A Strategy for Studying Environmental Engineering: Simple Hydrothermal Synthesis of Flower-Shaped Stannous Sulfide Nanomaterials for Efficient Cataluminescence Sensing of Diethyl Ether
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.2. Effect of Operating Temperature on CTL Intensity
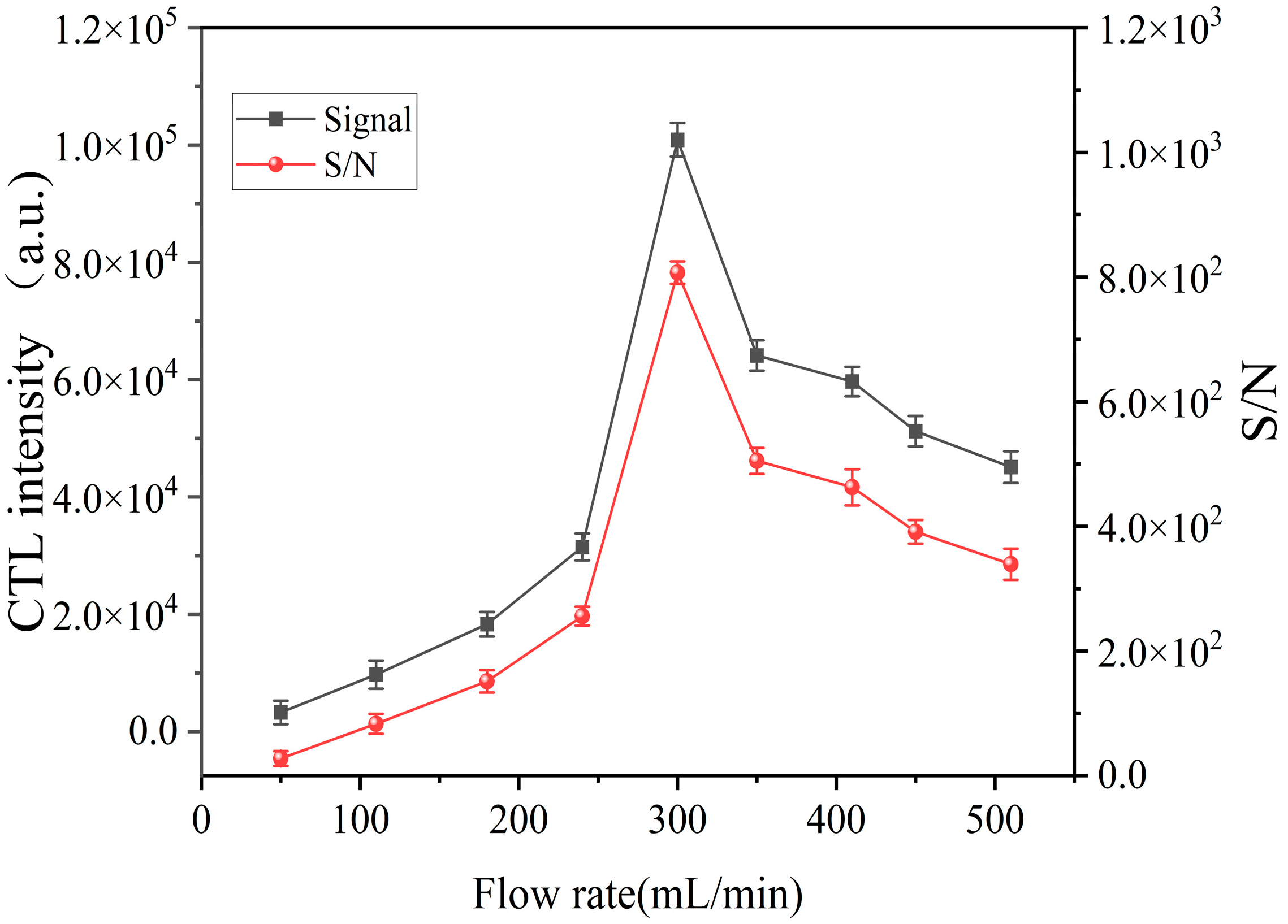
2.3. Effect of Flow Rate on CTL Intensity
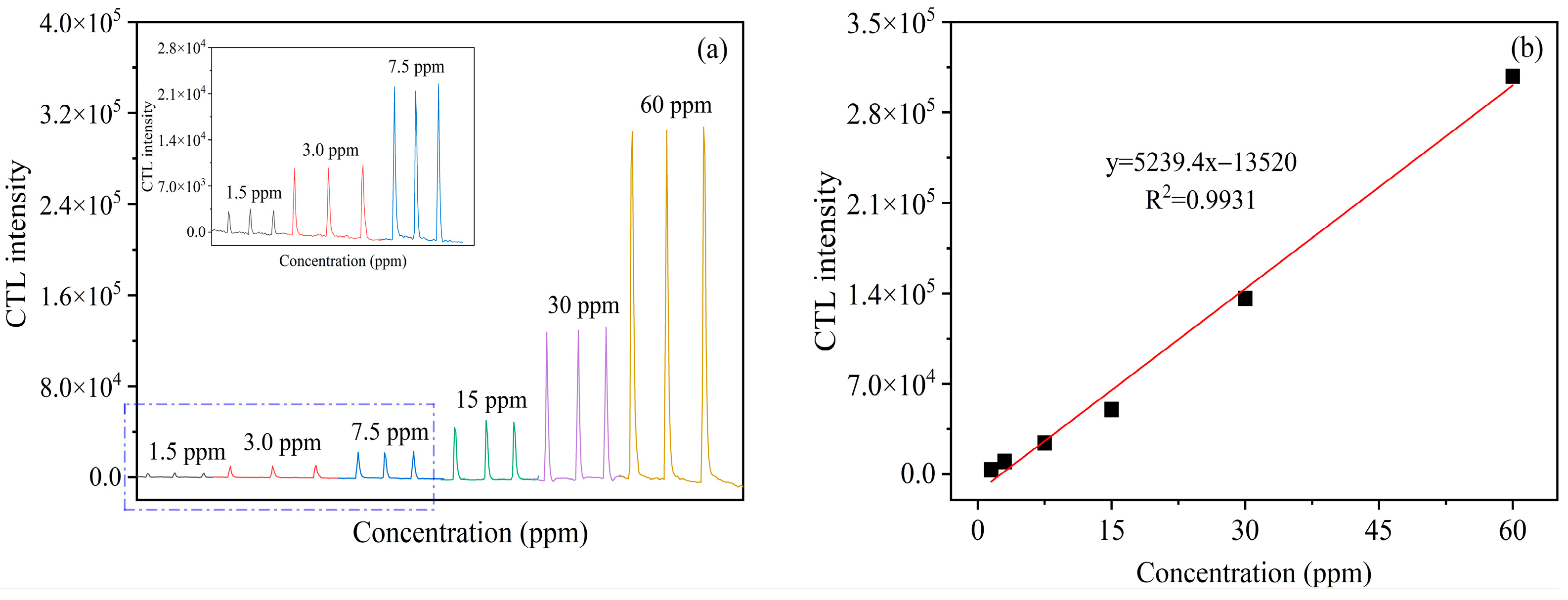
2.4. Effect of Concentration
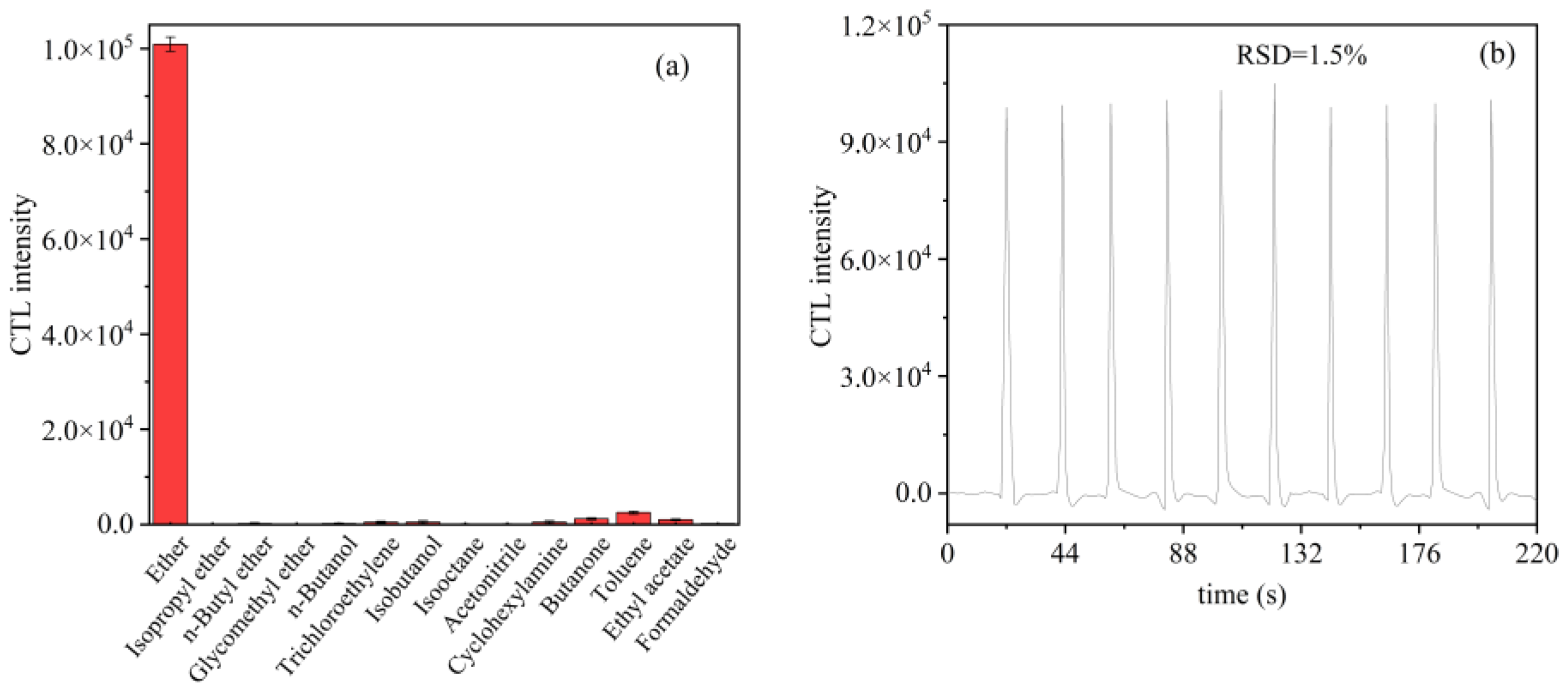
2.5. Selectivity and Stability of the Sensor
2.6. Advantages of SnS Sensors
| Test Samples | Sensing Material | Response Time (s) | Recovery Time (s) | Operating Temperature (°C) | Linear Range (ppm) | LOD (ppm) | References |
|---|---|---|---|---|---|---|---|
| diethyl ether | ZnWO4 | 3 | 7 | 330 | 20–3500 | 8.7 | [15] |
| SiO2/Fe3O4 | 5 | 30 | 320 | 10–3000 | 6.7 | [16] | |
| Mg-Al LDO | 2.5 | 15 | 205 | 7–593 | 1.5 | [17] | |
| α-MoO3 | 16 | 2 | 120 | 9–2000 | 7.5 | [37] | |
| CdO | 3 | 10 | 285 | 10–4000 | 6.5 | [38] | |
| Al-Fe composite oxide | 4 | 8 | 180 | 10–5800 | 4.3 | [39] | |
| TiO2 | — | — | 200 | 148–3706 | 111.2 | [40] | |
| SnS | 3 | 8 | 153 | 1.5–60 | 0.15 | This work |
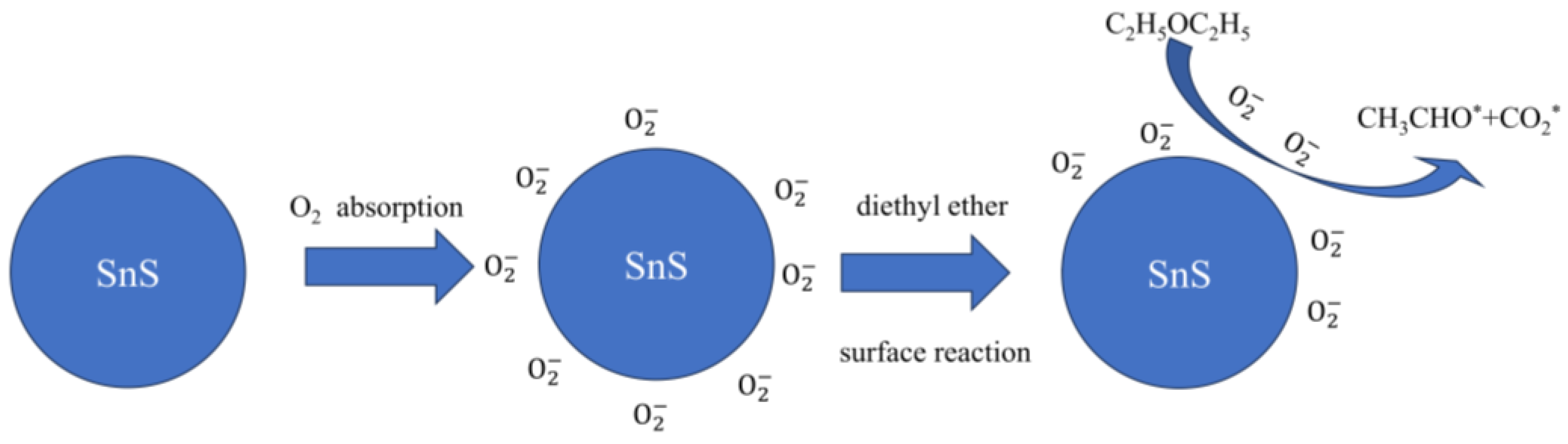
2.7. Mechanistic Discussion
3. Materials and Methods
3.1. Test Reagents and Instruments
3.2. Preparation of Nanomaterials
3.3. Main Analytical Instruments
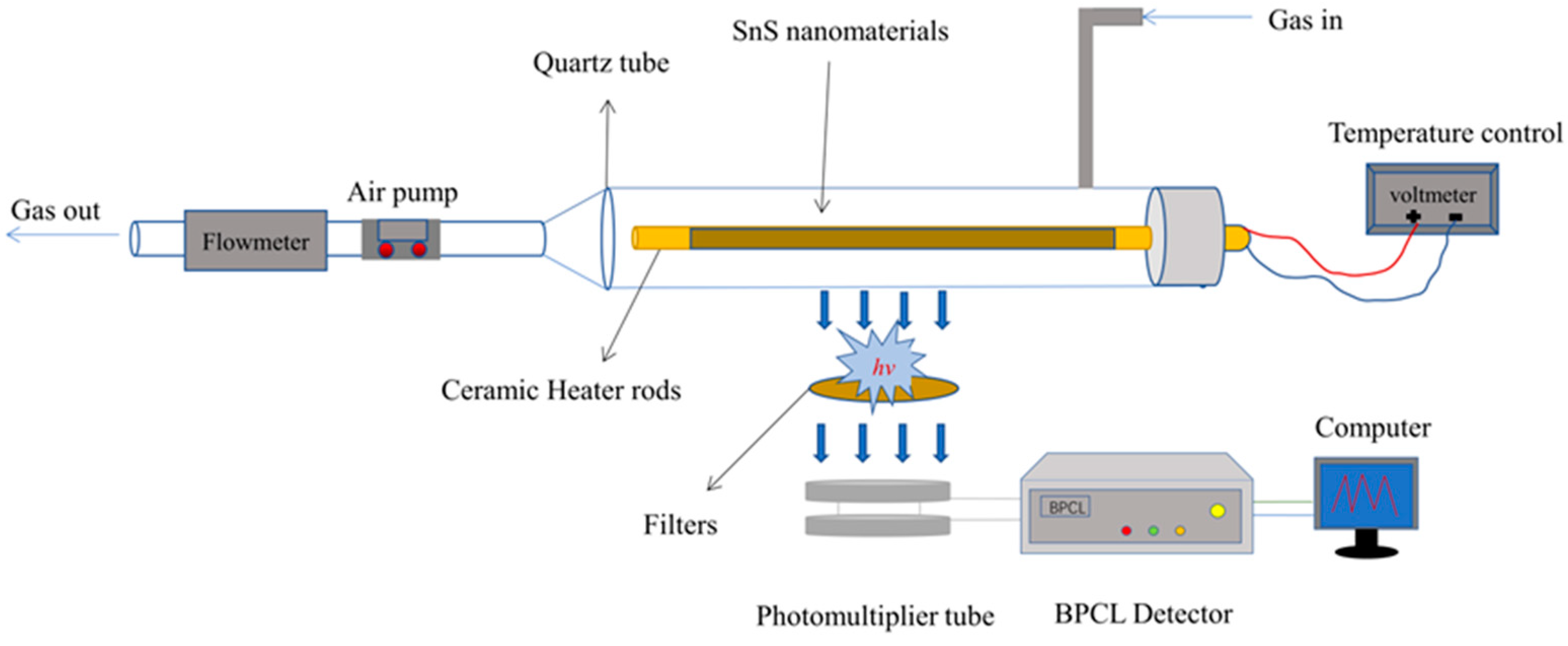
3.4. CTL Device and Detection Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, J.; Zhang, C.; Liu, W.; Bai, C.; Zhao, X.; Sun, B.; Liu, N. The explosion characteristics of diethyl ether-Al mixtures under different ambient conditions. Combust. Flame 2021, 227, 162–171. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.L.; Chang, Q.; Cheng, J.W. Measurements of explosion parameters for diethyl ether/air mixtures at pre-ignition quasi-isotropic turbulence. Fuel 2021, 292, 120224. [Google Scholar] [CrossRef]
