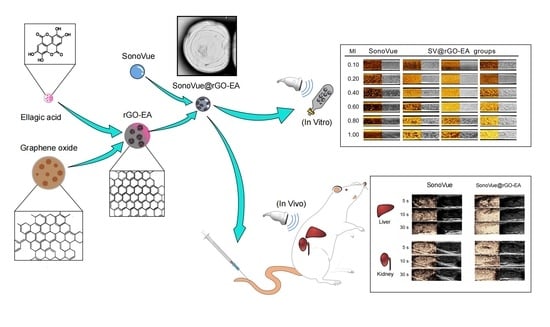
The Green Synthesis of Reduced Graphene Oxide Using Ellagic Acid: Improving the Contrast-Enhancing Effect of Microbubbles in Ultrasound
Abstract
:1. Introduction
2. Results and Discussion
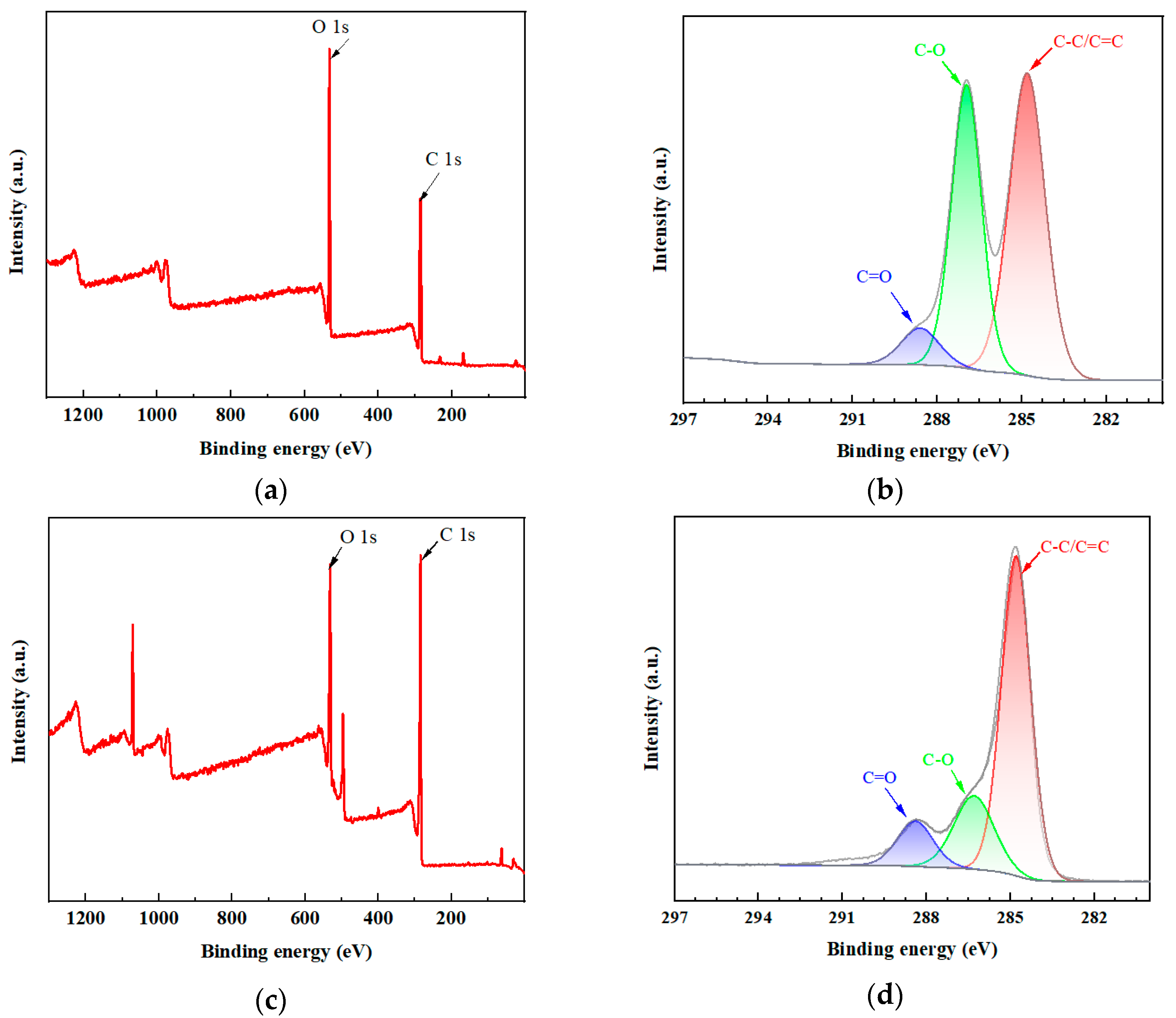
2.1. Characterization of rGO-EA
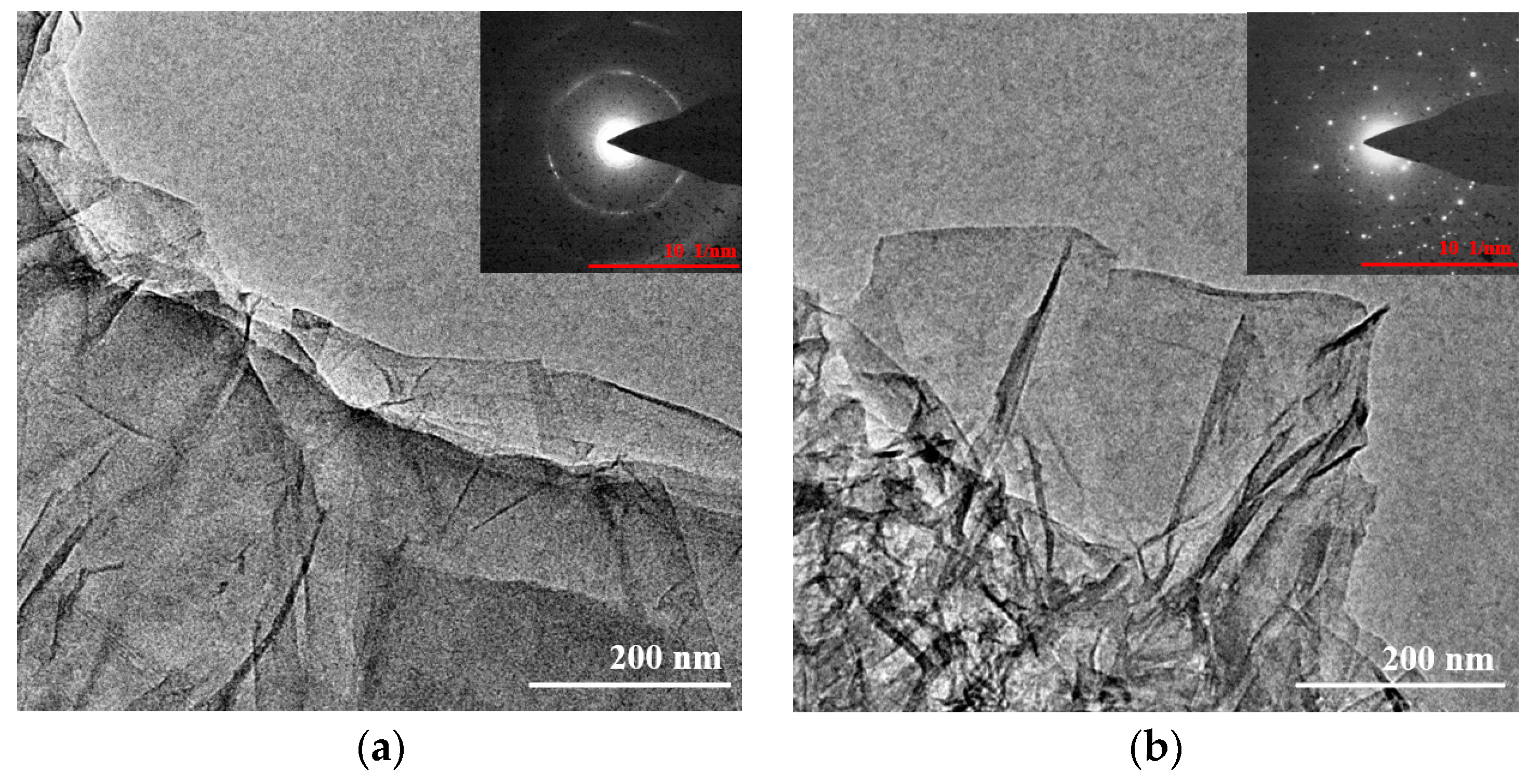
2.2. Morphology of GO and rGO-EA
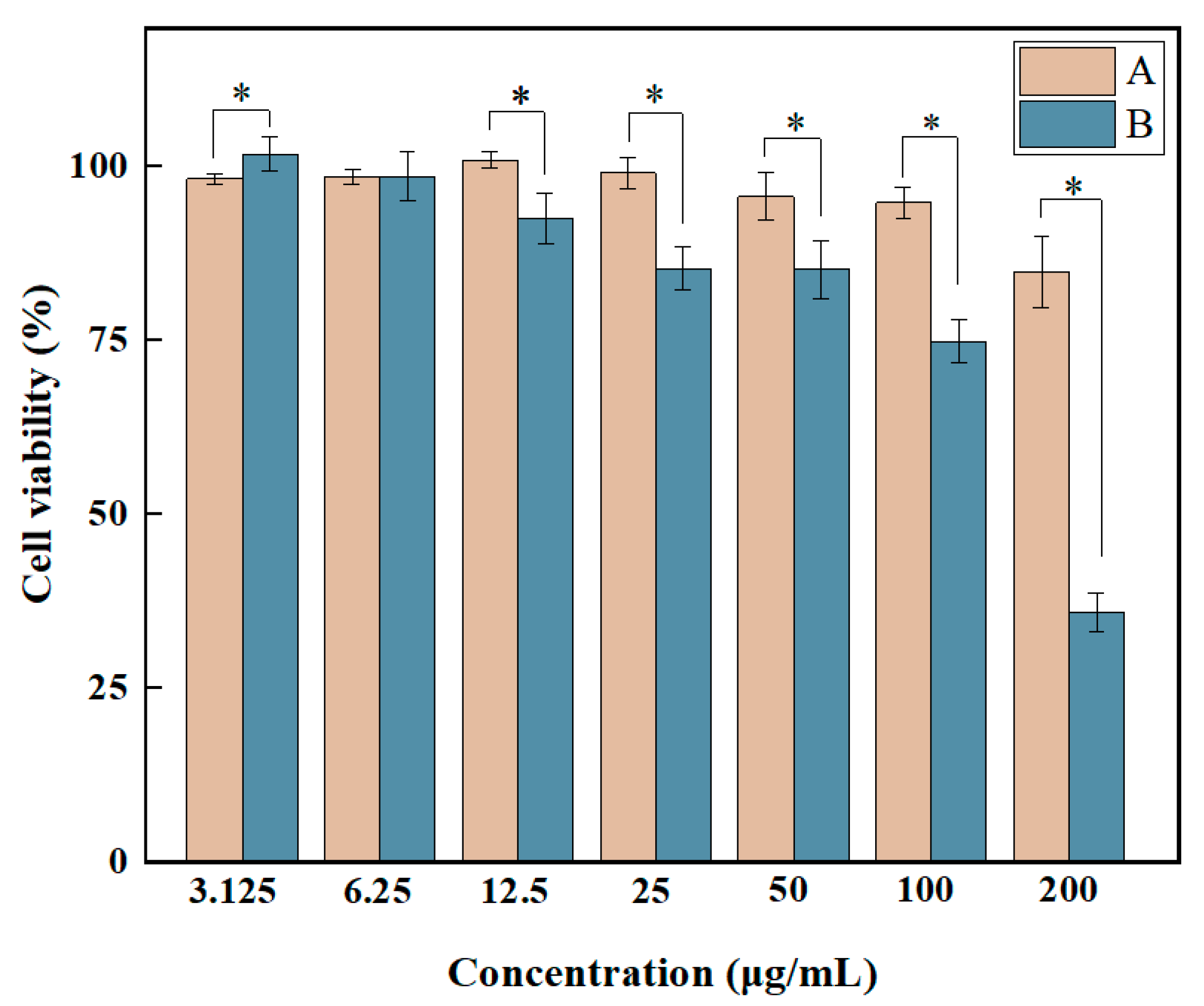
2.3. Cell Viability Assay
2.4. Hemolysis Assay
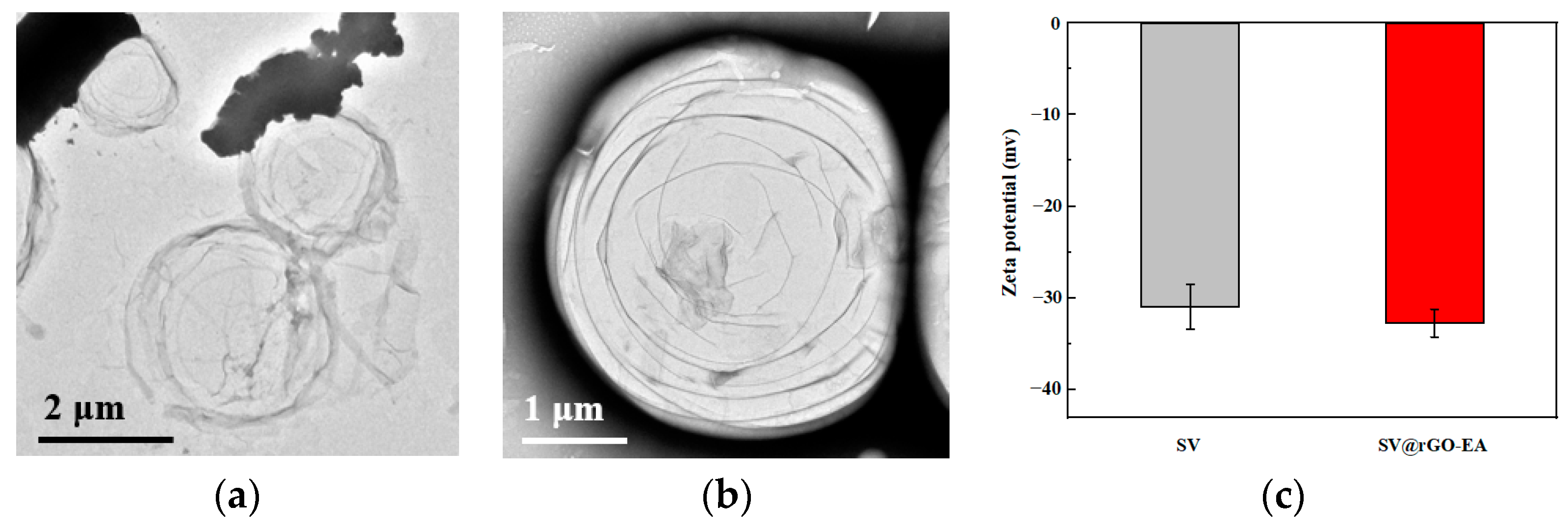
2.5. Morphology and Zeta Potential Values of MBs
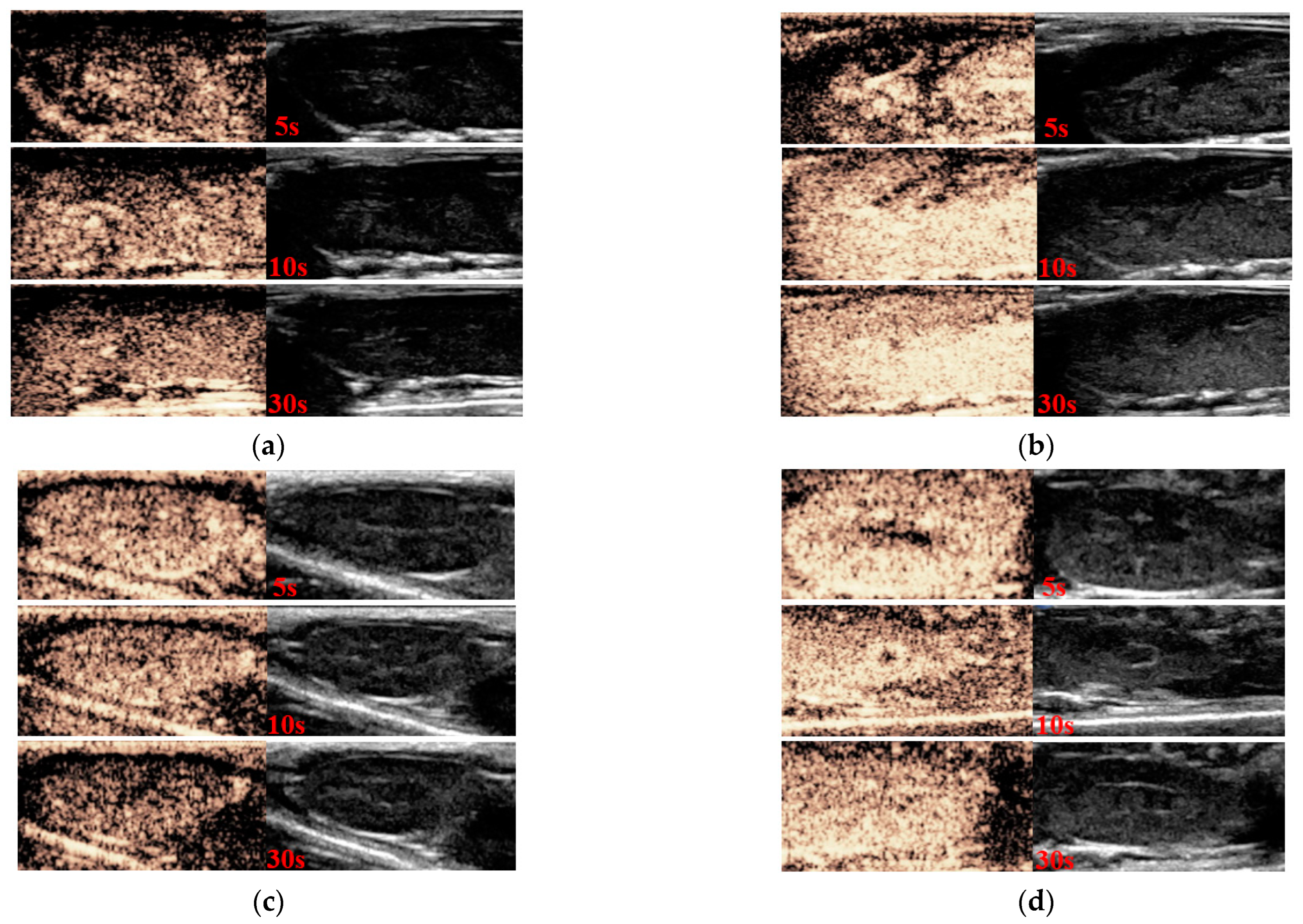
2.6. In Vitro Ultrasound Imaging
2.7. In Vivo Ultrasound Imaging
3. Materials and Methods
3.1. Materials
3.2. Cell Lines and Animals
3.3. Synthesis of rGO-EA
3.4. Characterization of rGO-EA
3.5. Synthesis of SV@rGO-EA
3.6. Characterization of SV@rGO-EA
3.7. Cell Viability Assay
3.8. Hemolysis Assay
3.9. In Vitro Ultrasound Imaging
3.10. In Vivo Ultrasound Imaging
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, J.L.; Fan, T.T. Flexible and stretchable transparent conductive graphene-based electrodes for emerging wearable electronics. Carbon 2023, 202, 495–527. [Google Scholar] [CrossRef]
- Yin, T.; Xu, L.; Gil, B.; Merali, N.; Sokolikova, M.S.; Gaboriau, D.C.A.; Liu, D.S.K.; Mustafa, A.N.M.; Alodan, S.; Chen, M.; et al. Graphene Sensor Arrays for Rapid and Accurate Detection of Pancreatic Cancer Exosomes