The Role of Catalyst Promotive Additives and Temperature in the Hydroisodewaxing Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics of Bifunctional Catalysts
2.2. Characteristics of Diesel Oil Fractions before the Experiments
2.3. Investigation of the Activity of the Synthesized Catalysts in the Process of Isodewaxing the Diesel Fraction
3. Materials and Methods
3.1. Bifunctional Catalysts Preparation
3.2. Study of the Physicochemical Characteristics of Bifunctional Catalysts
3.2.1. The Nitrogen Adsorption/Desorption Method
3.2.2. X-ray Wide-Angle Scattering Methods (XRD)
3.2.3. Diffuse Reflection Infrared Fourier Transform Spectroscopy (DRIFT)
3.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.5. Temperature-Programmed Desorption of Ammonia (TPD-NH3)
3.2.6. The Temperature-Programmed Reduction of Hydrogen (TPR-H2)
3.3. Study of the Composition and Physicochemical Characteristics of Diesel Fractions before and after Testing
3.3.1. The Chromatography-Mass Spectrometry Method
3.3.2. Determination of Pour Point of Diesel Fractions
3.3.3. The Closed Cup Flash Point Method
3.3.4. Method for Determining the CFPP
3.3.5. Method for Determining the Fractional Composition of the Diesel Fraction
3.3.6. Method for Determining the Sulfur Content in the Diesel Fraction
3.4. Testing of Catalysts in a Flow-Through Unit
3.5. Determination of Errors in Experimental Results
- n is the total number of values ;
- xi is the ith individual observation.
- is a mean value;
- n is the total number of values ;
- xi is the ith individual observation.
- s is a standard deviation;
- is a mean value;
- n is the total number of values ;
- xi is the ith individual observation.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erol, D.; Yeşilyurt, M.D.; Yaman, H.; Doğan, B. Evaluation of the use of diesel-biodiesel-hexanol fuel blends in diesel engines with exergy analysis and sustainability index. Fuel 2023, 337, 126892. [Google Scholar] [CrossRef]