- Paidi, M.K.; Attupuram, A.; Udata, K.S.; Mandal, S.K. Acetone diethyl ether-based biorefinery process for co-extraction of fucoxanthin, chlorophyll, DHA, and EPA from the diatom Thalassiosira lundiana. Algal Res. 2023, 74, 103215. [Google Scholar] [CrossRef]
- Yasmen, N.; Aziz, M.A.; Tajmim, A.; Akter, M.I.; Hazra, A.K.; Rahman, S.M.M. Analgesic and Anti-Inflammatory Activities of Diethyl Ether and n-Hexane Extract of Polyalthia suberosa Leaves. Evid.-Based Complement. Altern. Med. 2018, 2018, 5617234. [Google Scholar] [CrossRef]
- Pavlovič, A.; Libiaková, M.; Bokor, B.; Jakšová, J.; Petřík, I.; Novák, O.; Baluška, F. Anaesthesia with diethyl ether impairs jasmonate signalling in the carnivorous plant Venus flytrap (Dionaea muscipula). Ann. Bot. 2020, 125, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, F.; Kemmerling, R.; Schulz, K.; Keller, T. Another case of diethyl ether intoxication? A case report focusing on toxicological analysis. Leg. Med. 2011, 13, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yuan, Z.; Shen, Y.; Zhang, R.; Meng, F. Phosphorus-doped porous perovskite LaFe1-xPxO3-δ nanosheets with rich surface oxygen vacancies for ppb level acetone sensing at low temperature. Chem. Eng. J. 2022, 431, 134280. [Google Scholar] [CrossRef]
- Belhadj, N.; Benoit, R.; Lailliau, M.; Glasziou, V.; Dagaut, P. Oxidation of diethyl ether: Extensive characterization of products formed at low temperature using high resolution mass spectrometry. Combust. Flame 2021, 228, 340–350. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Xun, Z.; Du, W.; Guo, X.; Xian, Y.; Lin, S.; Tan, J. Simultaneous determination of glycol ethers and their acetates in cosmetics by gas chromatography with mass spectrometry. J. Sep. Sci. 2018, 41, 2354–2359. [Google Scholar] [CrossRef]