in Patients’ Blood Plasma Samples. ACS Nano 2023, 17, 14619–14631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qi, G.; Shi, G.; Zhang, M.; Hu, H.; Hao, L. Engineered Graphene Quantum Dots as a Magnetic Resonance Signal Amplifier for Biomedical Imaging. Molecules 2023, 28, 2363. [Google Scholar] [CrossRef] [PubMed]
- Mohebichamkhorami, F.; Faizi, M.; Mahmoudifard, M.; Hajikarim-Hamedani, A.; Mohseni, S.S.; Heidari, A.; Ghane, Y.; Khoramjouy, M.; Khayati, M.; Ghasemi, R.; et al. Microfluidic Synthesis of Ultrasmall Chitosan/Graphene Quantum Dots Particles for Intranasal Delivery in Alzheimer’s Disease Treatment. Small 2023, 19, e2207626. [Google Scholar] [CrossRef]
- Zhang, G.; Ye, H.R.; Sun, Y.; Guo, Z.Z. Ultrasound Molecular Imaging and Its Applications in Cancer Diagnosis and Therapy. ACS Sens. 2022, 7, 2857–2864. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Saiding, Q.; Cai, Z.W.; Cai, M.; Cui, W.G. Ultrasound technology and biomaterials for precise drug therapy. Mater. Today 2023, 63, 210–238. [Google Scholar] [CrossRef]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef]
- Borden, M.A.; Song, K.H. Reverse engineering the ultrasound contrast agent. Adv. Colloid Interface Sci. 2018, 262, 39–49. [Google Scholar] [CrossRef]