- The Production of Diesel Fuel in Kazakhstan Has Increased by a Third. Available online: https://mgorod.kz/nitem/proizvodstvo-dizelnogo-topliva-v-kazaxstane-uvechili-na-tret (accessed on 15 June 2023).
- Zhang, M.; Chen, Y.; Wang, L.; Zhang, Q.; Tsang, C.W.; Liang, C. Shape Selectivity in Hydroisomerization of Hexadecane over Pt Supported on 10-Ring Zeolites: ZSM-22, ZSM-23, ZSM-35, and ZSM-48. Ind. Eng. Chem. Res. 2016, 55, 6069–6078. [Google Scholar] [CrossRef]
- Soualah, A.; Lemberton, J.L.; Pinard, L.; Chater, M.; Magnoux, P.; Moljord, P. Hydroisomerization of long-chain n-alkanes on bifunctional Pt/zeolite catalysts: Effect of the zeolite structure on the product selectivity and on the reaction mechanism. Appl. Catal. A Gen. 2008, 336, 23–28. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, G.; Zhao, L.; Gao, J.; Xu, C. Effect of metal–acid balance and textual modifications on hydroisomerization catalysts for n-alkanes with different chain length: A mini-review. Fuel 2022, 315, 122809. [Google Scholar] [CrossRef]
- Vajglova, Z.; Kumar, N.; Peurla, M.; Hupa, L.; Semikin, K.; Sladkovskiy, D.A.; Murzin, D.Y. Effect of the Preparation of Pt-Modified Zeolite Beta-Bentonite Extrudates on Their Catalytic Behavior in n-Hexane Hydroisomerization. Ind. Eng. Chem. Res. 2019, 58, 10875–10885. [Google Scholar] [CrossRef]
- Park, K.C.; Ihm, S.K. Comparison of Pt/zeolite catalysts for n-hexadecane hydroisomerization. Appl. Catal. 2000, 203, 201–209. [Google Scholar] [CrossRef]
- Yu, F.; Yin, J.; Gang, S.; Liu, H.; Bao, X. Mechanistic pathways for olefin hydroisomerization and aromatization in fluid catalytic cracking gasoline hydro-upgrading. Energy Fuels 2009, 23, 3016–3023. [Google Scholar] [CrossRef]
- Claude, M.C.; Martens, J.A. Monomethyl-branching of long n-alkanes in the range from decane to tetra- cosane on Pt/H-ZSM-22 bifunctional catalyst. J. Catal. 2000, 190, 39–48. [Google Scholar] [CrossRef]
- Bedoya, J.C.; Valdez, R.; Cota, L.; Alvarez-Amparan, M.A.; Olivas, A. Performance of Al-MCM-41 nanospheres as catalysts for dimethyl ether production. Catal. Today 2022, 388, 55–62. [Google Scholar] [CrossRef]
