Visible-Light-Mediated Catalyst-Free [2+2] Cycloaddition Reaction for Dihydrocyclobuta[b]naphthalene-3,8-diones Synthesis under Mild Conditions
Abstract
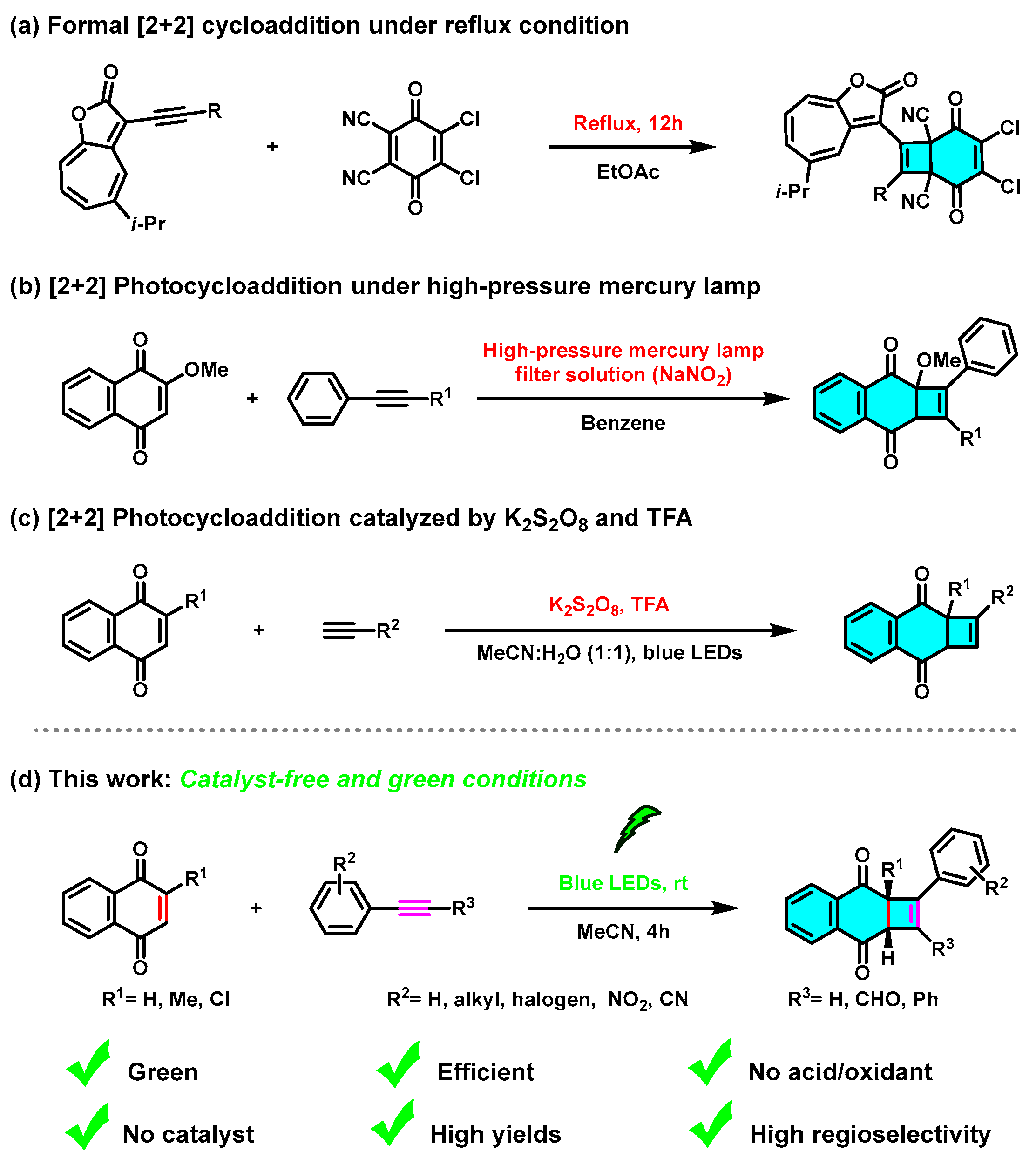
:1. Introduction
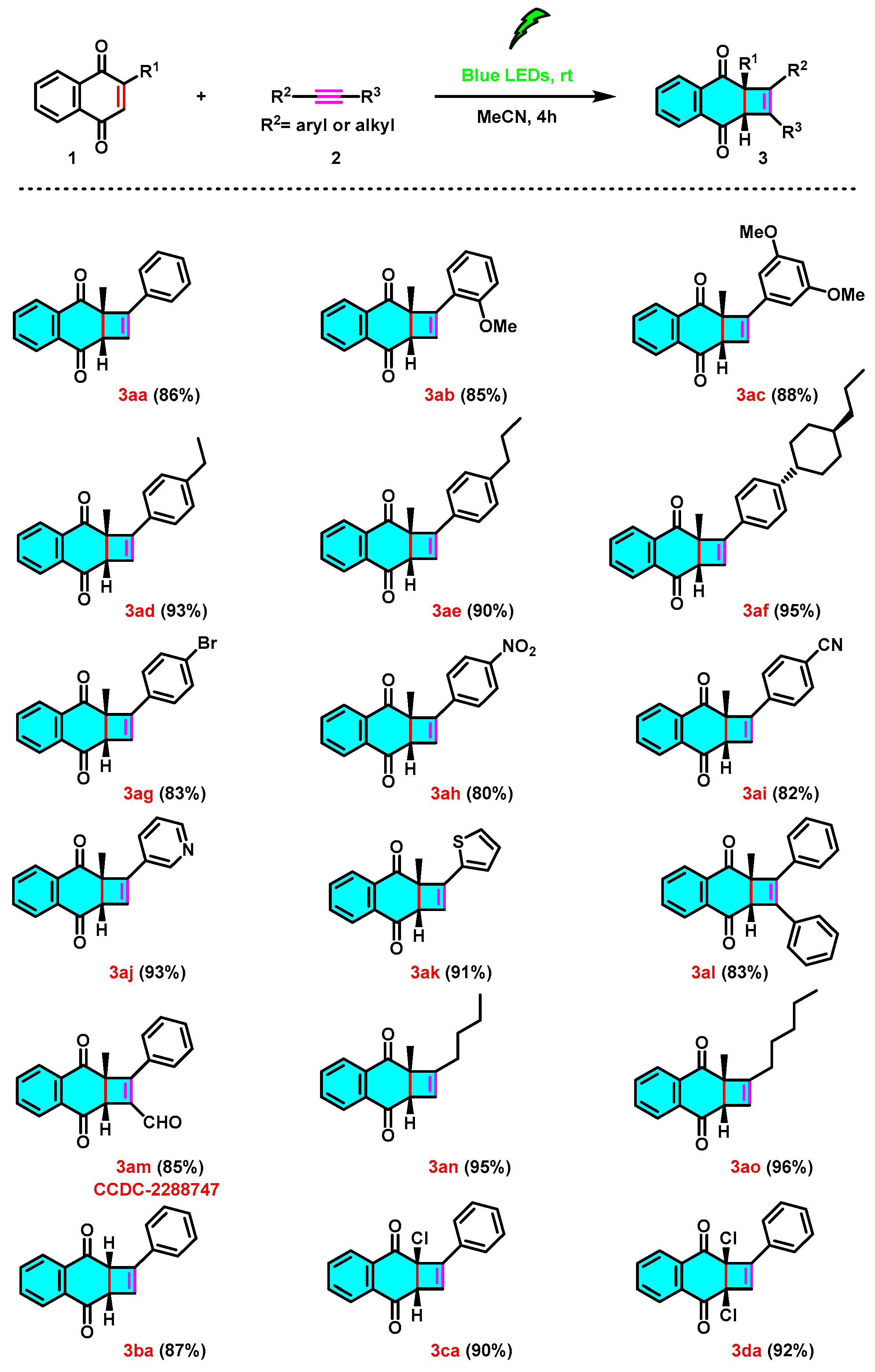
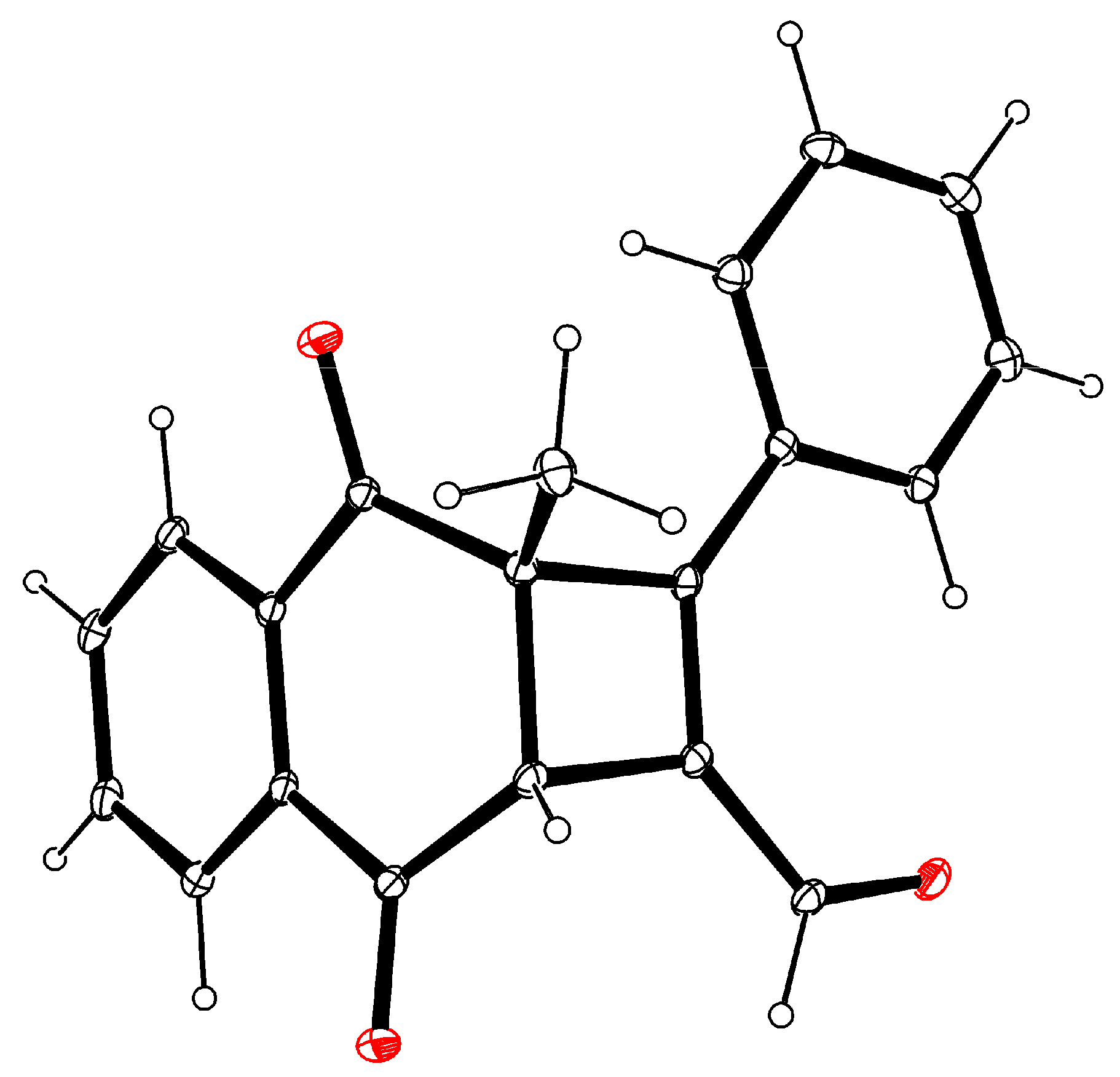
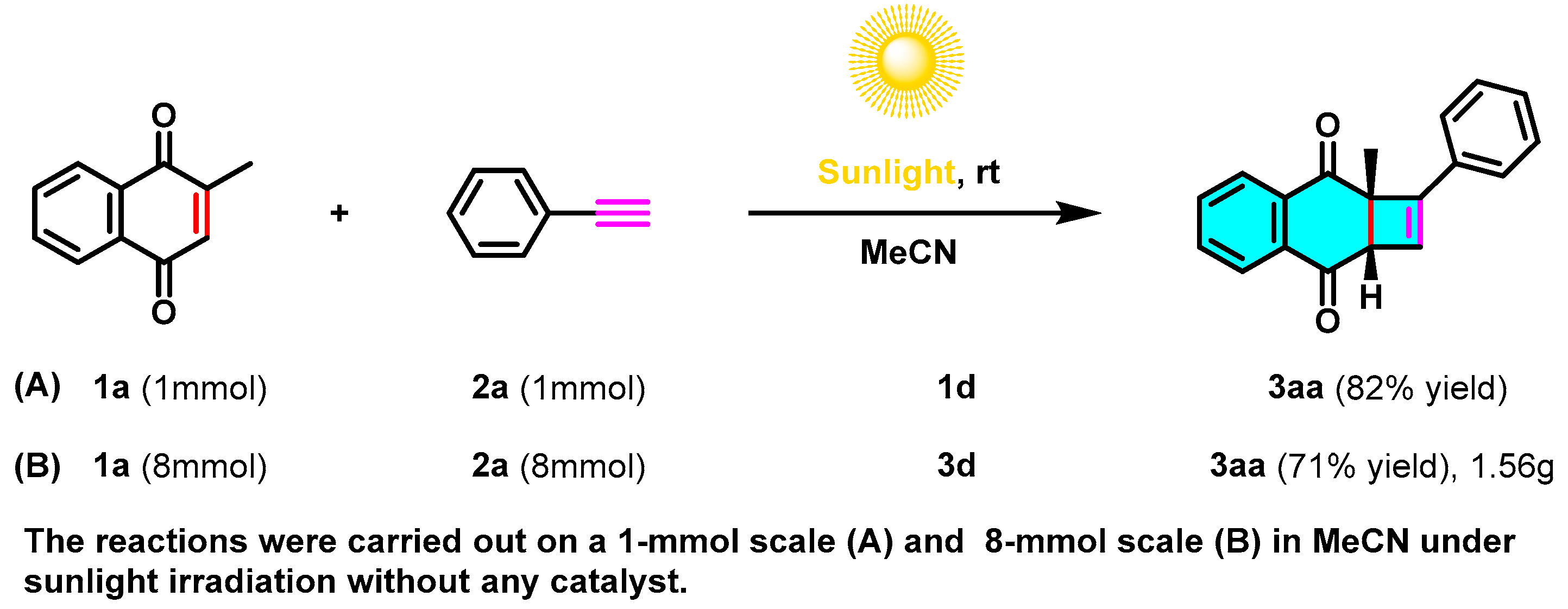
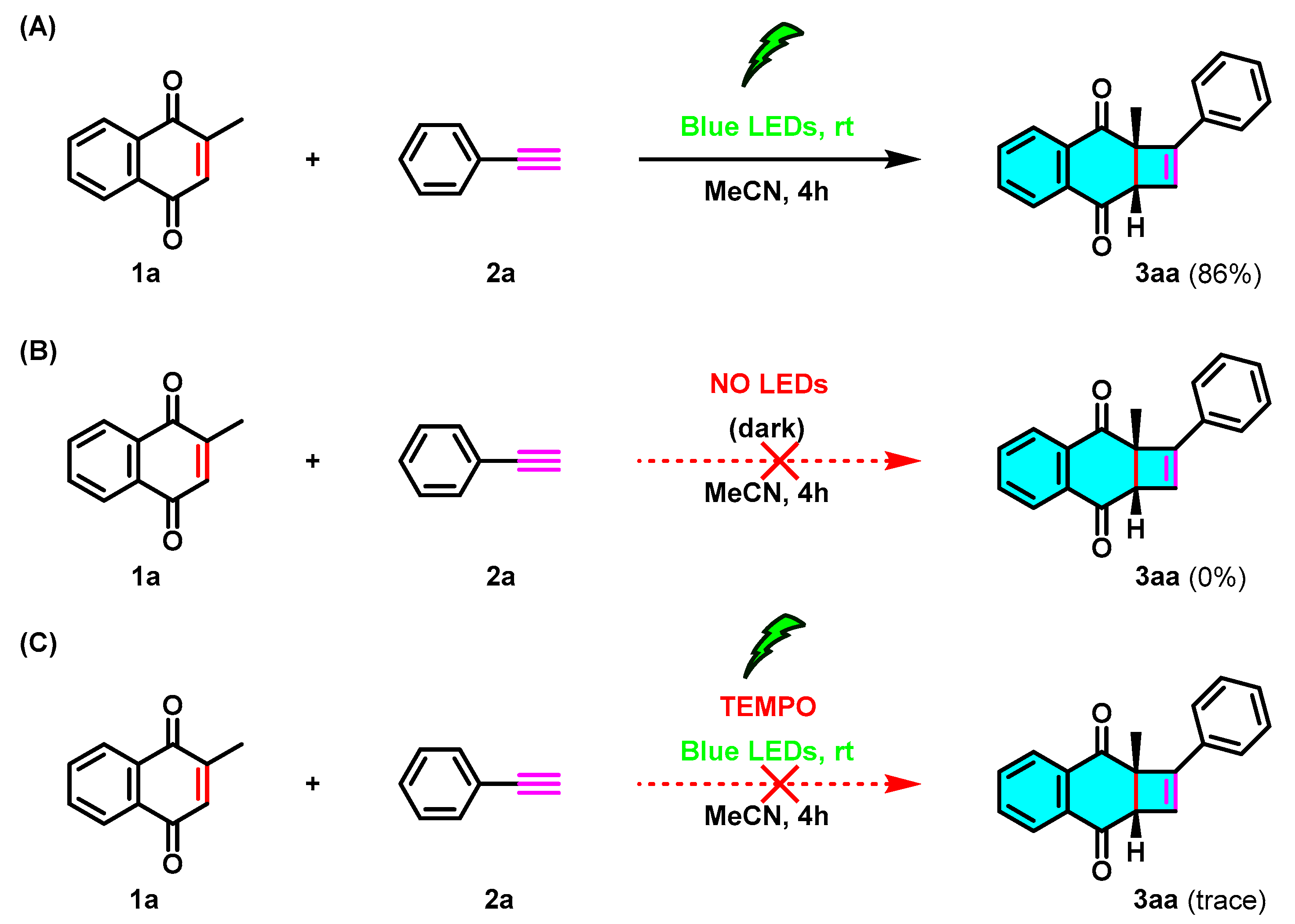
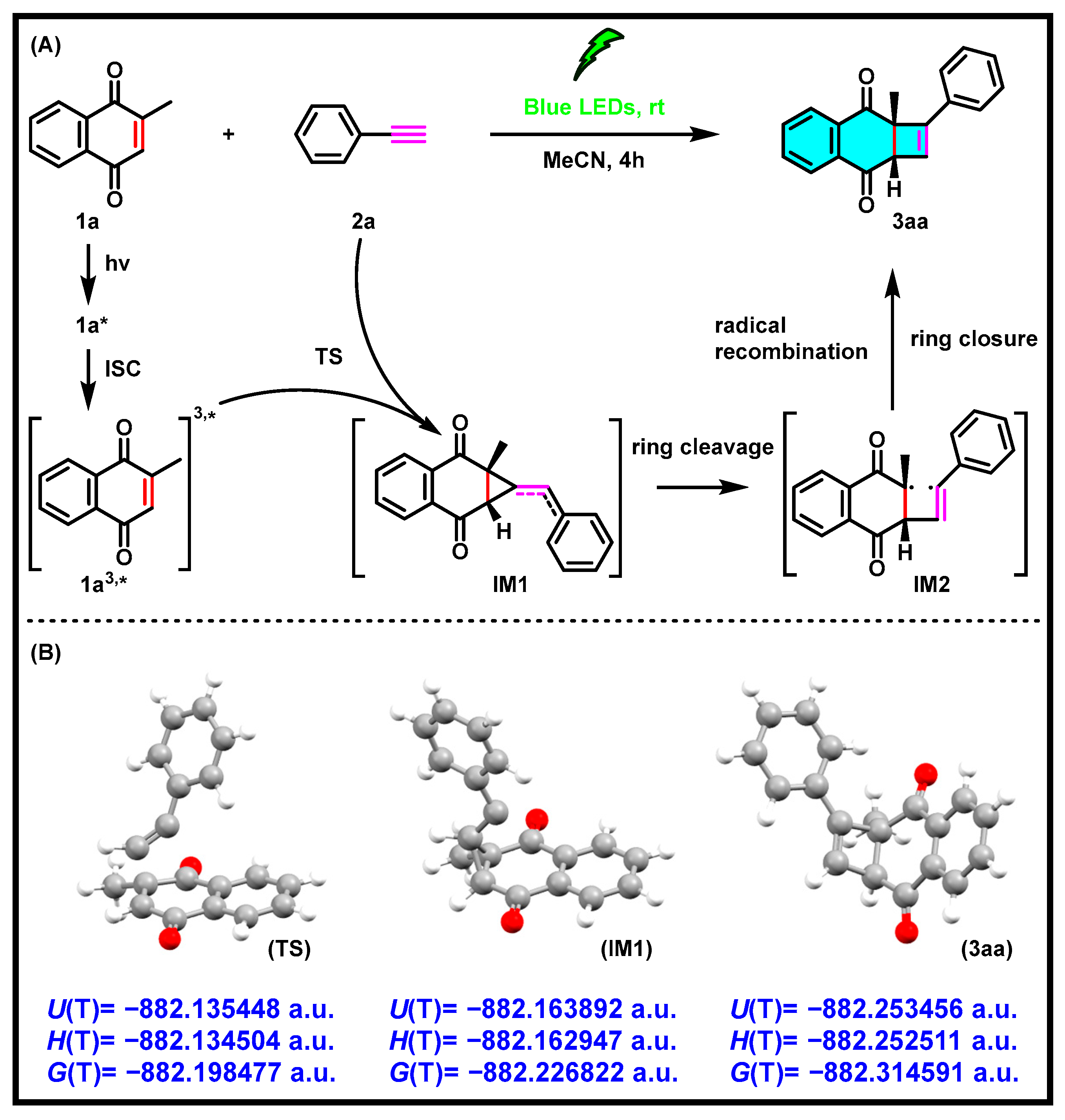
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.1.1. Experimental Section
3.1.2. Computational Section
3.2. General Procedure for the Preparation of DHCBNDOs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Gao, K.; Bian, M.; Ding, H. Recent advances in the total synthesis of cyclobutane-containing natural products. Org. Chem. Front. 2020, 7, 136–154. [Google Scholar] [CrossRef]
- Hill, R.A.; Sutherland, A. Hot off the press. Nat. Prod. Rep. 2011, 28, 1031–1034. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, Y.; Xuan, J.; Zhu, C.; Wang, B.; Ding, H. Diastereoselective Total Synthesis of Salvileucalin C. Org. Lett. 2014, 16, 3376–3379. [Google Scholar] [CrossRef] [PubMed]
- McLean, T.H.; Parrish, J.C.; Braden, M.R.; Marona-Lewicka, D.; Gallardo-Godoy, A.; Nichols, D.E. 1-Aminomethylbenzocycloalkanes: Conformationally Restricted Hallucinogenic Phenethylamine Analogues as Functionally Selective 5-HT2A Receptor Agonists. J. Med. Chem. 2006, 49, 5794–5803. [Google Scholar] [CrossRef] [PubMed]