- Ferraioli, G.; Meloni, M.F. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography 2018, 37, 25–35. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Duan, S.; Zhang, Y.; Wang, Y.; Zhang, B.; Ren, S.; Zhang, L. Cellulose nanocrystal-based enhancement of ultrasound microbubbles for increased tolerance of mechanical index values. Cellulose 2022, 29, 9531–9547. [Google Scholar] [CrossRef]
- Fournier, L.; de La Taille, T.; Chauvierre, C. Microbubbles for human diagnosis and therapy. Biomaterials 2023, 294, 122025. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, G.S.; Tamilselvi, R.; Geethalakshmi, R.; Kirupha, S.D.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Multifunctional oil-produced reduced graphene oxide—Silver oxide composites with photocatalytic, antioxidant, and antibacterial activities. J. Colloid Interface Sci. 2022, 608, 294–305. [Google Scholar] [CrossRef]
- Verma, S.; Das, T.; Pandey, V.K.; Verma, B. Facile and scalable synthesis of reduced-graphene oxide using different green reducing agents and its characterizations. Diam. Relat. Mater. 2022, 129, 109361. [Google Scholar] [CrossRef]
- Vatandost, E.; Ghorbani-HasanSaraei, A.; Chekin, F.; Naghizadeh Raeisi, S.; Shahidi, S.A. Green tea extract assisted green synthesis of reduced graphene oxide: Application for highly sensitive electrochemical detection of sunset yellow in food products. Food Chem. X 2020, 6, 100085. [Google Scholar] [CrossRef] [PubMed]