- Fedyna, M.; Zak, A.; Jaroszewska, K.; Mokrzycki, J.; Trawczyński, J. Composite of Pt/AlSBA-15+zeolite catalyst for the hydroisomerization of n-hexadecane: The effect of platinum precursor. Microporous Mesoporous Mater. 2020, 305, 110366. [Google Scholar] [CrossRef]
- Jokar, F.; Alavi, S.M.; Rezaei, M. Investigating the hydroisomerization of n-pentane using Pt supported on ZSM-5, desilicated ZSM-5, and modified ZSM-5/MCM-41. Fuel 2022, 324, 124511. [Google Scholar] [CrossRef]
- Abdi-Khanghah, M.; Adelizadeh, M.; Naserzadeh, Z.; Zhang, Z. n-Decane hydro-conversion over bi- and tri-metallic Al-HMS catalyst in a mini-reactor. Chin. J. Chem. Eng. 2018, 26, 1330–1339. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Yang, Z. An excellent universal catalyst support- mesoporous silica: Preparation, modification and applications in energy-related reactions. Int. J. Hydrog. Energy 2022, 47, 9537–9565. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Pawelec, B.; Obeso-Estrella, R.; Díaz de León, J.N.; Fuentes, S.; Alonso-Núnez, G.; Fierro, J.L.G. Competitive HDS and HDN reactions over NiMoS/HMS-Al catalysts: Diminishing of the inhibition of HDS reaction by support modification with P. Appl. Catal. B Environ. 2016, 180, 569–579. [Google Scholar] [CrossRef]
- Pirshahid, M.R.B.; Alavi, S.M.; Rezaei, M.; Akbari, E.; Varbar, M.; Khosravi, K. Novel highly efficient Ni-based mesoporous alumina-silica supported catalysts for methane dry reforming: Influence of nickel loading. J. Energy Inst. 2023, 110, 101361. [Google Scholar] [CrossRef]
- Zheng, J.; Impeng, S.; Liu, J.; Deng, J.; Zhang, D. Mo promoting Ni-based catalysts confined by halloysite nanotubes for dry reforming of methane: Insight of coking and H2S poisoning resistance. Appl. Catal. 2023, 342, 123369. [Google Scholar] [CrossRef]
- Méndeza, F.J.; Franco-López, O.E.; Díaz, G.; Gómez-Cortés, A.; Bokhimi, X.; Klimova, T.E. On the role of niobium in nanostructured Mo/Nb-MCM-41 and NiMo/Nb-MCM-41 catalysts for hydrodesulfurization of dibenzothiophene. Fuel 2020, 280, 118550. [Google Scholar] [CrossRef]
