurotus ostreatus spent substrate biochar enhances the removal of cadmium in aqueous solution: Characterization, performance, mechanism. J. Environ. Manag. 2022, 322, 115900. [Google Scholar] [CrossRef]
- Wang, S.; Wang, N.; Yao, K.; Fan, Y.C.; Li, W.H.; Han, W.H.; Yin, X.H.; Chen, D.Y. Characterization and interpretation of Cd (II) adsorption by different modified rice straws under contrasting conditions. Sci. Rep. 2019, 9, 17868. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.H.; Zhao, N.; Li, J.; Lv, Y.H. Coupling interaction between porous biochar and nano zero valent iron/nano alpha-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chmosphere 2019, 219, 493–503. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Kim, W.-K.; Shim, T.; Kim, Y.-S.; Hyun, S.; Ryu, C.; Park, Y.-K.; Jung, J. Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour. Technol. 2013, 138, 266–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Chen, H.; Quan, G.; Gao, B. Potassium permanganate modification of hydrochar enhances sorption of Pb(II), Cu(II), and Cd(II). Bioresour. Technol. 2023, 386, 129482. [Google Scholar] [CrossRef]
- Kim, H.-S.; Choi, H.-J. Design of a novel sericite-phosphoric acid framework for enhancement of Pb(II) adsorption. Molecules 2023, 28, 7395. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, H.; Fan, H.; Zhou, S.; Huang, J. One-step fabrication of recyclable konjac glucomannan-based magnetic nanoparticles for highly efficient Cr (VI) adsorption. Molecules 2023, 28, 7100. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, J.; Xiao, R. Catalytic transformation of lignin to aromatic hydrocarbons over solid-acid catalyst: Effect of lignin sources and catalyst species. Energy Convers. Manag. 2016, 124, 61–72. [Google Scholar] [CrossRef]
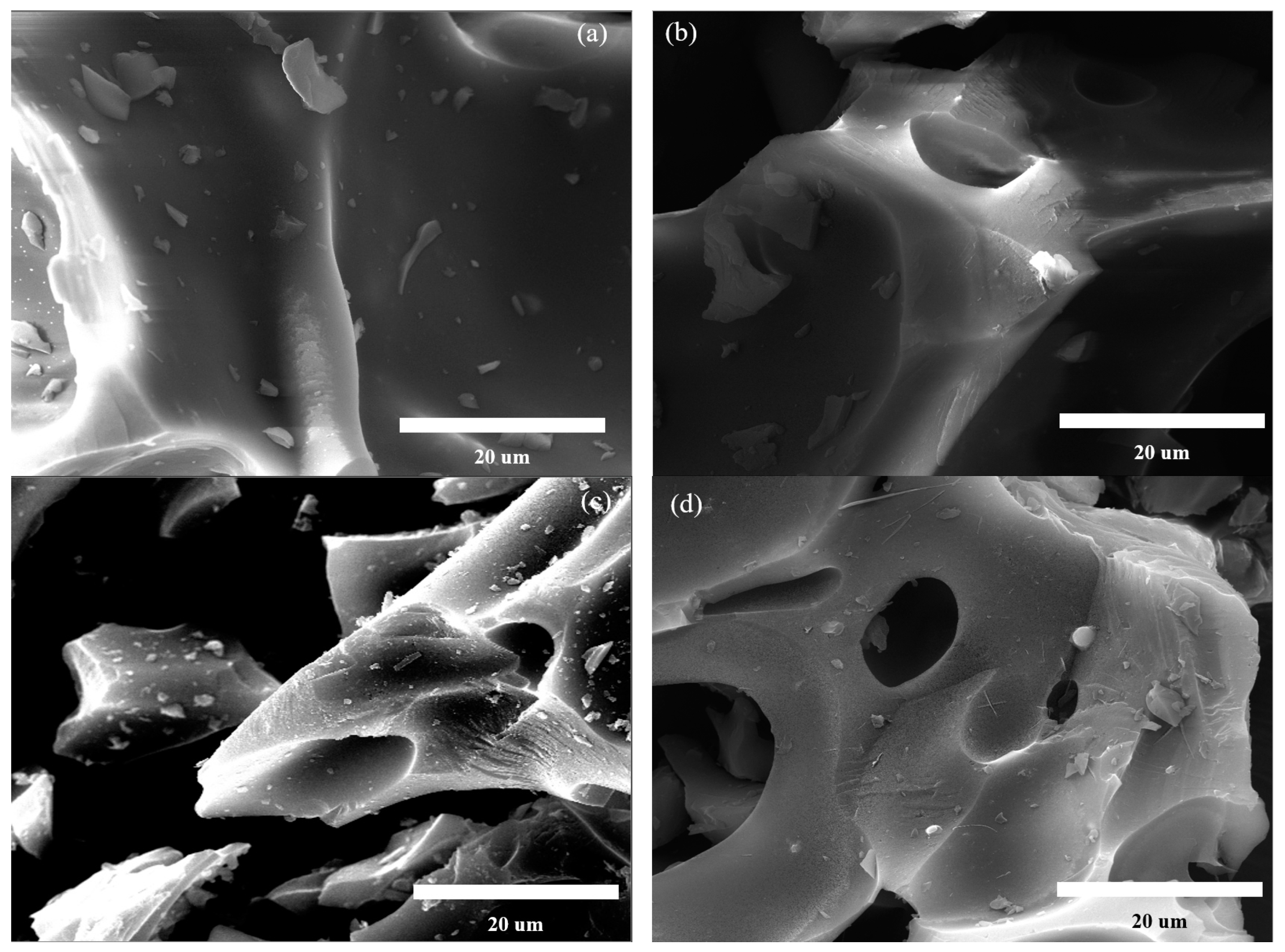
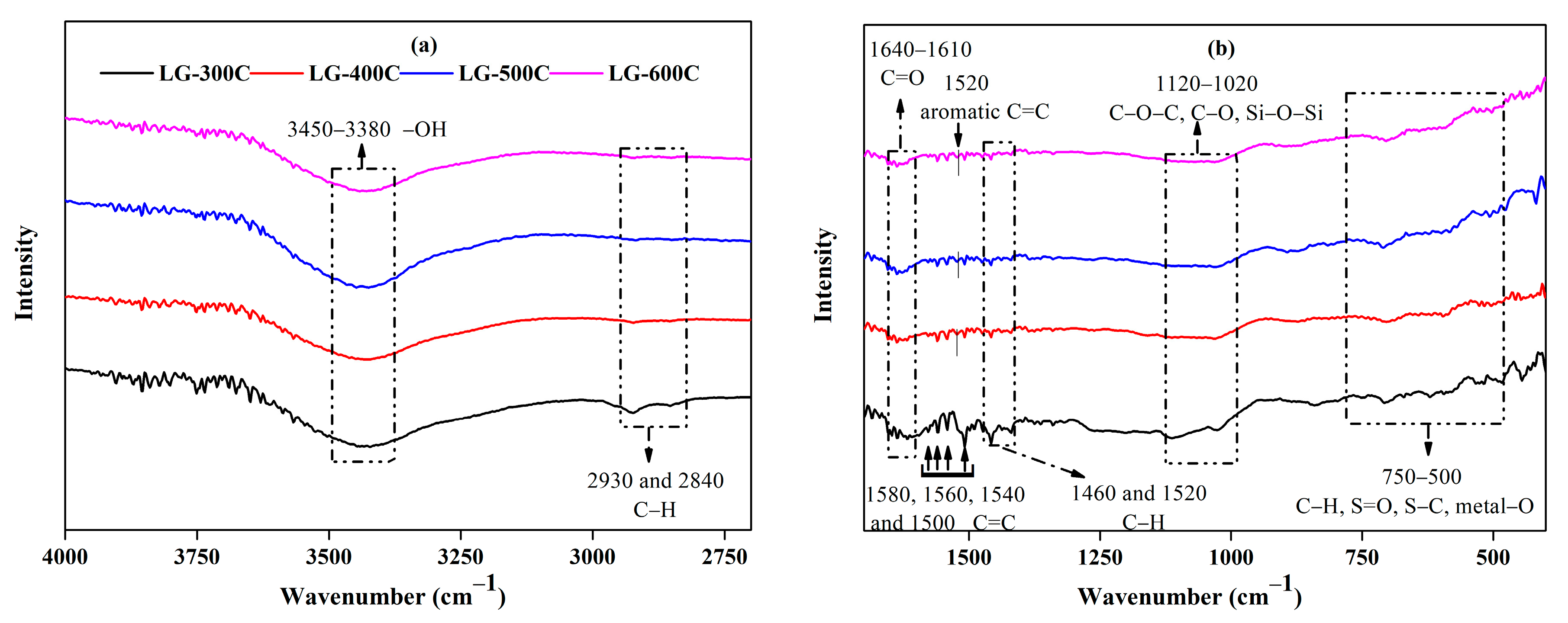
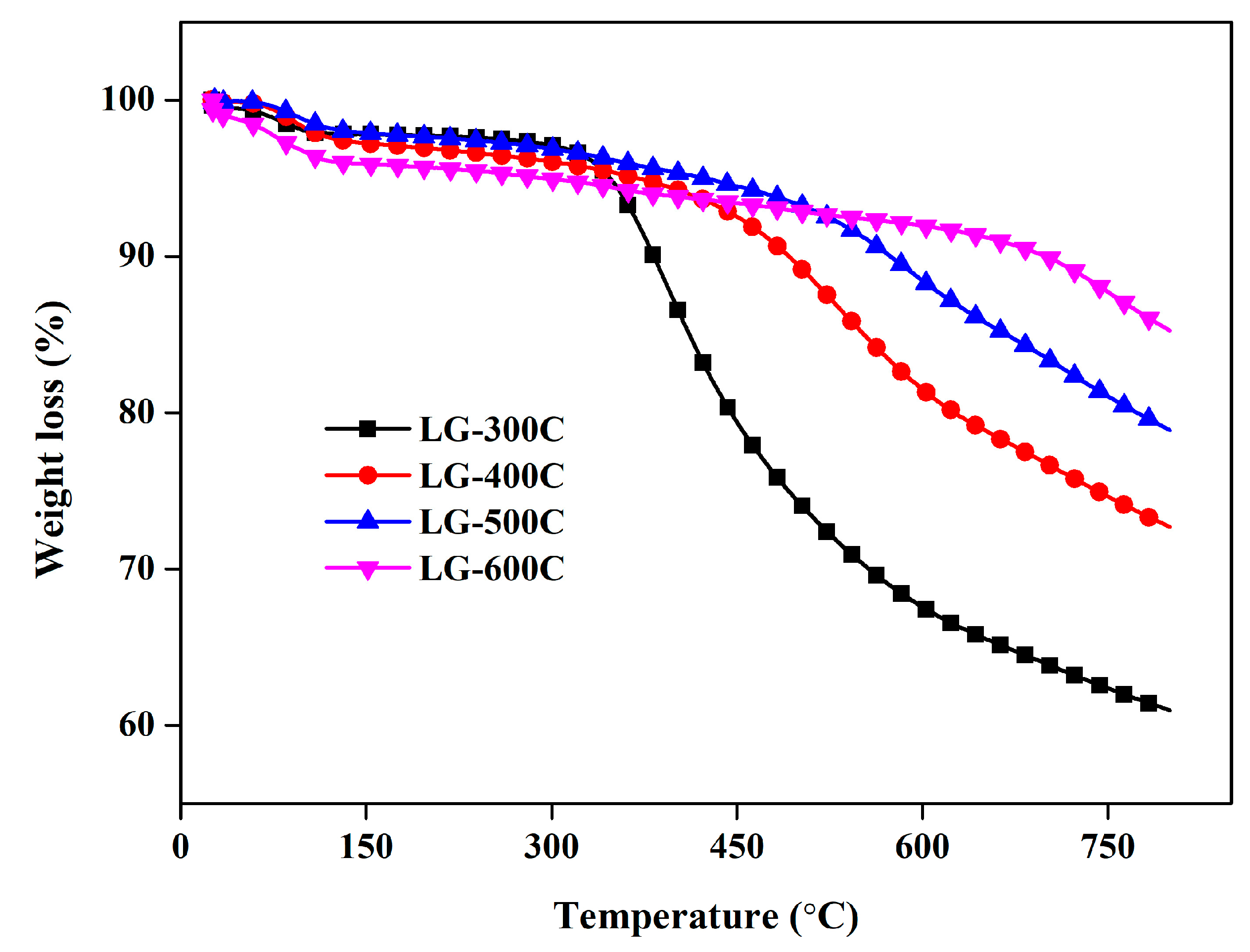
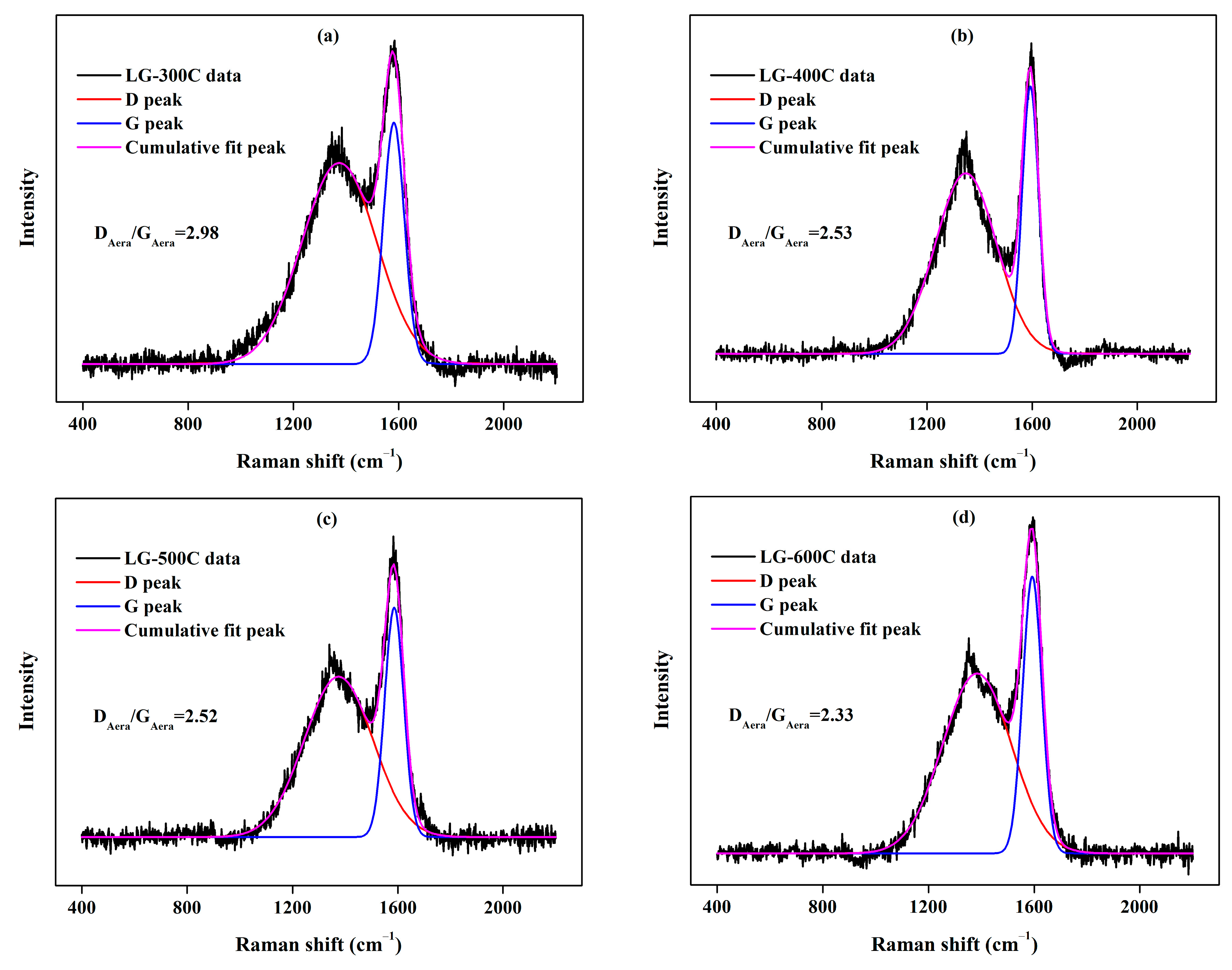

| Specific Surface Area (m2/g) | Micropore Specific Surface Area (m2/g) | Total Pore Volume (m3/g) | Micropore Pore Volume (m3/g) | Average Pore Size (nm) | Elemental Content (%) | Atomic Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | S | H/C | O/C | ||||||
| LG-300C | 3.14 | 1.07 | 0.005 | 0.004 | 64.73 | 60.21 | 5.48 | 29.44 | 1.45 | 0.09 | 0.49 |