- Yoganathan, S.; Alagaratnam, A.; Acharekar, N.; Kong, J. Ellagic Acid and Schisandrins: Natural Biaryl Polyphenols with Therapeutic Potential to Overcome Multidrug Resistance in Cancer. Cells 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ghosh, S.; Kumar, P.; Basu, B.; Nagpal, K. Ellagic acid-loaded, tween 80-coated, chitosan nanoparticles as a promising therapeutic approach against breast cancer: In-vitro and in-vivo study. Life Sci. 2021, 284, 119927. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Ibarra-Lara, L.; Cuevas-Magaña, M.Y.; Sánchez-Mendoza, A.; Armada, E. Protective activities of ellagic acid and urolithins against kidney toxicity of environmental pollutants: A review. Env. Toxicol Pharmacol. 2022, 95, 103960. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, C.; Fu, C.; Lu, H.; Jin, H.; Chen, Q.; Pan, J. The protective effect of Ellagic acid (EA) in osteoarthritis: An in vitro and in vivo study. Biomed Pharmacother. 2020, 125, 109845. [Google Scholar] [CrossRef]
- Han, W.; Niu, W.Y.; Sun, B.; Shi, G.C.; Cui, X.Q. Biofabrication of polyphenols stabilized reduced graphene oxide and its anti-tuberculosis activity. J. Photochem. Photobiol. B 2016, 165, 305–309. [Google Scholar] [CrossRef]
- Parihar, A.; Sharma, P.; Choudhary, N.K.; Khan, R.; Gupta, A.; Sen, R.K.; Prasad, H.C.; Ashiq, M. Green Synthesis of CdS and CdS/rGO Nanocomposites: Evaluation of Electrochemical, Antimicrobial, and Photocatalytic Properties. ACS Appl. Biol. Mater. 2023, 6, 3706–3716. [Google Scholar] [CrossRef]