- Batalha, N.; Pinard, L.; Bouchy, C.; Guillon, E.; Guisnet, M. n-Hexadecane Hydroisomerization over Pt-HBEA Catalysts. Quantification and Effect of the Intimacy between Metal and Protonic Sites. J. Catal. 2013, 307, 122–131. [Google Scholar] [CrossRef]
- Vassilina, G.; Abdildina (Umbetkaliyeva), K.; Oktar, N.; Karaman, B.T.; Vassilina, T. Characterization and catalytic activity of Ni/mesoporous aluminosilicate HMS and Mo/mesoporous aluminosilicate HMS in the conversion of n-hexadecane. Mater. Today Proc. 2020, 30, 580–583. [Google Scholar] [CrossRef]
- Vassilina, G.; Abdildina (Umbetkaliyeva), K.; Abdrassilova, A.; Vassilina, T.; Zakirov, Z. The mesoporous aluminosilicate application as support for bifunctional catalysts for n-hexadecane hydroconversion. Open Chem. 2022, 20, 225–236. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X.; Xue, W.; Ge, L.; Wang, T.; Qiu, M.; Wei, W.; Gao, P.; Sun, Y. Melting-assisted solvent-free synthesis of SAPO-11 for improving the hydroisomerization performance of n-dodecane. Chin. J. Catal. 2020, 41, 622–630. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Pawelec, B.; Loricera, C.V.; Rivera-Muñoz, E.M.; Nava, R.; Torres, B.; Fierro, J.L.G. Comparison of the morphology and HDS activity of ternary Ni(Co)-Mo-W catalysts supported on Al-HMS and Al-SBA-16 substrates. Appl. Catal. B 2012, 125, 473–485. [Google Scholar] [CrossRef]
- Garg, S.; Soni, K.; Prabhu, T.A.; Rama, K.S.; Murali, G. Effect of ordered mesoporous Zr SBA-15 support on catalytic functionalities of hydrotreating catalysts 2. Variation of molybdenum and promoter loadings. Catal. Today 2016, 261, 128–136. [Google Scholar] [CrossRef]
- Wang, M.; He, M.; Fang, Y.; Baeyens, J.; Tan, T. The Ni-Mo/γ-Al2O3 catalyzed hydrodeoxygenation of FAME to aviation fuel. Catal. Commun. 2017, 100, 237–241. [Google Scholar] [CrossRef]
- Vivenes, A.; Méndez, F.J.; Bastardo-González, E.; Brito, J.L. Catalizadores M-Mo/HMS (M = Fe, Co o Ni) en sus formas sulfuradas y nitruradas como potenciales sistemas catalíticos de hidrodesulfuración. Av. Quim. 2015, 10, 55–65. [Google Scholar]