| LG-400C | 8.42 | 1.26 | 0.006 | 0.004 | 25.68 | 67.08 | 4.82 | 23.04 | 1.02 | 0.07 | 0.34 |
| LG-500C | 67.32 | 25.80 | 0.12 | 0.04 | 14.15 | 73.41 | 4.20 | 16.38 | 0.47 | 0.06 | 0.22 |
| LG-600C | 89.76 | 44.77 | 0.15 | 0.09 | 5.36 | 77.68 | 3.99 | 11.75 | 0.36 | 0.05 | 0.15 |
| pH | pHpzc | Proximate Analysis | Minerals | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FC a | VM b | Ash | K | Na | Ca | Mg | Cd | |||
| % | mmol/kg | |||||||||
| LG-300C | 5.98 | 5.08 | 29.99 | 64.49 | 3.42 | 4.56 | 44.67 | 0.42 | 1.78 | - c |
| LG-400C | 6.84 | 5.92 | 39.78 | 54.22 | 4.04 | 6.98 | 51.87 | 0.98 | 1.98 | - |
| LG-500C | 7.34 | 6.76 | 46.32 | 46.38 | 5.54 | 7.76 | 53.06 | 1.87 | 2.28 | - |
| LG-600C | 7.87 | 6.96 | 71.67 | 21.08 | 6.22 | 9.22 | 65.09 | 2.25 | 3.69 | - |
| Sample | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | Kl (L/mg) | R2 | Kf (mg(1−n)Ln/g) | n | R2 | |
| LG-300C | 2.12 | 0.04 | 0.984 | 0.17 | 1.88 | 0.993 |
| LG-400C | 3.26 | 0.03 | 0.985 | 0.16 | 1.63 | 0.992 |
| LG-500C | 9.15 | 0.23 | 0.971 | 2.55 | 2.99 | 0.965 |
| LG-600C | 18.54 | 0.21 | 0.926 | 4.79 | 2.65 | 0.967 |