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of Metal Oxide Semiconductor Gas Sensors: A Review. IEEE Sens. J. 2022, 22, 5470–5481. [Google Scholar] [CrossRef]
- Lin, H.-B.; Shih, J.-S. Fullerene C60-cryptand coated surface acoustic wave quartz crystal sensor for organic vapors. Sens. Actuators B Chem. 2003, 92, 243–254. [Google Scholar] [CrossRef]
- Lun, D.; Xu, K. Recent Progress in Gas Sensor Based on Nanomaterials. Micromachines 2022, 13, 919. [Google Scholar] [CrossRef]
- Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Correa, D.S. Electrospun Ceramic Nanofibers and Hybrid-Nanofiber Composites for Gas Sensing. ACS Appl. Nano Mater. 2019, 2, 4026–4042. [Google Scholar] [CrossRef]
- Raya, I.; Kzar, H.H.; Mahmoud, Z.H.; Al Ayub Ahmed, A.; Ibatova, A.Z.; Kianfar, E. A review of gas sensors based on carbon nanomaterial. Carbon Lett. 2022, 32, 339–364. [Google Scholar] [CrossRef]
- Cao, X.; Wu, W.; Chen, N.; Peng, Y.; Liu, Y. An ether sensor utilizing cataluminescence on nanosized ZnWO4. Sens. Actuators B Chem. 2009, 137, 83–87. [Google Scholar] [CrossRef]
- Shi, G.; Sun, B.; Jin, Z.; Liu, J.; Li, M. Synthesis of SiO2/Fe3O4 nanomaterial and its application as cataluminescence gas sensor material for ether. Sens. Actuators B Chem. 2012, 171–172, 699–704. [Google Scholar] [CrossRef]
- Zhang, L.; He, N.; Shi, W.; Lu, C. A cataluminescence sensor with fast response to diethyl ether based on layered double oxide nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8787–8793. [Google Scholar] [CrossRef]
- Jamal, F.; Rafique, A.; Moeen, S.; Haider, J.; Nabgan, W.; Haider, A.; Imran, M.; Nazir, G.; Alhassan, M.; Ikram, M.; et al. Review of Metal Sulfide Nanostructures and their Applications. ACS Appl. Nano Mater. 2023, 6, 7077–7106. [Google Scholar] [CrossRef]