- Wu, P.; Tatsumi, T.; Komatsu, T.; Yashima, T. A novel titanosilicate with MWW structure: II. Catalytic properties in the selective oxidation of alkenes. J. Catal. 2001, 202, 245–255. [Google Scholar] [CrossRef]
- Van, O.C.J.; Góra-Marek, K.; Sadowska, K.; Mertens, M.; Meynen, V.; Datka, J.; Cool, P. In situ IR spectroscopic study to reveal the impact of the synthesis conditions of zeolite b nanoparticles on the acidic properties of the resulting zeolite. J. Chem. Eng. 2014, 237, 372–379. [Google Scholar] [CrossRef]
- Ma, D.; Lu, Y.; Su, L.L.; Xu, Z.S.; Tian, Z.J.; Xu, Y.D.; Lin, L.W.; Bao, X.H. Remarkable improvement on the methane aromatization reaction: A highly selective and cokingresistant catalyst. J. Phys. Chem. B 2002, 106, 8524–8530. [Google Scholar] [CrossRef]
- Xiong, Z.T.; Zhang, H.B.; Lin, G.D.; Zeng, J.L. Study of W/HZSM-5-based catalysts for dehydroaromatization of CH4 in absence of O2. Action of promoters Zn and Li. Catal. Lett. 2001, 74, 233–239. [Google Scholar] [CrossRef]
- Perea, L.A.; Felischak, M.; Wolff, T.; Gaonad, J.A.L.; Hamel, C.; Morgenstern, S.A. Propene production at low temperature by bimetallic Ni-Mo and Ni-Re T catalysts on mesoporous MCM-41 prepared using template ion exchange. Fuel 2021, 284, 119031. [Google Scholar] [CrossRef]
- Heba, M. Characterization and catalytic activity of NiO/mesoporous aluminosilicate Al-SBA-15 in conversion of some hydrocarbons. Egypt. J. Pet. 2012, 21, 1–10. [Google Scholar] [CrossRef]
- Zhuang, J.; Yan, S.; Zhang, P.; Liu, X.; Zhao, Y.; Yu, Y.; Wang, Y.; Zhao, Q.; Wu, H.; Zhu, X.; et al. Regulating the states of Ni species by controlling the silanols of MCM-41 support to promote the hydrogenation of maleic anhydride. Fuel 2023, 335, 127030. [Google Scholar] [CrossRef]
- Bukhari, C.C.; Chong, H.D.; Setiabudi, N.; Ainirazali, M.A.; Aziz, A.A.; Jalil, S.Y. Optimal Ni loading towards efficient CH4 production from H2 and CO2 over Ni supported onto fibrous SBA-15. Int. J. Hydrog. Energy 2019, 44, 7228–7240. [Google Scholar] [CrossRef]