- Taniselass, S.; Md Arshad, M.K.; Gopinath, S.C.B. Current state of green reduction strategies: Solution-processed reduced graphene oxide for healthcare biodetection. Mater. Sci. Eng. C 2019, 96, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Archana, V.; Xia, Y.; Fang, R.; Gnana Kumar, G. Hierarchical CuO/NiO-Carbon Nanocomposite Derived from Metal Organic Framework on Cello Tape for the Flexible and High Performance Nonenzymatic Electrochemical Glucose Sensors. ACS Sustain. Chem. Eng. 2019, 7, 6707–6719. [Google Scholar] [CrossRef]
- Mei, X.; Ouyang, J. Ultrasonication-assisted ultrafast reduction of graphene oxide by zinc powder at room temperature. Carbon 2011, 49, 5389–5397. [Google Scholar] [CrossRef]
- Krishna, R.; Titus, E.; Okhay, O.; Gil, J.C.; Ventura, J.; Ramana, E.V.; Gracio, J.J. Rapid Electrochemical Synthesis of Hydrogenated Graphene Oxide Using Ni Nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 4054–4069. [Google Scholar] [CrossRef]
- Rani, P.; Dahiya, R.; Bulla, M.; Devi, R.; Jeet, K.; Jatrana, A.; Kumar, V. Hydrothermal-assisted green synthesis of reduced graphene oxide nanosheets (rGO) using lemon (Citrus Limon) peel extract. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; Abudoleh, S.M.; Hussein, M.Z.; Bullo, S.; Palanisamy, A. Graphene oxide-ellagic acid nanocomposite as effective anticancer and antimicrobial agent. IET Nanobiotechnol. 