- Norton, K.J.; Alam, F.; Lewis, D.J. A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS). Appl. Sci. 2021, 11, 2062. [Google Scholar] [CrossRef]
- Qing, L.; Li, R.; Su, W.; Zhao, W.; Li, Y.; Chen, G.; Liu, N.; Chen, J. Nanostructures of Carbon Nanofiber-Constrained Stannous Sulfide with High Flexibility and Enhanced Performance for Sodium-Ion Batteries. Energy Fuels 2022, 36, 2179–2188. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Jiao, M.; Mou, K.; Zhang, X.; Liu, L. Engineering Electronic Structure of Stannous Sulfide by Amino-Functionalized Carbon: Toward Efficient Electrocatalytic Reduction of CO2 to Formate. Adv. Energy Mater. 2020, 10, 1903664. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Ying, S.; Yang, C.; Zhao, J.; Hu, N.; Peng, C. In-situ sulfidation-derived three-dimensional cobalt sulfide nanoflower/graphene nanosheet hybrid for ultrasensitive room-temperature NO2 gas sensor. J. Alloys Compd. 2022, 926, 166868. [Google Scholar] [CrossRef]
- Feng, J.; Li, X.; Zhu, G.; Wang, Q. Emerging High-Performance SnS/CdS Nanoflower Heterojunction for Ultrafast Photonics. ACS Appl. Mater. Interfaces 2020, 12, 43098–43105. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bai, C.; Qiu, P. Alkalized SnS Nanoflakes with Enhanced Sensing Properties towards Methanol Vapor. ECS J. Solid State Sci. Technol. 2020, 9, 121013. [Google Scholar] [CrossRef]
- Babaei, P.; Safaei-Ghomi, J. l-proline covered N doped graphene quantum dots modified CuO/ZnO hexagonal nanocomposite as a robust retrievable catalyst in synthesis of substituted chiral 2-amino-4H-chromenes. Mater. Chem. Phys. 2021, 267, 124668. [Google Scholar] [CrossRef]
- Cheng, P.; Dang, F.; Wang, Y.; Gao, J.; Xu, L.; Wang, C.; Lv, L.; Li, X.; Zhang, B.; Liu, B. Gas sensor towards n-butanol at low temperature detection: Hierarchical flower-like Ni-doped Co3O4 based on solvent-dependent synthesis. Sens. Actuators B Chem. 2021, 328, 129028. [Google Scholar] [CrossRef]