- Tsotinis, A.; Afroudakis, P.A.; Garratt, P.J.; Bocianowska-Zbrog, A.; Sugden, D. Benzocyclobutane, Benzocycloheptane and Heptene Derivatives as Melatonin Agonists and Antagonists. ChemMedChem 2014, 9, 2238–2243. [Google Scholar] [CrossRef]
- Juliane, S.; Karen, W.; Georg, S.; Andreas, L.; Franz, H. Bradycardic and Proarrhythmic Properties of Sinus Node Inhibitors. Mol. Pharmacol. 2006, 69, 1328. [Google Scholar]
- Kuo, G.-H.; Gaul, M.D.; Liang, Y.; Xu, J.Z.; Du, F.; Hornby, P.; Xu, G.; Qi, J.; Wallace, N.; Lee, S.; et al. Synthesis and biological evaluation of benzocyclobutane-C-glycosides as potent and orally active SGLT1/SGLT2 dual inhibitors. Bioorganic Med. Chem. Lett. 2018, 28, 1182–1187. [Google Scholar] [CrossRef]
- Levchenko, K.S.; Chudov, K.A.; Adamov, G.E.; Poroshin, N.O.; Shmelin, P.S.; Grebennikov, E.P.; Parshikov, Y.G. Photocurable and Thermosetting Polymer Materials on the Basis of Benzocyclobutene and Its Derivatives for Electronics. Russ. J. Gen. Chem. 2018, 88, 2793–2812. [Google Scholar] [CrossRef]
- Adachi, K.; Hirose, S.; Ueda, Y.; Uekusa, H.; Hamura, T. Thermodynamically Stable o-Quinodimethane: Synthesis, Structure, and Reactivity. Chem.-A Eur. J. 2021, 27, 3665–3669. [Google Scholar] [CrossRef]
- Murakami, M.; Ishida, N. Cleavage of Carbon–Carbon σ-Bonds of Four-Membered Rings. Chem. Rev. 2021, 121, 264–299. [Google Scholar] [CrossRef]