| No. | Biochar Sources | Prepared Conditions | Adsorption Conditions | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|
| 1 | Pleurotus ostreatus spent substrate | Pyrolysis at 500 °C for 2 h | Initial concentration in the range of 10~300 mg/L at 25 °C with solution pH of 7 for 24 h | 12.63 | [44] |
| 2 | Rice husk | Pyrolysis at 500 °C for 2 h | Initial concentration in the range of 20~800 mg/L at 25 °C for 24 h | 5.55 | [45] |
| 3 | Corn stalk | Pyrolysis at 600 °C for 2 h | Initial concentration in the range of 5~50 mg/L at 25 °C with solution pH of 6 for 24 h | 7.02 | [46] |
| 4 | Rice straw | Pyrolysis at 400 °C for 2 h | Initial concentration in the range of 1~60 mg/L at 25 °C with solution pH of 6 for 24 h | 10.07 | [47] |
| 5 | Giant Miscanthus | Pyrolysis at 600 °C for 1 h | Initial concentration in the range of 1~50 mg/L at 25 °C with solution pH of 7 for 48 h | 12.96 | [48] |
| 6 | Wheat straw | Hydrothermal carbonization at 600 °C for 4 h | Initial concentration in the range of 10~100 mg/L for 24 h | 1.70 | [49] |
| 7 | Black liquor lignin | Pyrolysis at 600 °C for 2 h | Initial concentration in the range of 5~60 mg/L at 25 °C with solution pH of 5 for 8 h | 18.54 | Present work |