- Jusoh, N.W.; Jalil, A.A.; Triwahyono, S.; Setiabudi, H.D.; Sapawe, N.; Satar, M.A.; Karim, A.H.; Kamarudin, N.H.; Jusoh, R.; Jaafar, N.F.; et al. Sequential desilication-isomorphous substitution route to prepare mesostructured silica nanoparticles loaded with ZnO and their photocatalytic activity. Appl. Catal. A Gen. 2013, 468, 276–287. [Google Scholar] [CrossRef]
- Sangsong, S.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Phongaksorn, M.; Eric, C.E. The Demonstration of the Superiority of the Dual Ni-Based Catalytic System for the Adjustment of the H2/CO Ratio in Syngas for Green Fuel Technologies. Catalyst 2020, 10, 1056. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo, M.S.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Yang, E.; Noh, Y.S.; Hong, G.H.; Moon, D.J. Combined steam and CO2 reforming of methane over La1−xSrxNiO3 perovskite oxides. Catal. Today 2018, 299, 242–250. [Google Scholar] [CrossRef]
- Patcas, F.; Honicke, D. Effect of alkali doping on catalytic properties of alumina-supported nickel oxide in the selective oxidehydrogenation of cyclohexane. Catal. Commun. 2005, 6, 23–27. [Google Scholar] [CrossRef]
- Miletić, N.; Izquierdo, U.; Obregón, I.; Bizkarra, K.; Agirrezabal-Telleria, I.; Bario, L.V.; Arias, P.L. Oxidative steam reforming of methane over nickel catalysts supported on Al2O3–CeO2–La2O3. Catal. Sci. Technol. 2015, 5, 1704–1715. [Google Scholar] [CrossRef]
- Stroud, T.; Smith, T.J.; Saché, E.; Santos, J.L.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Chemical CO2 recycling via dry and bi reforming of methane using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 catalysts. Appl. Catal. B 2018, 224, 125–135. [Google Scholar] [CrossRef]
- Li, P.P.; Lang, W.Z.; Xia, K.; Yan, X.; Guo, Y.J. The promotion effects of Ni on the properties of Cr/Al catalysts for propane dehydrogenation reaction. Appl. Catal. A 2016, 522, 172–179. [Google Scholar] [CrossRef]
