Effect of Na versus Ca Sulfate Salts on the Hydration of Calcium Sulfoaluminate Clinker
Abstract
:1. Introduction
2. Results
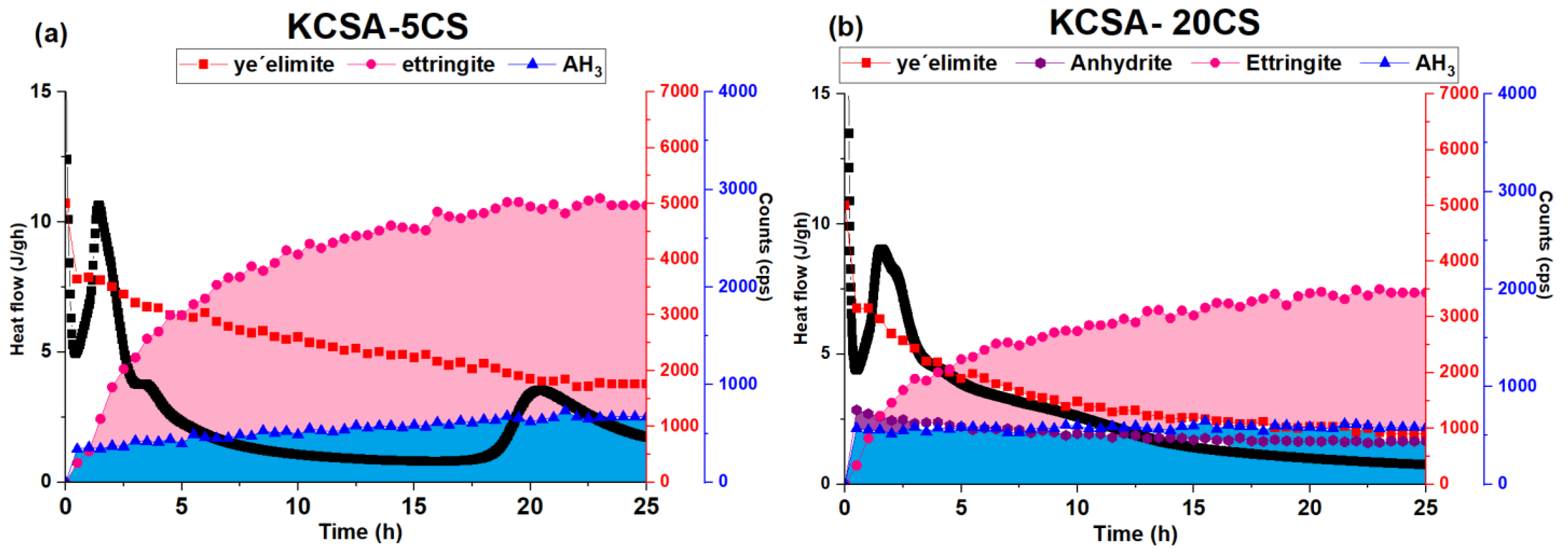
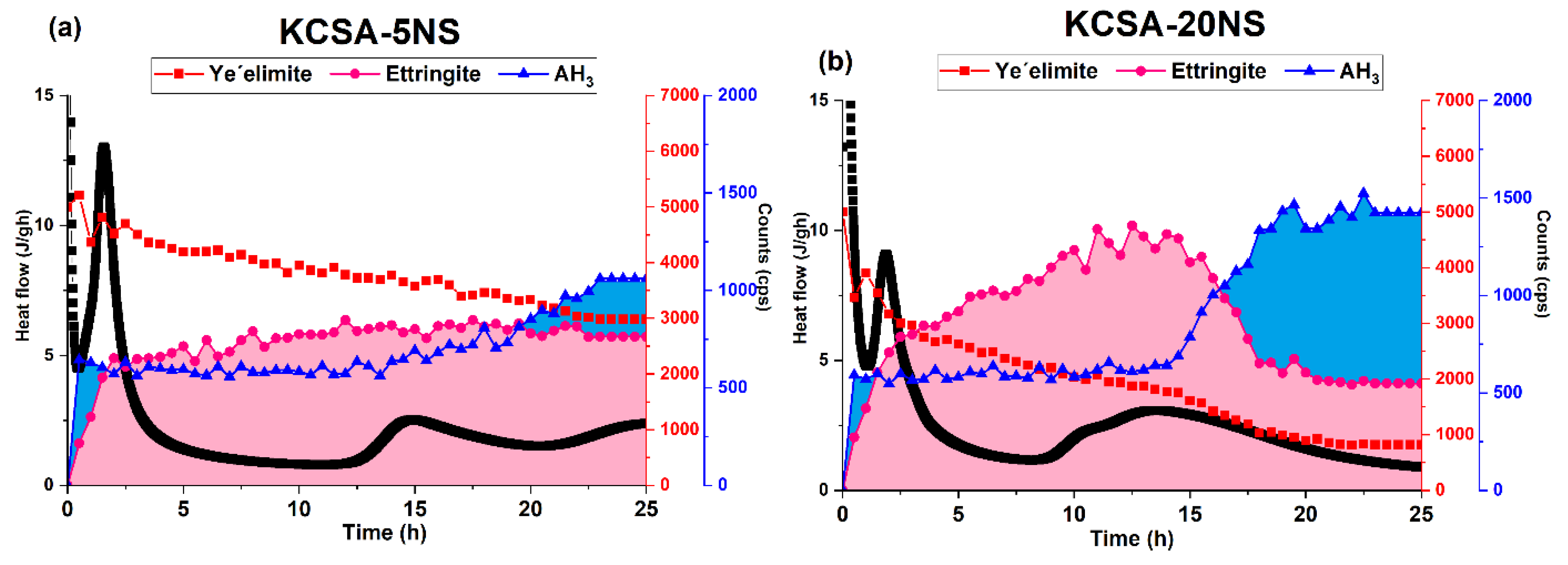
2.1. Isothermal Conduction Calorimetry
2.2. Mechanical Behavior
2.3. Mineralogical Characterization: Continuous X-ray Diffraction (XRD)
2.4. Mineralogical Characterization: Discontinuous XRD
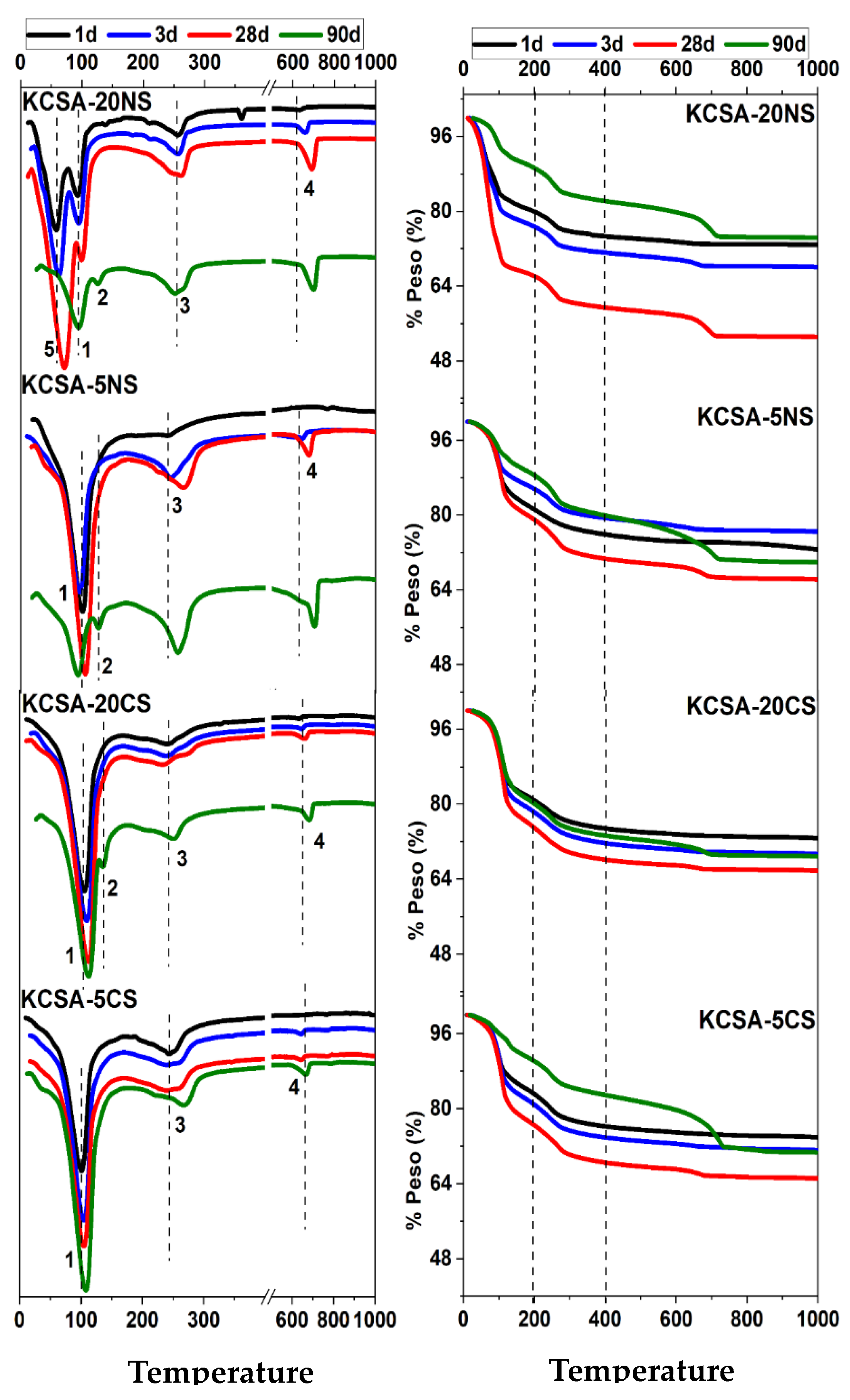
2.5. Differential Thermal Analysis and Thermogravimetry (DTA/TG)
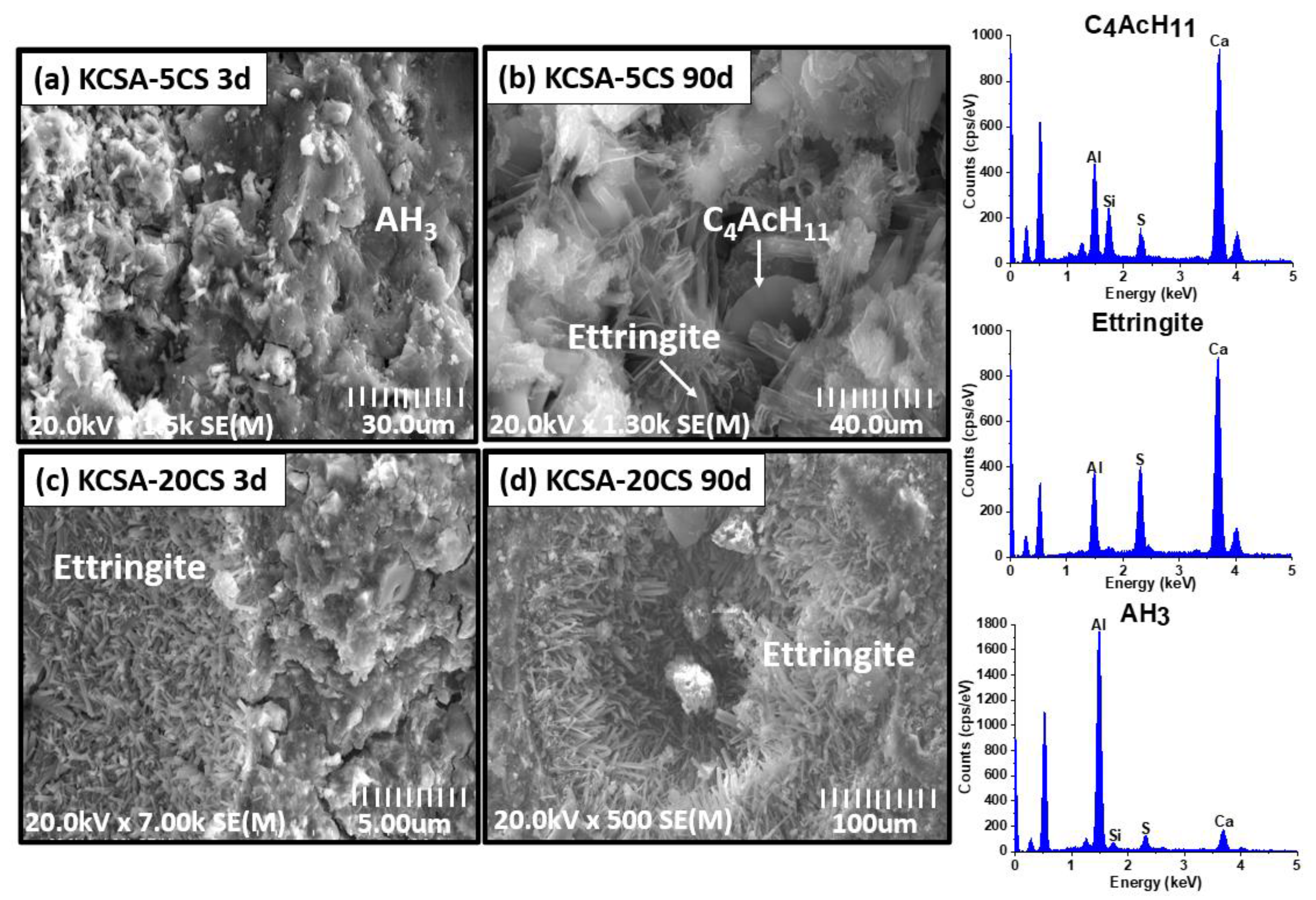
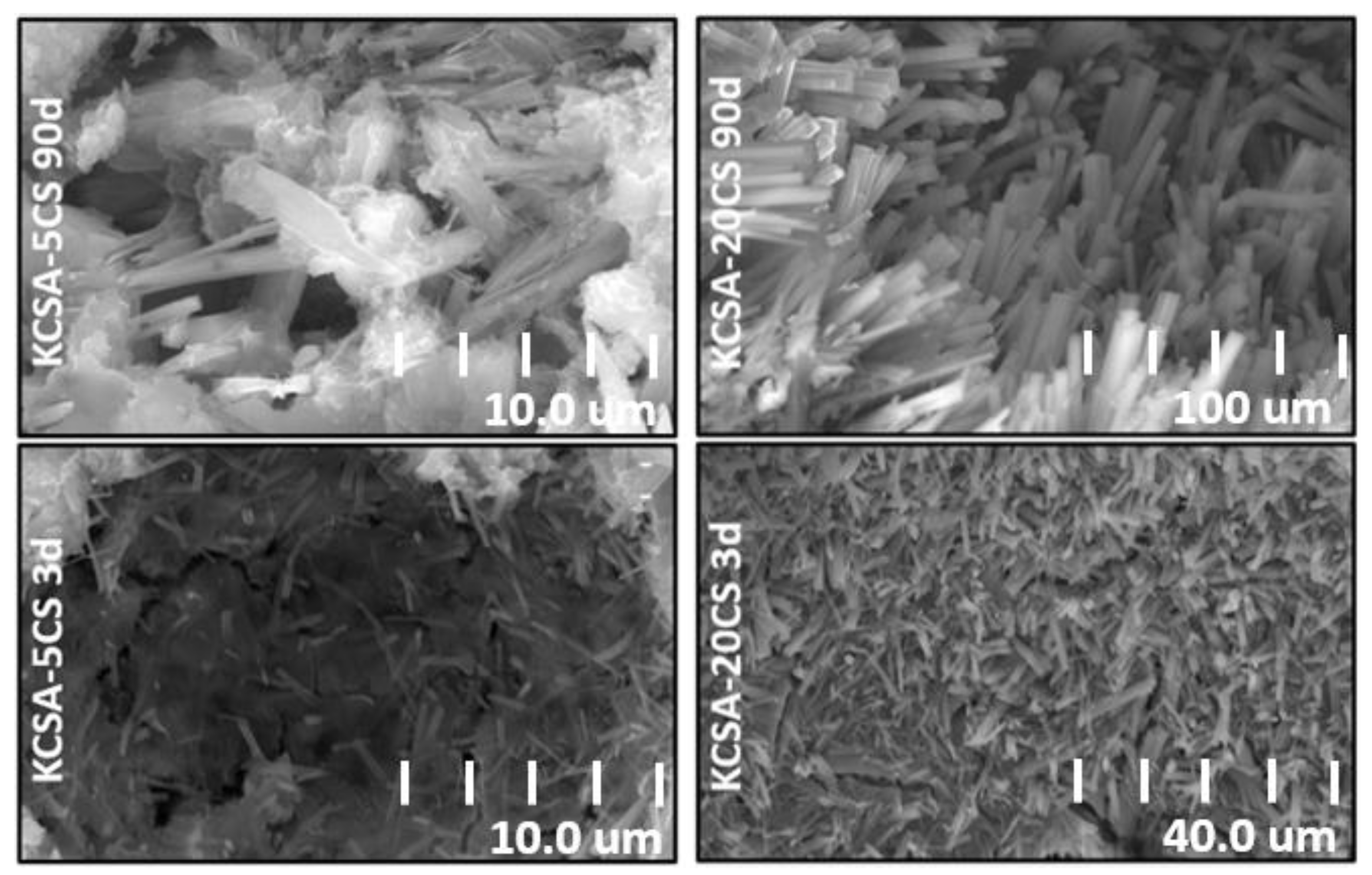
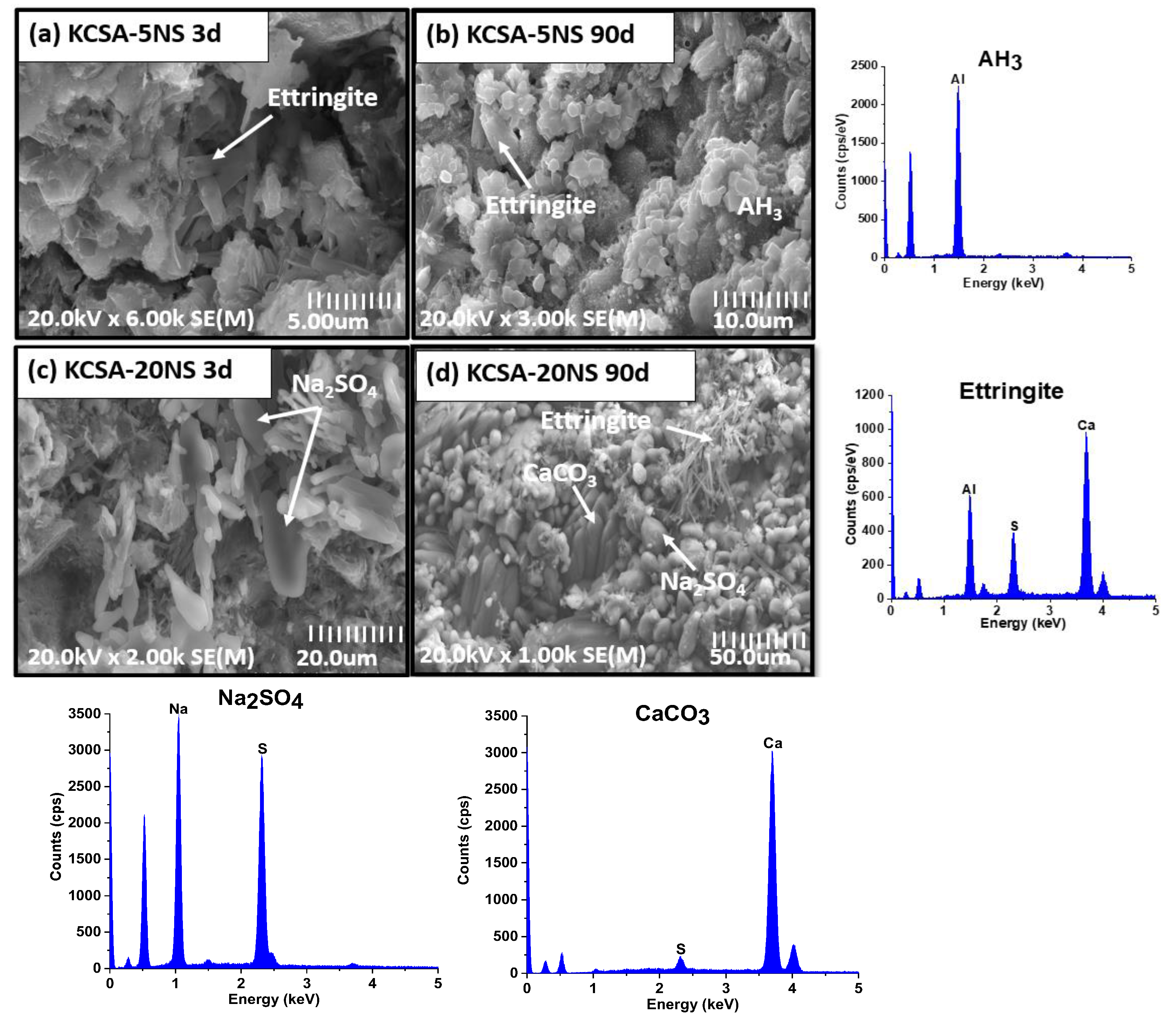
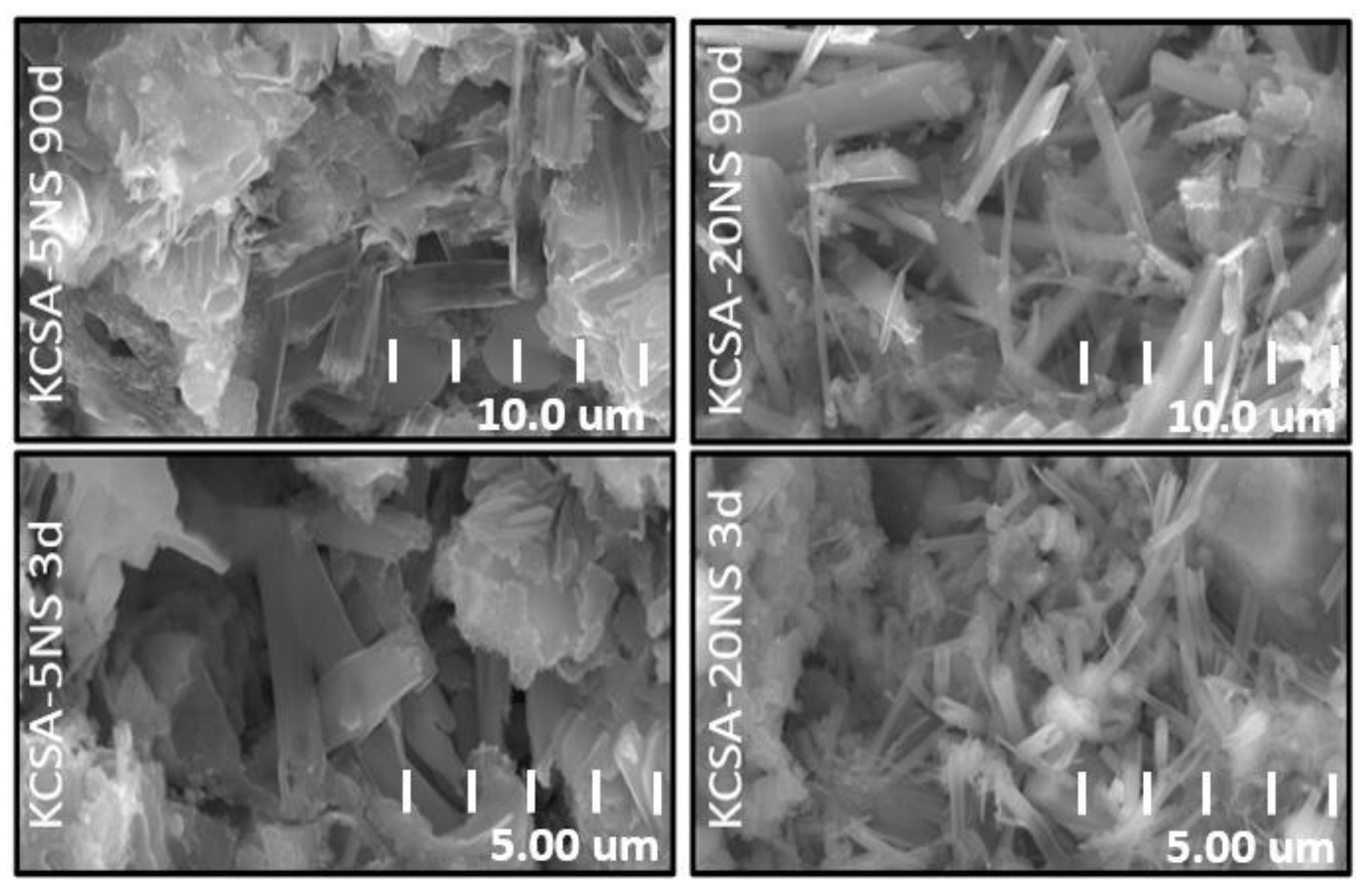
2.6. Scanning Electron Microscopy (SEM/EDX)
3. Discussion
3.1. Hydration of the KCSA Containing Anhydrite
3.2. Hydration of the KCSA Containing Na2SO4
4. Materials and Methods
4.1. Materials
4.2. Procedure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palomo, A.; Monteiro, P.; Martauz, P.; Bilek, V.; Fernandez-Jimenez, A. Hybrid Binders: A Journey from the Past to a Sustainable Future (Opus Caementicium Futurum). Cem. Concr. Res. 2019, 124, 105829. [Google Scholar] [CrossRef]