- Biletskyi, B.; Colonna, P.; Masson, K.; Parrain, J.-L.; Commeiras, L.; Chouraqui, G. Small rings in the bigger picture: Ring expansion of three- and four-membered rings to access larger all-carbon cyclic systems. Chem. Soc. Rev. 2021, 50, 7513–7538. [Google Scholar] [CrossRef]
- Álvarez-García, J.; Rubio-Pisabarro, V.; Silva-López, C.; Cid, M.M. Photochemically Driven Tandem Process in the Construction of a Biscyclopropylcage from 2,5-Dimethoxy-p-benzoquinone and Terminal Acetylenes. Org. Lett. 2020, 22, 4527–4531. [Google Scholar] [CrossRef]
- Kato, S.-I.; Beels, M.T.R.; La Porta, P.; Schweizer, W.B.; Boudon, C.; Gisselbrecht, J.-P.; Biaggio, I.; Diederich, F. Homoconjugated Push–Pull and Spiro Systems: Intramolecular Charge-Transfer Interactions and Third-Order Optical Nonlinearities. Angew. Chem. Int. Ed. 2010, 49, 6207–6211. [Google Scholar] [CrossRef]
- Ansari, S.M.; Khanum, G.; Bhat, M.-U.-S.; Rizvi, M.A.; Reshi, N.U.D.; Ganie, M.A.; Javed, S.; Shah, B.A. Studies towards investigation of Naphthoquinone-based scaffold with crystal structure as lead for SARS-CoV-19 management. J. Mol. Struct. 2023, 1283, 135256. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-B.; Huang, C.; Chen, X.; Wu, Y.; Zhou, M.; Zhang, C.; Zhang, Y. Small molecular inhibitors of miR-1 identified from photocycloadducts of acetylenes with 2-methoxy-1,4-naphthalenequinone. Bioorganic Med. Chem. 2013, 21, 6124–6131. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, C.; Zhang, W.; Wu, Y.; Chen, X.; Zhang, C.-Y.; Zhang, Y. A universal activator of microRNAs identified from photoreaction products. Chem. Commun. 2012, 48, 6432–6434. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, B.A.; Sobenina, L.N.; Stepanova, Z.V.; Ushakov, I.A.; Sinegovskaya, L.M.; Vakul’skaya, T.I.; Mikhaleva, A.B.I. Facile[2+2] Cycloadditionof DDQ to an Alkyne: Synthesis of Pyrrolyl- and Indolylbicyclo[4.2.0]octadienesfrom C-Ethynylpyrroles or C-Ethynylindoles. Synthesis 2010, 2010, 470–476. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.-Y.; Zhou, P.; Li, G.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Merging [2+2] Cycloaddition with Radical 1,4-Addition: Metal-Free Access to Functionalized Cyclobuta[a]naphthalen-4-ols. Angew. Chem. Int. Ed. 2017, 56, 15570–15574. [Google Scholar] [CrossRef]
- Qin, X.-Y.; Wang, J.-Y.; Geng, F.-Z.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Engaging yne-allenones in tunable catalytic silane-mediated conjugate transfer reductions. Chem. Commun. 2021, 57, 5394–5397. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Xie, F.-L.; Hu, J.-Q.; Yang, S.-Z.; Wang, Y.-J.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Atom-economic synthesis of cyclobuta[a]naphthalen-4-ols via a base-promoted [2+2] cycloaddition/1,6-nucleophilic addition cascade. Org. Biomol. Chem. 2018, 16, 7104–7108. [Google Scholar] [CrossRef]