2021, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Sheet, S.; Sathishkumar, Y.; Lee, Y.S.; Phang, S.-M.; Periasamy, V.; Gnana Kumar, G. Titania/reduced graphene oxide composite nanofibers for the direct extraction of photosynthetic electrons from microalgae for biophotovoltaic cell applications. Appl. Phys. A 2018, 124, 769. [Google Scholar] [CrossRef]
- Cherian, R.S.; Sandeman, S.; Ray, S.; Savina, I.N.; Ashtami, J.; Mohanan, P.V. Green synthesis of Pluronic stabilized reduced graphene oxide: Chemical and biological characterization. Colloids Surf. B Biointerfaces 2019, 179, 94–106. [Google Scholar] [CrossRef]
- Gómez-Mancebo, M.B.; Fernández-Martínez, R.; Ruiz-Perona, A.; Rubio, V.; Bastante, P.; García-Pérez, F.; Borlaf, F.; Sanchez, M.; Hamada, A.; Velasco, A.; et al. Comparison of Thermal and Laser-Reduced Graphene Oxide Production for Energy Storage Applications. Nanomaterials 2023, 13, 1391. [Google Scholar] [CrossRef]
- Wu, J.; Liu, B.; Ren, Z.; Ni, M.; Li, C.; Gong, Y.; Qin, W.; Huang, Y.; Sun, C.Q.; Liu, X. CuS/RGO hybrid photocatalyst for full solar spectrum photoreduction from UV/Vis to near-infrared light. J. Colloid Interface Sci. 2018, 517, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.P.; Lai, C.W.; Lee, K.M.; Hamid, S.B.A. Advanced Chemical Reduction of Reduced Graphene Oxide and Its Photocatalytic Activity in Degrading Reactive Black 5. Materials 2015, 8, 7118–7128. [Google Scholar] [CrossRef] [PubMed]