- Pan, F.; Sun, B.; Tang, Z.; Zhu, S. A fast response cataluminescence ether gas sensor based on GO/Mo2TiC2Tx at low working temperature. RSC Adv. 2022, 12, 8361–8367. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Alamri, H.R.; Ansari, M.O.; Khan, Z.; Cho, M.H. Facile Synthesis of SnS2 Nanostructures with Different Morphologies for High-Performance Supercapacitor Applications. ACS Omega 2018, 3, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Govindarajan, D.; Dar, G.N. Facile synthesis of SnS nanostructures with different morphologies for supercapacitor and dye-sensitized solar cell applications. J. Mater. Sci. Mater. Electron. 2021, 32, 20394–20409. [Google Scholar] [CrossRef]
- Matmin, J.; Jalani, M.A.; Osman, H.; Omar, Q.; Ab’lah, N.; Elong, K.; Kasim, M.F. Photochemical Synthesis of Nanosheet Tin Di/Sulfide with Sunlight Response on Water Pollutant Degradation. Nanomaterials 2019, 9, 264. [Google Scholar] [CrossRef]
- Anfimov, N.; Fedoseev, D.; Rybnikov, A.; Selyunin, A.; Sokolov, S.; Sotnikov, A. Study of silicon photomultiplier performance at different temperatures. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 997, 165162. [Google Scholar] [CrossRef]
- Sun, B.; Shi, G.; Tang, Z.; Zhang, P.; Guo, Y.; Zhu, S.; Liu, J. Rapid Gas-Sensing Detection of Carbon Disulfide by a CdS/SnS Nanocomposite-Based Cataluminescence Sensor. Chemosensors 2023, 11, 10. [Google Scholar] [CrossRef]
- Peng, X.; Han, Y.; Zhang, Q.; Feng, P.; Jia, P.; Cui, H.; Wang, L.; Duan, S. Performance Improvement of MoS2 Gas Sensor at Room Temperature. IEEE Trans. Electron Devices 2021, 68, 4644–4650. [Google Scholar] [CrossRef]