- Fadeev, A.; Kotora, M. Catalytic vs. Uncatalyzed [2+2] Photocycloadditions of Quinones with Alkynes. Org. Biomol. Chem. 2023, 21, 6174–6179. [Google Scholar] [CrossRef] [PubMed]
- Dengiz, C.; Prange, C.; Gawel, P.; Trapp, N.; Ruhlmann, L.; Boudon, C.; Diederich, F. Push–pull chromophores by reaction of 2,3,5,6-tetrahalo-1,4-benzoquinones with 4-(N,N-dialkylanilino)acetylenes. Tetrahedron 2016, 72, 1213–1224. [Google Scholar] [CrossRef]
- Shoji, T.; Maruyama, M.; Shimomura, E.; Maruyama, A.; Ito, S.; Yasunami, M.; Higashi, J.; Toyota, K.; Morita, N. Synthesis, Properties, and Crystal Structure of DDQ-Adducts of Ethynylated 2H-Cyclohepta[b]furan-2-ones. Heterocycles 2014, 88, 319–329. [Google Scholar] [CrossRef]
- Erden, K.; Savaş, İ.; Dengiz, C. Synthesis of triazene-substituted homoconjugated push-pull chromophores by formal [2+2] cycloadditions. Tetrahedron Lett. 2019, 60, 1982–1985. [Google Scholar] [CrossRef]
- Sultan, S.; Bhat, M.-u.-S.; Rizvi, M.A.; Shah, B.A. Visible Light-Mediated [2+2] Cycloaddition Reactions of 1,4-Quinones and Terminal Alkynes. J. Org. Chem. 2019, 84, 8948–8958. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-B.; Yu, Y.-H.; Qi, Z.-H.; Zhang, X.; Xu, Z.-G.; Tang, D.-Y.; Chen, Z.-Z.; Wang, B.-C.; Qu, X.-Y. Photochemical diastereoselective synthesis and spectral characterization of (E)-3-(2-benzoylstyryl)-4H-chromen-4-ones. J. Mol. Struct. 2023, 1283, 135308. [Google Scholar] [CrossRef]