- Moreno de los Reyes, A.M.; Suárez-Navarro, J.A.; Alonso, M.d.M.; Gascó, C.; Sobrados, I.; Puertas, F. Hybrid Cements: Mechanical Properties, Microstructure and Radiological Behavior. Molecules 2022, 27, 498. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Saavedra, W.; Robayo-Salazar, R.; Mejía de Gutiérrez, R. Alkali-Activated Hybrid Cements Based on Fly Ash and Construction and DemolitionWastes Using Sodium Sulfate and Sodium Carbonate. Molecules 2021, 26, 7572. [Google Scholar] [CrossRef] [PubMed]
- Amer, I.; Kohail, M.; El-Feky, M.S.; Rashad, A.; Khalaf, M.A. Characterization of alkali-activated hybrid slag/cement concrete. Ain Shams Eng. J. 2021, 12, 135–144. [Google Scholar] [CrossRef]
- Vance, K.; Aguayo, M.; Oey, T.; Sant, G.; Neithalath, N. Hydration and strength development in ternary portland cement blends containing limestone and fly ash or metakaolin. Cem. Concr. Compos. 2013, 39, 93–103. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A. Eco-Efficient Concrete, 439th ed.; Chapter 17; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Allevi, S.; Marchi, M.; Scotti, F.; Bertini, S.; Cosentino, C. Hydration of calcium sulphoaluminate clinker with additions of different calcium sulphate sources. Mater. Struct. 2016, 49, 453–466. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2009, 101, 949–957. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker. ZKG Int. 2009, 62, 42–53. [Google Scholar]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements-experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Jansen, D.; Wolf, J.; Fobbe, N. The hydration of nearly pure ye’elimite with a sulfate carrier in a stoichiometric ettringite binder system. Implications for the hydration process based on in-situ XRD, 1H-TD-NMR, pore solution analysis, and thermodynamic modeling. Cem. Concr. Res. 2020, 127, 105923. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Fernández-Jiménez, A.; Palomo, A. Alkaline Hydration of Tricalcium Aluminate. J. Am. Ceram. Soc. 2012, 95, 3317–3324. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J. La Activación Alcalina Como Procedimiento para el Desarrollo de Nuevos Cementos Belíticos. Ph.D. Thesis, Universidad Nacional de Educación a Distancia, Madrid, Spain, 2017; p. 376. [Google Scholar]
- Palou, M.T.; Majling, J. Effects of sulphate, calcium and aluminum ions upon the hydration of sulphoaluminate belite cement. J. Therm. Anal. 1996, 46, 549–556. [Google Scholar] [CrossRef]
- Tambara, L.U.D.; Cheriaf, M.; Rocha, J.C.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis content on calcium sulfoaluminate (CSA) cement hydration. Cem. Concr. Res. 2020, 128, 105953. [Google Scholar] [CrossRef]