- Sedlák, P.; Kuberský, P. The Effect of the Orientation Towards Analyte Flow on Electrochemical Sensor Performance and Current Fluctuations. Sensors 2020, 20, 1038. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B Chem. 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Wu, T.; Shen, J.; Li, Z.; Xing, F.; Xin, W.; Wang, Z.; Liu, G.; Han, X.; Man, Z.; Fu, S. Microfluidic-integrated graphene optical sensors for real-time and ultra-low flow velocity detection. Appl. Surf. Sci. 2021, 539, 148232. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H.; Fu, F.; Zhang, Y. A cataluminescence sensor based on α-MoO3 nanobelts with low working temperature for the detection of diethyl ether. J. Mater. Sci. Mater. Electron. 2019, 30, 3722–3728. [Google Scholar] [CrossRef]
- Sha, W.; Gu, P.; Zhang, B.; Zheng, C. A cataluminescence sensor system for diethyl ether based on CdO nanostructure. Meas. Sci. Technol. 2014, 25, 085102. [Google Scholar] [CrossRef]
- Shi, G.; He, Y.; Zhang, Y.; Yin, B.; Ali, F. Detection and determination of harmful gases in confined spaces for the Internet of Things based on cataluminescence sensor. Sens. Actuators B Chem. 2019, 296, 126686. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Yuan, Z.; Lu, C. A controllable selective cataluminescence sensor for diethyl ether using mesoporous TiO2 nanoparticles. Sens. Actuators B Chem. 2016, 230, 242–249. [Google Scholar] [CrossRef]
- Chatterjee, B.; Bandyopadhyay, A. Review on the synthesis of metal sulfides gas sensors and their performances at room temperature. Mater. Sci. Eng. B 2023, 297, 116781. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, H.; Sun, T.; Zhang, L.; Lv, Y. Cataluminescence on 2D WS2 nanosheets surface for H2S sensing. Sens. Actuators B Chem. 2022, 353, 131111. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Su, Y.; Lv, Y. Recent advances in methodologies and applications of cataluminescence sensing. Luminescence 2020, 35, 1174–1184. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Liu, B.; Zhou, K. Novel Diethyl Ether Gas Sensor Based on Cataluminescence on Nano-Pd/ZnNi3Al2O7. ACS Omega 2021, 6, 17576–17583. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Qi, T.; Zhang, J.; Zhu, H.; Yuan, Z.; Liu, C.; Qin, W.; Ding, M. MoS2-Templated Porous Hollow MoO3 Microspheres for Highly Selective Ammonia Sensing via a Lewis Acid-Base Interaction. IEEE Trans. Ind. Electron. 2021, 69, 960–970. [Google Scholar] [CrossRef]
- Jiang, R.; Jia, L.; Guo, X.; Zhao, Z.; Du, J.; Wang, X.; Wang, P.; Xing, F. Dimethyl sulfoxide-assisted hydrothermal synthesis of Co3O4-based nanorods for selective and sensitive diethyl ether sensing. Sens. Actuators B Chem. 2019, 290, 275–284. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, K.; Zhang, Y.; Ding, X. Dendritic fibrous nano-silica & titania (DFNST) spheres as novel cataluminescence sensing materials for the detection of diethyl ether. RSC Adv. 2019, 9, 39622–39630. [Google Scholar]
- Zhou, Q.; Yan, S.; Zhang, L. Fe-doped MOF-derived N-rich porous carbon nanoframe for H2S cataluminescence sensing. Luminescence 2022, 37, 1135–1144. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Sun, J.; Zhu, X.; Xin, Y.; Sun, B. Study on cracking of n-hexadecane by dielectric barrier discharge with diethyl ether addition. Chem. Eng. J. 2023, 475, 146045. [Google Scholar] [CrossRef]
- Liu, G.; Cui, X.; Yao, L.; Rongjun, Z.; Tian, X.; Li, D.; Sun, C.; Wang, Y. ZnO-SnO2 nanocomposites modified by PdO nanoparticles named PdO-ZSO as gas sensing material for hydrogen and butane with the excellent response time and recovery time. J. Mater. Sci. Mater. Electron. 2021, 32, 28891–28908. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Liu, F.; Duan, T.; Sun, B. Non-Invasive Rapid Detection of Lung Cancer Biomarker Toluene with a Cataluminescence Sensor Based on the Two-Dimensional Nanocomposite Pt/Ti3C2Tx-CNT. Chemosensors 2022, 10, 333. [Google Scholar] [CrossRef]