- Hatamie, S.; Akhavan, O.; Sadrnezhaad, S.K.; Ahadian, M.M.; Shirolkar, M.M.; Wang, H.Q. Curcumin-reduced graphene oxide sheets and their effects on human breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Utkan, G.; Yumusak, G.; Tunali, B.C.; Ozturk, T.; Turk, M. Production of Reduced Graphene Oxide by Using Three Different Microorganisms and Investigation of Their Cell Interactions. ACS Omega 2023, 8, 31188–31200. [Google Scholar] [CrossRef] [PubMed]
- Maktedar, S.S.; Avashthi, G.; Singh, M. Ultrasound assisted simultaneous reduction and direct functionalization of graphene oxide with thermal and cytotoxicity profile. Ultrason. Sonochem. 2017, 34, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Dai, J.; Li, J.; Di, Z.; Liu, X. Antibacterial ability and hemocompatibility of graphene functionalized germanium. Sci. Rep. 2016, 6, 37474. [Google Scholar] [CrossRef]
- Dat, N.M.; Cong, C.Q.; Phuc, N.M.; Dat, N.T.; Huong, L.M.; Tai, L.T.; Hai, N.G.; Thinh, D.B.; Dat, T.D.; Phong, M.T.; et al. Facile phytosynthesis of gold nanoparticles-doped graphene oxide using Mangifera indica leaf extract: Characterization, antibacterial activity, and catalytic reduction of organic dyes. Mater. Today Sustain. 2022, 19, 100216. [Google Scholar] [CrossRef]