- Millán-Corrales, G.; González-López, J.R.; Palomo, A.; Fernández-Jiménez, A. Replacing fly ash with limestone dust in hybrid cements. Constr. Build. Mater. 2020, 243, 118169. [Google Scholar] [CrossRef]
- Al-Kutti, W.; Nasir, M.; Saiful Islam, A.B.M.; Manda, A.A.; Blaisi, N.I. An overview and experimental study on hybrid binders containing date palm ash, fly ash, OPC and activator composites. Constr. Build. Mater. 2018, 159, 567–577. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–155. [Google Scholar] [CrossRef]
- Padilla-Encinas, P.; Palomo, A.; Blanco-Varela, M.T.; Fernandez-Jimenez, A. Calcium sulfoaluminate clinker hydration at different alkali concentrations. Cem. Concr. Res. 2020, 138, 106251. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Palomo, A.; Fernández-Jiménez, A. C4A3Š hydration in different alkaline media. Cem. Concr. Res. 2013, 46, 41–49. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Peng, Y.; Tang, S.; Wang, L.; Shi, Y.; Li, Y.; Wang, Y.; Geng, Z.; Wu, K. Hydration and Fractal Analysis on Low-Heat Portland Cement Pastes Using Thermodynamics-Based Methods. Fractal Fract. 