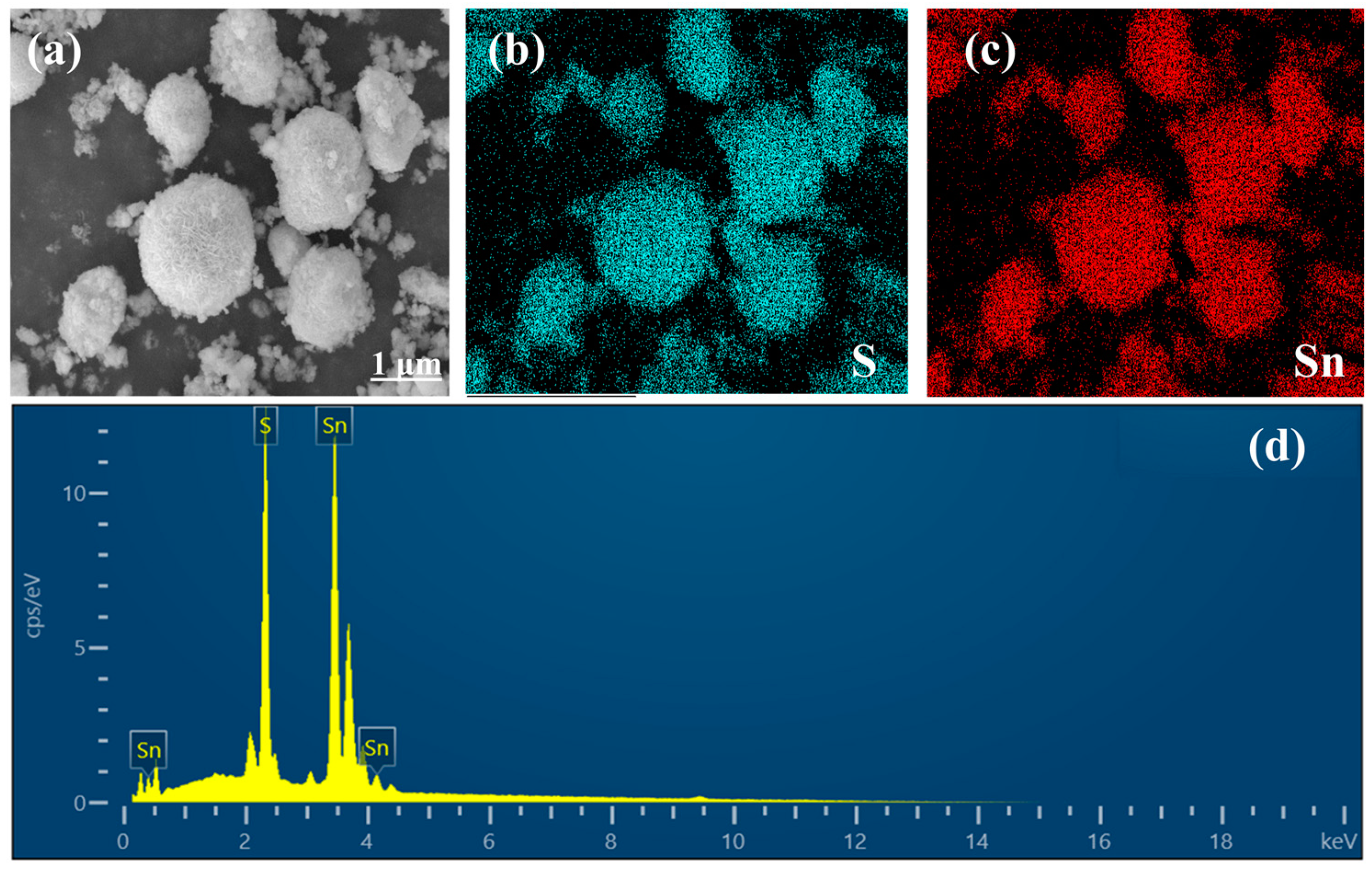
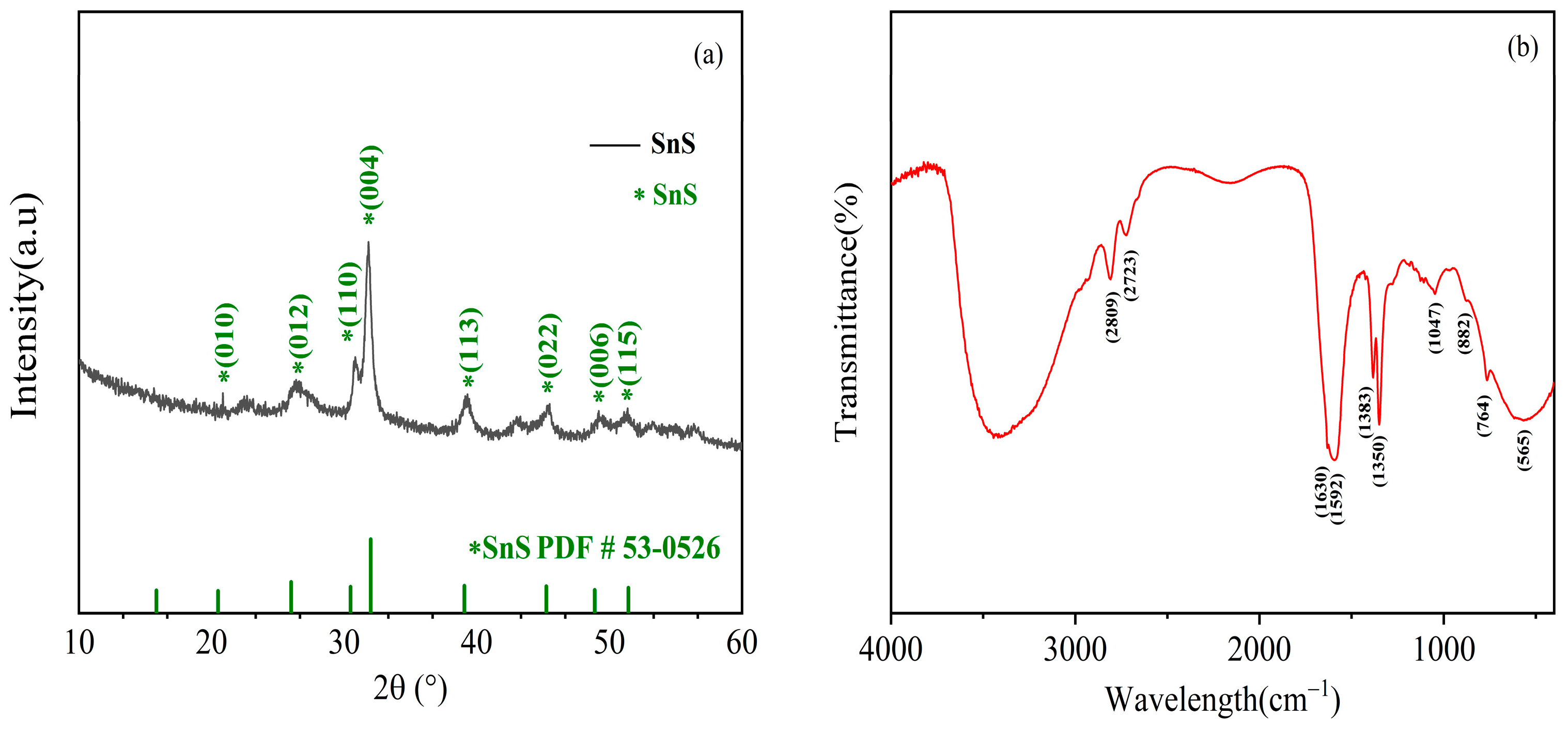

| Elements | Atomic% |
|---|---|
| S | 52.87 |
| Sn | 47.13 |
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Fan, J.; Tang, Z.; Shi, G.; Yi, M.; Wang, Y.; Wang, X.; Guo, Y.; Zhu, S. A Strategy for Studying Environmental Engineering: Simple Hydrothermal Synthesis of Flower-Shaped Stannous Sulfide Nanomaterials for Efficient Cataluminescence Sensing of Diethyl Ether. Molecules 2023, 28, 7621. https://doi.org/10.3390/molecules28227621
Sun B, Fan J, Tang Z, Shi G, Yi M, Wang Y, Wang X, Guo Y, Zhu S. A Strategy for Studying Environmental Engineering: Simple Hydrothermal Synthesis of Flower-Shaped Stannous Sulfide Nanomaterials for Efficient Cataluminescence Sensing of Diethyl Ether. Molecules. 2023; 28(22):7621. https://doi.org/10.3390/molecules28227621
Chicago/Turabian StyleSun, Bai, Jingjie Fan, Zhuo Tang, Guoji Shi, Mingjian Yi, Yun Wang, Xiangxiang Wang, Yuxian Guo, and Shuguang Zhu. 2023. "A Strategy for Studying Environmental Engineering: Simple Hydrothermal Synthesis of Flower-Shaped Stannous Sulfide Nanomaterials for Efficient Cataluminescence Sensing of Diethyl Ether" Molecules 28, no. 22: 7621. https://doi.org/10.3390/molecules28227621
APA StyleSun, B., Fan, J., Tang, Z., Shi, G., Yi, M., Wang, Y., Wang, X., Guo, Y., & Zhu, S. (2023). A Strategy for Studying Environmental Engineering: Simple Hydrothermal Synthesis of Flower-Shaped Stannous Sulfide Nanomaterials for Efficient Cataluminescence Sensing of Diethyl Ether. Molecules, 28(22), 7621. https://doi.org/10.3390/molecules28227621