| Sample | Qe,exp/(mg/g) | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|---|
| k1 (1/h) | Qe (mg/g) | R2 | k2 (g/mg h) | Qe (mg/g) | R2 | ||
| LG-300C | 0.90 | 4.84 | 0.91 | 0.979 | 7.26 | 0.99 | 0.930 |
| LG-400C | 1.08 | 4.84 | 1.08 | 0.978 | 6.26 | 1.16 | 0.948 |
| LG-500C | 5.98 | 1.80 | 5.94 | 0.998 | 0.32 | 6.76 | 0.980 |
| LG-600C | 8.06 | 1.59 | 8.12 | 0.995 | 0.20 | 9.35 | 0.972 |
| Sample | ΔG0(kJ/mol) | ΔH0/kJ/mol | ΔS0/J/(mol·K) | |||
|---|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | 318 K | |||
| LG-300C | −2.23 | −4.20 | −5.18 | −7.55 | 46.49 | 169.26 |
| LG-400C | −3.19 | −4.36 | −5.50 | −8.18 | 42.94 | 159.21 |
| LG-500C | −6.74 | −10.75 | −11.64 | −13.40 | 53.53 | 170.19 |
| LG-600C | −9.99 | −13.18 | −14.02 | −15.83 | 43.00 | 185.64 |
| 2K(I) | 2Na(I) | Ca(II) | Mg(II) | TEC a | AC b | TEC/AC(%) c | |
|---|---|---|---|---|---|---|---|
| mmol/kg | |||||||
| LG-300C-Cd | 0.66 | 6.21 | 0.04 | 0.03 | 6.94 | 18.93 | 36.66 |
| LG-300C-H2O | 0.15 | 0.85 | - d | 0.01 | 1.01 | / e | / |
| LG-400C-Cd | 0.76 | 5.98 | 0.04 | 0.05 | 6.83 | 29.11 | 23.46 |
| LG-400C-H2O | 0.10 | 0.62 | 0.01 | 0.01 | 0.74 | / | / |