2023, 7, 606. [Google Scholar] [CrossRef]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Montes, M.; Pato, E.; Carmona-Quiroga, P.M. Blanco-Varela, M.T. Can calciumaluminates activate ternesite hydration? Cem. Concr. Res. 2018, 103, 204–215. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. (Eds.) A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kharchenko, I.; Alekseev, V. Effect of ettringite morphology on the properties of expanding cement systems. E3S Web Conf. 2019, 110, 01037. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Pei, M.; Deng, D. The role of carbonation in the occurrence of MgSO4 crystallization distress on concrete. Constr. Build. Mater. 2018, 192, 167–178. [Google Scholar] [CrossRef]
- Ke, G.; Zhang, J.; Liu, Y.; Xie, S. Pore characteristics of calcium sulfoaluminate cement paste with impact of supplementary cementitious materials and water to binder ratio. Powder Technol. 2021, 387, 146–155. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Telesca, A.; Marchon, D.; Xu, K.; Marroccoli, M.; Jiang, Z.; Monteiro, P.J.M. Effect of polycarboxylate ether on the expansion of ye’elimite hydration in the presence of anhydrite. Cem. Concr. Res. 2021, 140, 106321. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J. Microstructural evolution of aluminum hydroxide gel during the hydration of calcium sulfoaluminate under different alkali concentrations. Constr. Build. Mater. 2018, 180, 655–664. [Google Scholar] [CrossRef]