- Tan, H.-B.; Wang, Y.-F. Synthesis, NMR analysis and X-ray crystal structure of 3, 9-bis(4-(trifluoromethyl) phenyl)-3,9-diazatetraasterane. J. Mol. Struct. 2020, 1220, 128751. [Google Scholar] [CrossRef]
- Tan, H.-B.; Zhao, Z.-C.; Ma, Z.-S.; Yan, H. Highly regioselective photodimerization of 1,4-dihydropyridines: An efficient synthesis of novel 3,6-diazatetraasteranes. Tetrahedron 2018, 74, 529–534. [Google Scholar] [CrossRef]
- Tan, H.; Qi, Z.; Yu, Y.; Zhang, X.; Xiang, Y.; Huang, J.; Xu, Z.; Tang, D.; Chen, Z.; Wang, B. An Efficient Synthesis of Naphtho[2,3-b]furan-4,9-diones via Visible-Light-Mediated [3+2] Cycloaddition Reaction. Molecules 2023, 28, 4751. [Google Scholar] [CrossRef]
- Hebah, A.-W.; Joseph, B. Gaussian M-062x/6-31+g (d,p) Calculation of Standard Enthalpy, Entropy and Heat Capacity of Some Fluorinated Alcohol’s and Its Radicals at Different Temperatures. Am. J. Phys. Chem. 2020, 9, 101–111. [Google Scholar]
- Liashuk, O.S.; Grygorenko, O.O.; Volovenko, Y.M.; Waser, J. Photochemical [2+2] Cycloaddition of Alkynyl Boronates. Chem.-Eur. J. 2023, 29, e202301650. [Google Scholar] [CrossRef]