- Nahar, A.; Hanium Maria, K.; Liba, S.I.; Anwaruzzaman, M.; Khan, M.N.I.; Islam, A.; Choudhury, S.; Hoque, S.M. Surface-modified CoFe2O4 nanoparticles using Folate-Chitosan for cytotoxicity Studies, hyperthermia applications and Positive/Negative contrast of MRI. J. Magn. Magn. Mater. 2022, 554, 169282. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Wang, Y.; Zhou, Q.; Duan, S.; Zhang, B.; Li, Y.; Zhang, L. The Green Synthesis of Reduced Graphene Oxide Using Ellagic Acid: Improving the Contrast-Enhancing Effect of Microbubbles in Ultrasound. Molecules 2023, 28, 7646. https://doi.org/10.3390/molecules28227646
Cheng Q, Wang Y, Zhou Q, Duan S, Zhang B, Li Y, Zhang L. The Green Synthesis of Reduced Graphene Oxide Using Ellagic Acid: Improving the Contrast-Enhancing Effect of Microbubbles in Ultrasound. Molecules. 2023; 28(22):7646. https://doi.org/10.3390/molecules28227646
Chicago/Turabian StyleCheng, Qiwei, Yuzhou Wang, Qi Zhou, Shaobo Duan, Beibei Zhang, Yaqiong Li, and Lianzhong Zhang. 2023. "The Green Synthesis of Reduced Graphene Oxide Using Ellagic Acid: Improving the Contrast-Enhancing Effect of Microbubbles in Ultrasound" Molecules 28, no. 22: 7646. https://doi.org/10.3390/molecules28227646
APA StyleCheng, Q., Wang, Y., Zhou, Q., Duan, S., Zhang, B., Li, Y., & Zhang, L. (2023). The Green Synthesis of Reduced Graphene Oxide Using Ellagic Acid: Improving the Contrast-Enhancing Effect of Microbubbles in Ultrasound. Molecules, 28(22), 7646. https://doi.org/10.3390/molecules28227646