- Padilla-Encinas, P.; Fernández-Carrasco, L.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalinity on early-age hydration in calcium sulfoaluminate clinker. Cem. Concr. Res. 2022, 155, 106781. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J.; Ji, J. AH3 phase in the hydration product system of AFt-AFm-AH3 in calcium sulfoaluminate cements: A microstructural study. Constr. Build. Mater. 2018, 167, 587–596. [Google Scholar] [CrossRef]
- Kudryavtsev, A.B.; Kouznetsova, T.V.; Pyatkova, A.V. Proton relaxation and high-resolution solid-state Al 27 NMR study of hydration of calcium oxide sulfoaluminate. Cem. Concr. Res. 1990, 20, 407–418. [Google Scholar] [CrossRef]
- Tambara Júnior LU, D.; Rocha, J.C.; Cheriaf, M.; Padilla-Encinas, P.; Fernández-Jiménez, A.; Palomo, A. Effect of Alkaline Salts on Calcium Sulfoaluminate Cement Hydration. Molecules 2021, 26, 1938. [Google Scholar] [CrossRef] [PubMed]
- Picandet, V.; Rangeard, D.; Perrot, A.; Lecompte, T. Permeability measurement of fresh cement paste. Cem. Concr. Res. 2011, 41, 330–338. [Google Scholar] [CrossRef]