| LG-500C-Cd | 0.78 | 7.83 | 0.08 | 0.07 | 8.76 | 81.70 | 10.72 |
| LG-500C-H2O | 0.02 | 0.65 | 0.01 | - | 0.68 | / | / |
| LG-600C-Cd | 1.26 | 8.02 | 0.07 | 0.34 | 9.35 | 165.54 | 5.65 |
| LG-600C-H2O | 0.02 | 0.31 | - | 0.02 | 0.35 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, J. The Physicochemical Characteristics and Heavy Metal Retention Capability of Black Liquor Lignin-Based Biochars. Molecules 2023, 28, 7694. https://doi.org/10.3390/molecules28237694
Wang Z, Li J. The Physicochemical Characteristics and Heavy Metal Retention Capability of Black Liquor Lignin-Based Biochars. Molecules. 2023; 28(23):7694. https://doi.org/10.3390/molecules28237694
Chicago/Turabian StyleWang, Zhanghong, and Jiale Li. 2023. "The Physicochemical Characteristics and Heavy Metal Retention Capability of Black Liquor Lignin-Based Biochars" Molecules 28, no. 23: 7694. https://doi.org/10.3390/molecules28237694
APA StyleWang, Z., & Li, J. (2023). The Physicochemical Characteristics and Heavy Metal Retention Capability of Black Liquor Lignin-Based Biochars. Molecules, 28(23), 7694. https://doi.org/10.3390/molecules28237694