- Alibakhshi, A.; Hartke, B. Improved prediction of solvation free energies by machine-learning polarizable continuum solvation model. Nat. Commun. 2021, 12, 3584. [Google Scholar] [CrossRef]
- Skyner, R.E.; McDonagh, J.L.; Groom, C.R.; van Mourik, T.; Mitchell, J.B.O. A review of methods for the calculation of solution free energies and the modelling of systems in solution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef] [PubMed]
- Shoaf, A.L.; Bayse, C.A. The effect of nitro groups on N2 extrusion from aromatic azide-based energetic materials. New J. Chem. 2019, 43, 15326–15334. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction Path Following in Mass-Weighted Internal Coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]

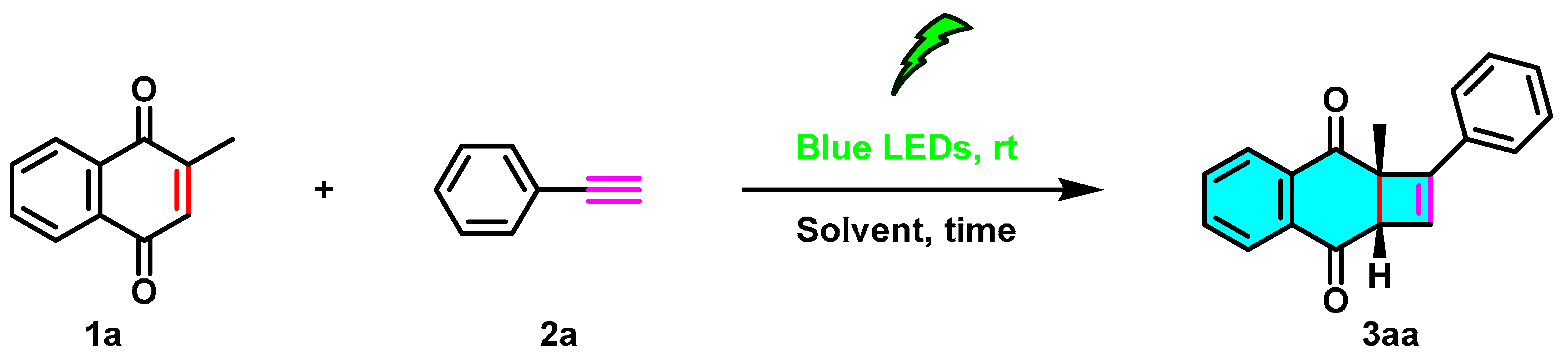 | ||||
|---|---|---|---|---|
| Entry | Solvent | Time | Eqn (2a) | Yield b |
| 1 | MeCN | 3 h | 1.0 | 81% |
| 2 | DCM | 3 h | 1.0 | 59% |
| 3 | Acetone | 3 h | 1.0 | 67% |
| 4 | Dioxane | 3 h | 1.0 | 63% |
| 5 | Chlorobenzene | 3 h | 1.0 | 60% |
| 6 | MeOH | 3 h | 1.0 | 71% |
| 7 | THF | 3 h | 1.0 | 75% |
| 8 | Toluene | 3 h | 1.0 | 68% |
| 9 | ClCH2CH2Cl | 3 h | 1.0 | 64% |
| 10 | MeCN | 4 h | 1.0 | 86% |
| 11 | MeCN | 5 h | 1.0 | 85% |
| 12 | MeCN | 4 h | 1.1 | 83% |
| 13 | MeCN | 4 h | 1.2 | 84% |
| 14 | MeCN | 4 h | 1.4 | 82% |
| 15 | MeCN | 4 h | 1.0 | 0% c |