- Caruso, F.; Mantellato, S.; Palacios, M.; Flatt, R.J. ICP-OES method for the characterization of cement pore solutions and their modification by polycarboxylate-based superplasticizers. Cem. Concr. Res. 2017, 91, 52–60. [Google Scholar] [CrossRef]
- Li, G.; Bescop, P.L.; Moranville, M. The U phase formation in cement-based systems containing high amounts of Na2SO4. Cem. Concr. Res. 1996, 26, 27–33. [Google Scholar] [CrossRef]

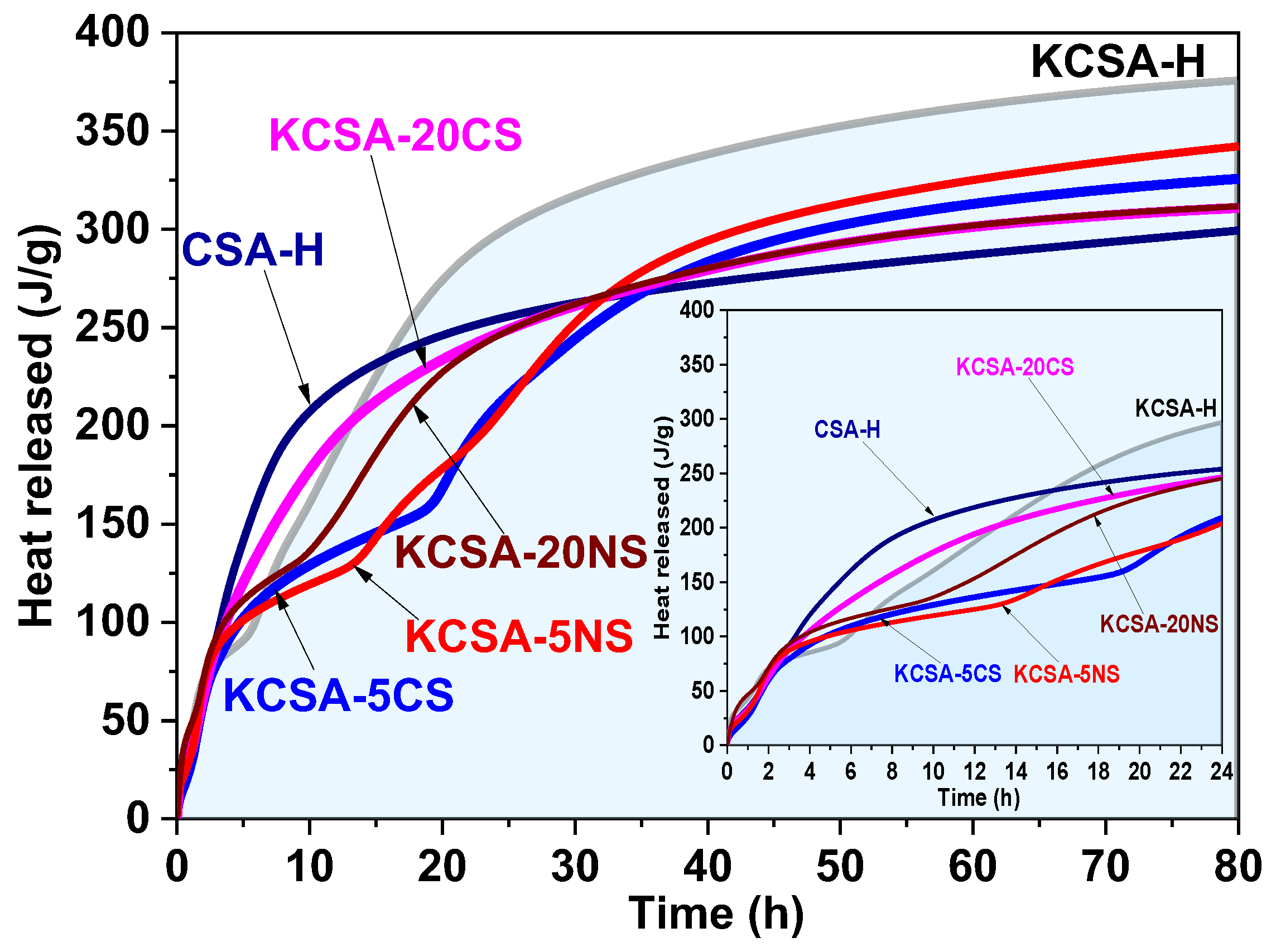

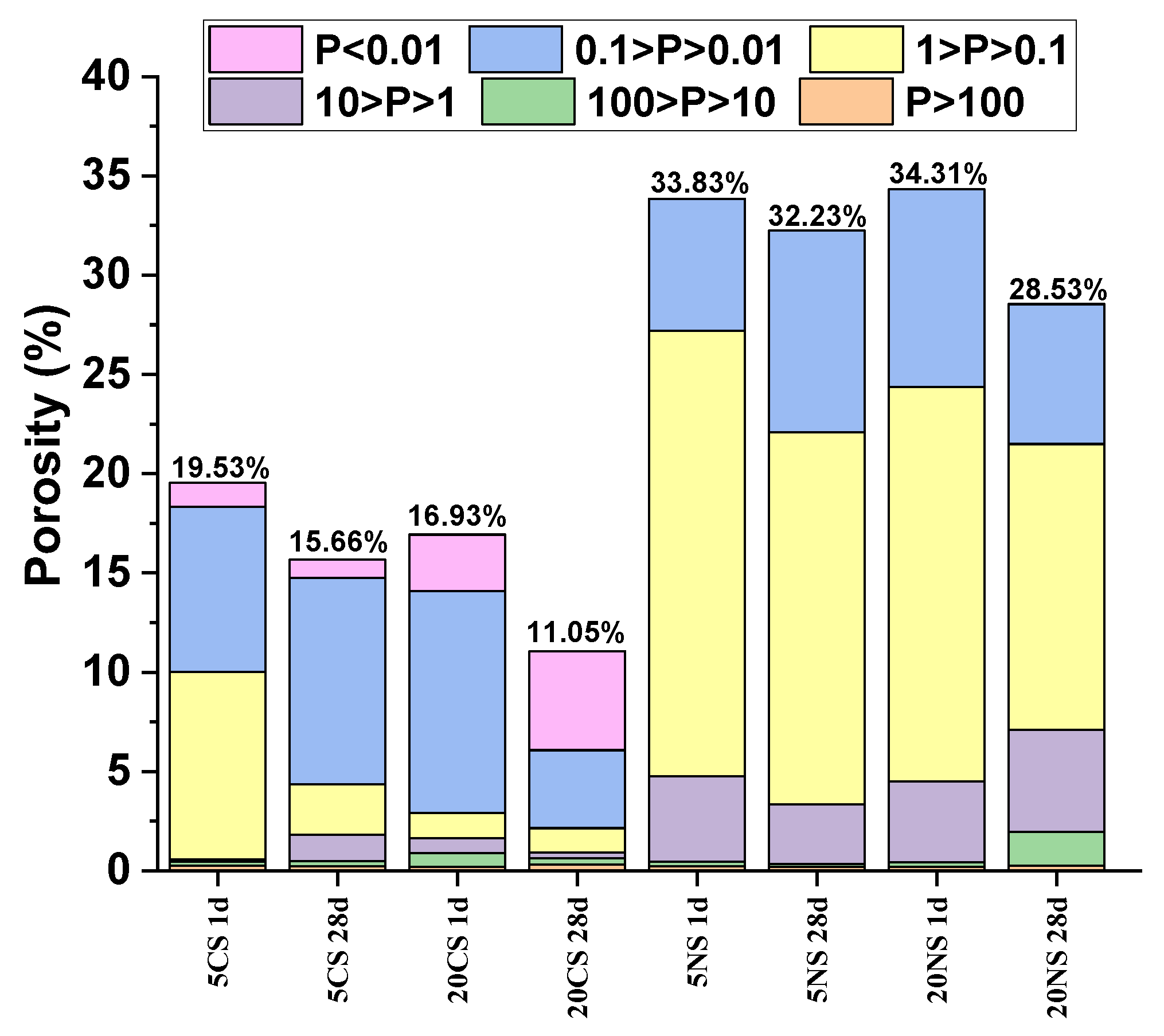
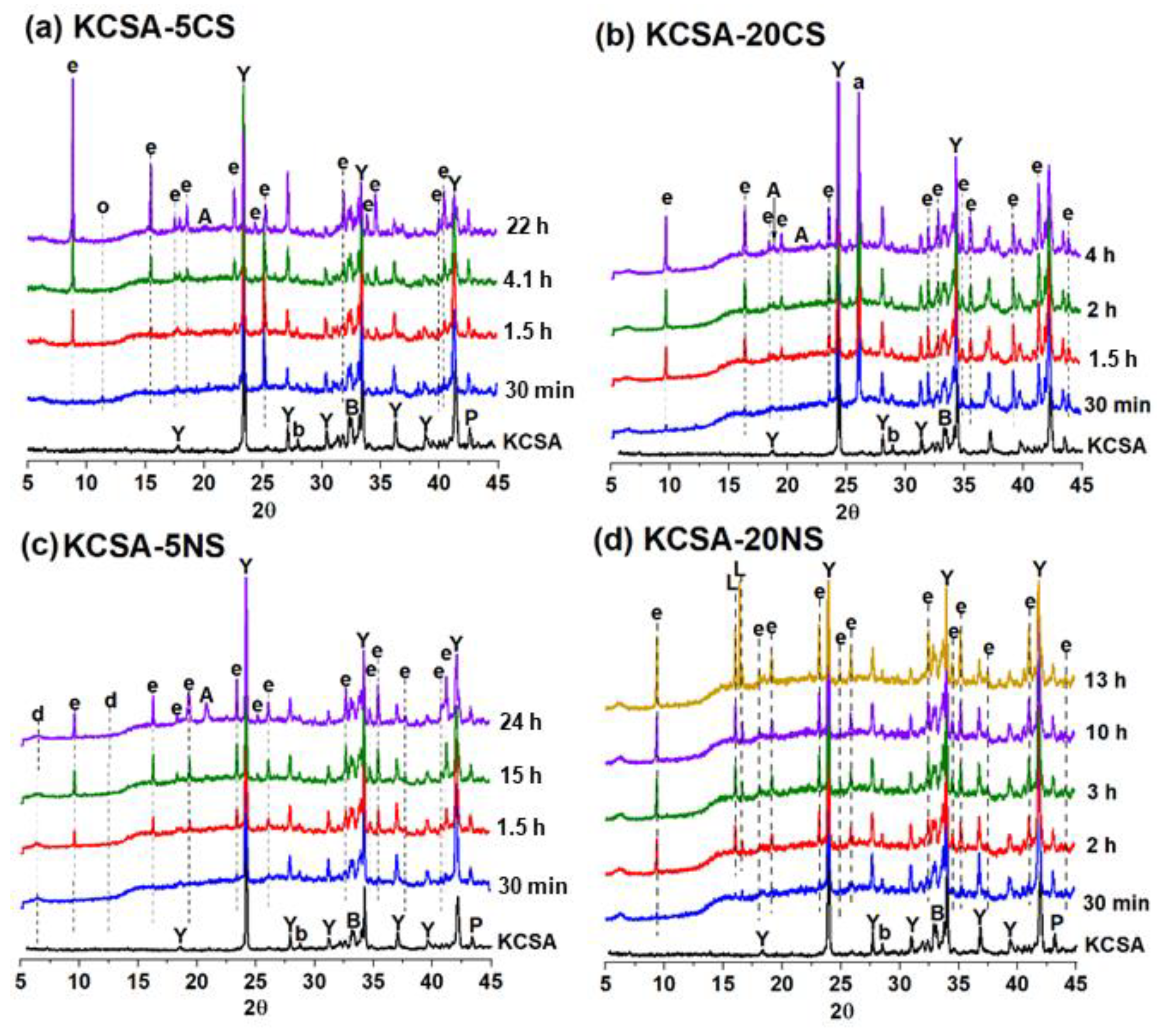
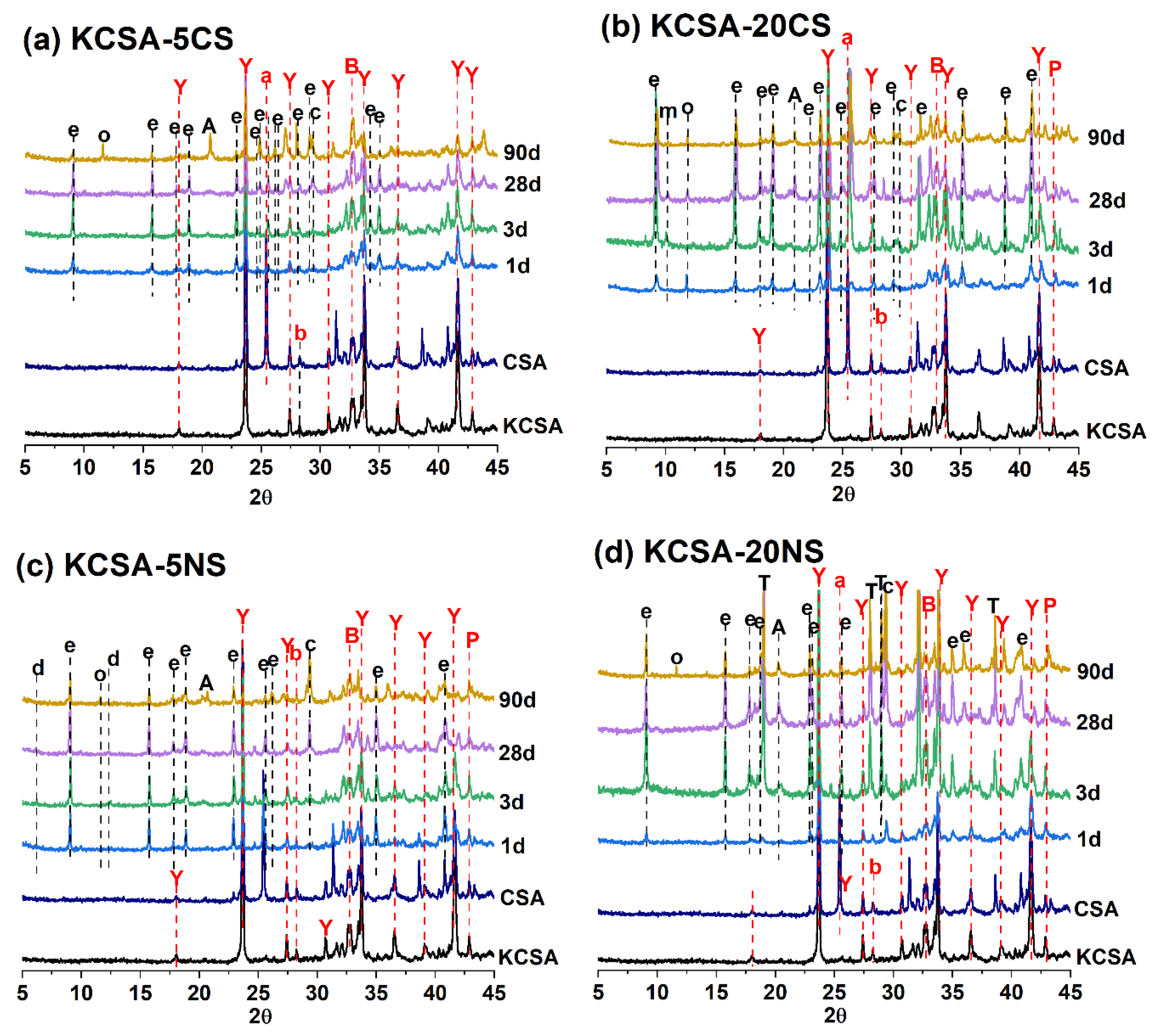
| pH MEASUREMENTS | ||||||
|---|---|---|---|---|---|---|
| 30 min | 1 h | 2 h | 5 h | 7 h | 24 h | |
| KCSA-20NS | 11.39 | 11.40 | 11.64 | 12.42 | 12.51 | 13.19 |
| Chemical Composition | Mineralogical Composition | ||||
|---|---|---|---|---|---|
| Oxides | KCSA | CSA | 2 ICDD PDF | KCSA | CSA |
| CaO | 40.82% | 41.50% | Ye’elimite, C4A3s, (PDF = 33-0256) | 68.4 ± 0.9% | 52.6 ± 0.4% |
| SiO2 | 9.51% | 8.14% | Brediggite, Ba0.293Ca13.467Mg1.81Mn0.43O32Si8 (PDF = 36-399) | 7.40 ± 0.4% | 5.0 ± 0.5% |
| Al2O3 | 29.37% | 23.20% | Belite, C2S, (PDF = 86-0398) | 16.90 ± 1.0% | 14.9 ± 0.4% |
| Fe2O3 | 1.32% | 1.05% | Periclase, MgO, (PDF = 4-829) | 3.67 ± 0.2% | 2.8 ± 0.3% |
| MgO | 4.13% | 3.22% | Gehlenite, Al2Ca2O7Si, (PDF = 35-755) | 1.90 ± 0.4% | 1.6 ± 0.5% |
| Na2O | 1.06% | 0.86% | C3A, (PDF = 70-0839) | 1.75 ± 0.4% | 1.0 ± 0.2% |
| K2O | 0.47% | 0.44% | Anhydrite, CaSO4 (PDF = 72-0916) | 22.1 ± 0.4% | |
| TiO2 | 0.39% | 0.32% | |||
| SO3 | 9.92% | 18.36% | |||
| Others | 1.70% | 1.35% | |||
| 1 LOI | 1.31% | 1.45% | Crystallography Open Database | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Encinas, P.; Palomo, A.; Fernández-Jiménez, A. Effect of Na versus Ca Sulfate Salts on the Hydration of Calcium Sulfoaluminate Clinker. Molecules 2023, 28, 7651. https://doi.org/10.3390/molecules28227651
Padilla-Encinas P, Palomo A, Fernández-Jiménez A. Effect of Na versus Ca Sulfate Salts on the Hydration of Calcium Sulfoaluminate Clinker. Molecules. 2023; 28(22):7651. https://doi.org/10.3390/molecules28227651
Chicago/Turabian StylePadilla-Encinas, Pilar, Angel Palomo, and Ana Fernández-Jiménez. 2023. "Effect of Na versus Ca Sulfate Salts on the Hydration of Calcium Sulfoaluminate Clinker" Molecules 28, no. 22: 7651. https://doi.org/10.3390/molecules28227651
APA StylePadilla-Encinas, P., Palomo, A., & Fernández-Jiménez, A. (2023). Effect of Na versus Ca Sulfate Salts on the Hydration of Calcium Sulfoaluminate Clinker. Molecules, 28(22), 7651. https://doi.org/10.3390/molecules28227651