| 16 | MeCN | 4 h | 1.0 | 65% d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.-B.; Zhou, J.-Y.; Liu, Y.-S.; Lei, T.; Wang, S.-Y.; Hu, S.-S.; Zhang, X.; Xu, Z.-G.; Tang, D.-Y.; Chen, Z.-Z.; et al. Visible-Light-Mediated Catalyst-Free [2+2] Cycloaddition Reaction for Dihydrocyclobuta[b]naphthalene-3,8-diones Synthesis under Mild Conditions. Molecules 2023, 28, 7654. https://doi.org/10.3390/molecules28227654
Tan H-B, Zhou J-Y, Liu Y-S, Lei T, Wang S-Y, Hu S-S, Zhang X, Xu Z-G, Tang D-Y, Chen Z-Z, et al. Visible-Light-Mediated Catalyst-Free [2+2] Cycloaddition Reaction for Dihydrocyclobuta[b]naphthalene-3,8-diones Synthesis under Mild Conditions. Molecules. 2023; 28(22):7654. https://doi.org/10.3390/molecules28227654
Chicago/Turabian StyleTan, Hong-Bo, Jia-Ying Zhou, Ying-Shan Liu, Tong Lei, Shi-Yu Wang, Shuang-Shuang Hu, Xu Zhang, Zhi-Gang Xu, Dian-Yong Tang, Zhong-Zhu Chen, and et al. 2023. "Visible-Light-Mediated Catalyst-Free [2+2] Cycloaddition Reaction for Dihydrocyclobuta[b]naphthalene-3,8-diones Synthesis under Mild Conditions" Molecules 28, no. 22: 7654. https://doi.org/10.3390/molecules28227654
APA StyleTan, H.-B., Zhou, J.-Y., Liu, Y.-S., Lei, T., Wang, S.-Y., Hu, S.-S., Zhang, X., Xu, Z.-G., Tang, D.-Y., Chen, Z.-Z., & Wang, B.-C. (2023). Visible-Light-Mediated Catalyst-Free [2+2] Cycloaddition Reaction for Dihydrocyclobuta[b]naphthalene-3,8-diones Synthesis under Mild Conditions. Molecules, 28(22), 7654. https://doi.org/10.3390/molecules28227654