- Saché, E.; Johnson, S.; Pastor-Pérez, L.; Horri, B.A.; Reina, T.R. Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source. Energies 2019, 12, 1007. [Google Scholar] [CrossRef]
- Muthu, G.; Garg, S.; Soni, K.; Kumar, M.; Sharma, L.D.; Rama, K.S.; Murali, G. Effect of Al-SBA-15 Support on Catalytic Functionalities of Hydrotreating Catalysts. II. Effect of Variation of Molybdenum and Promoter Contents on Catalytic Functionalities. Ind. Eng. Chem. Res. 2007, 46, 4747–4754. [Google Scholar]
- Behnejad, B.; Abdouss, M.; Tavasoli, A. Comparison of performance of Ni–Mo/γ-alumina catalyst in HDS and HDN reactions of main distillate fractions. Pet. Sci. 2019, 16, 645–656. [Google Scholar] [CrossRef]
- Qu, L. MAS NMR, TPR, and TEM studies of the interaction of NiMo with alumina and silica–alumina supports. J. Catal. 2003, 215, 7–13. [Google Scholar] [CrossRef]
- Ressler, T.; Wienold, J.; Jentoft, R.E. Formation of bronzes during temperature-programmed reduction of MoO3 with hydrogen—An in situ XRD and XAFS study. Solid State Ion. 2001, 141, 243–251. [Google Scholar] [CrossRef]
- Maity, S.K.; Rana, M.S.; Srinivas, B.N.; Bej, S.K.; Murali, G.; Prasada, S.R. Characterization and evaluation of ZrO2 supported hydrotreating catalysts. J. Mol. Catal. A Chem. 2000, 153, 121–127. [Google Scholar] [CrossRef]
- Alibouri, M.; Seyyed, M.; Ghoreishi, K.; Hamid, R.; Aghabozorg, H.R. Hydrodesulfurization Activity of NiMo/Al-HMS Nanocatalst Synthesized by Supercritical Impregnation. Ind. Eng. Chem. Res. 2009, 48, 4283–4292. [Google Scholar] [CrossRef]
- Zulkepli, S.; Lee, H.V.; Rahman, N.A.; Chuan, L.T.; Show, P.L.; Wei-Hsin Chen, W.H.; Juan, J.C. Highly active iron-promoted hexagonal mesoporous silica (HMS) for deoxygenation of triglycerides to green hydrocarbon-like biofuel. Fuel 2022, 308, 121860. [Google Scholar] [CrossRef]
- Peters, C.A. Statistics for Analysis of Experimental Data. Available online: https://gradebuddy.com/doc/2600243/statistics-for-analysis-of-experimental (accessed on 5 August 2020).


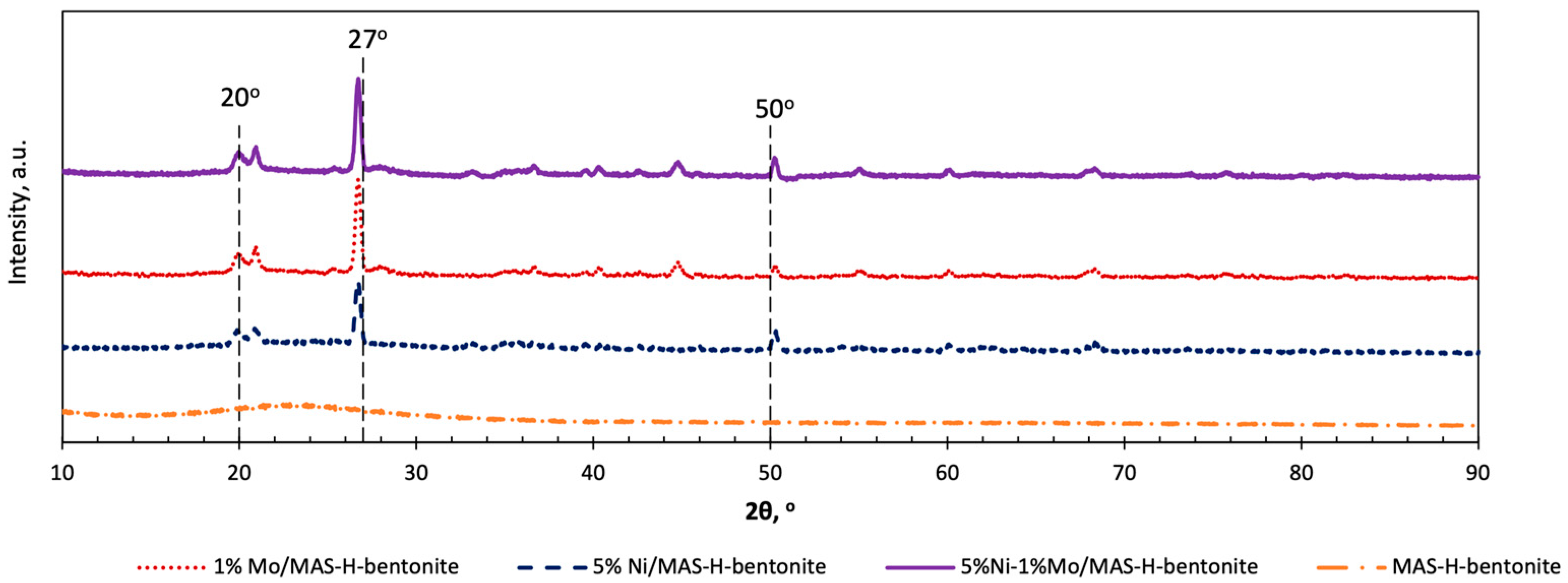
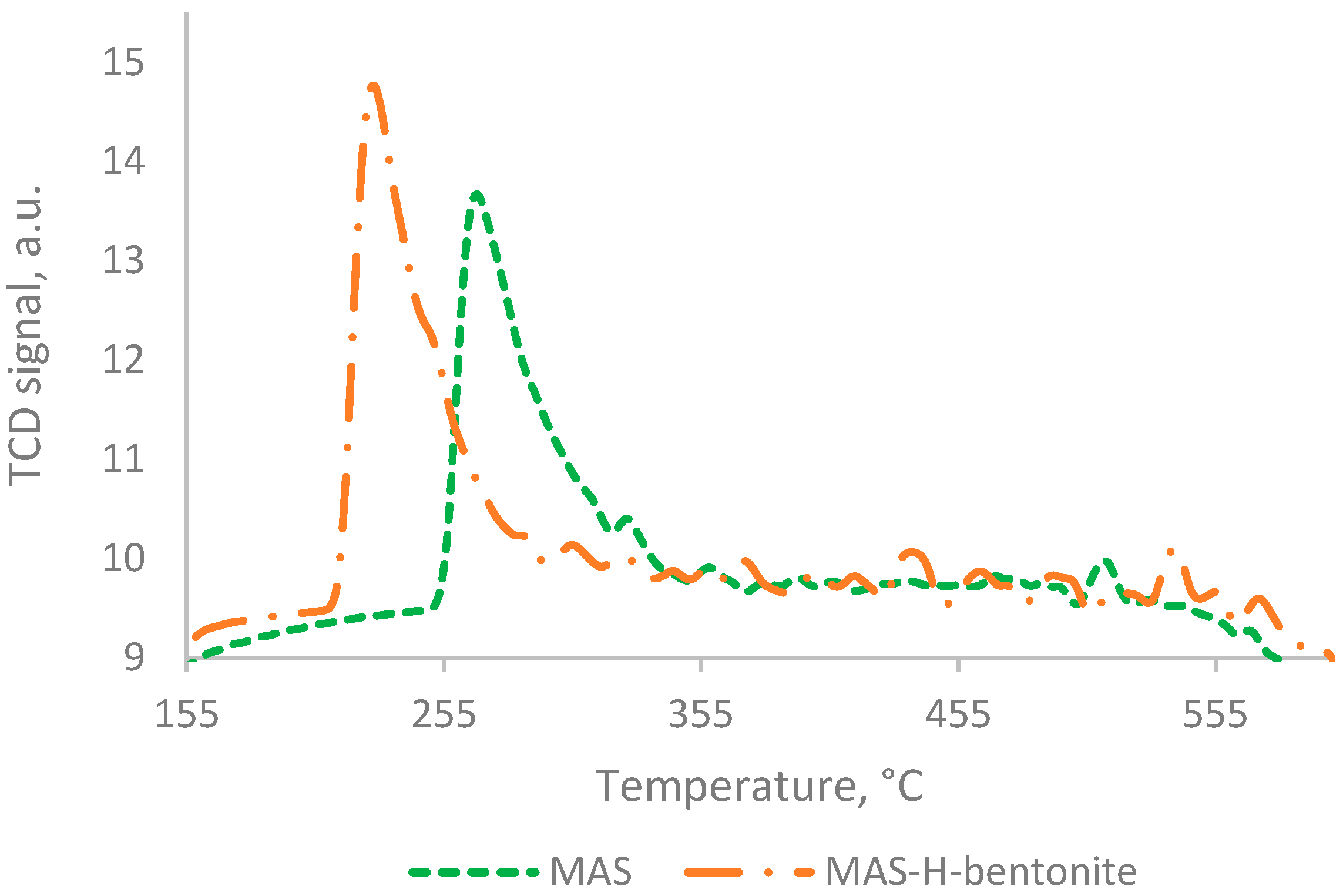
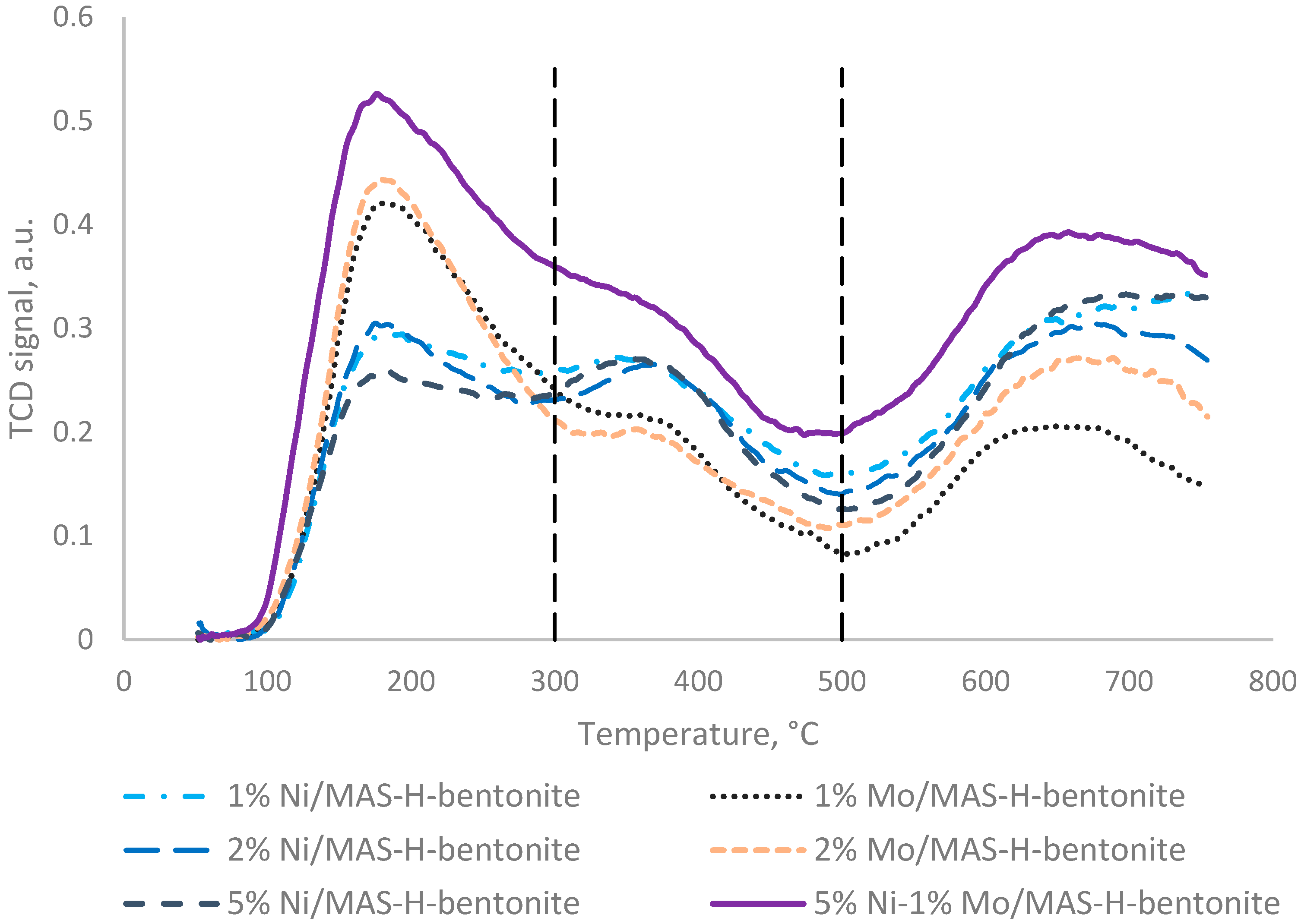
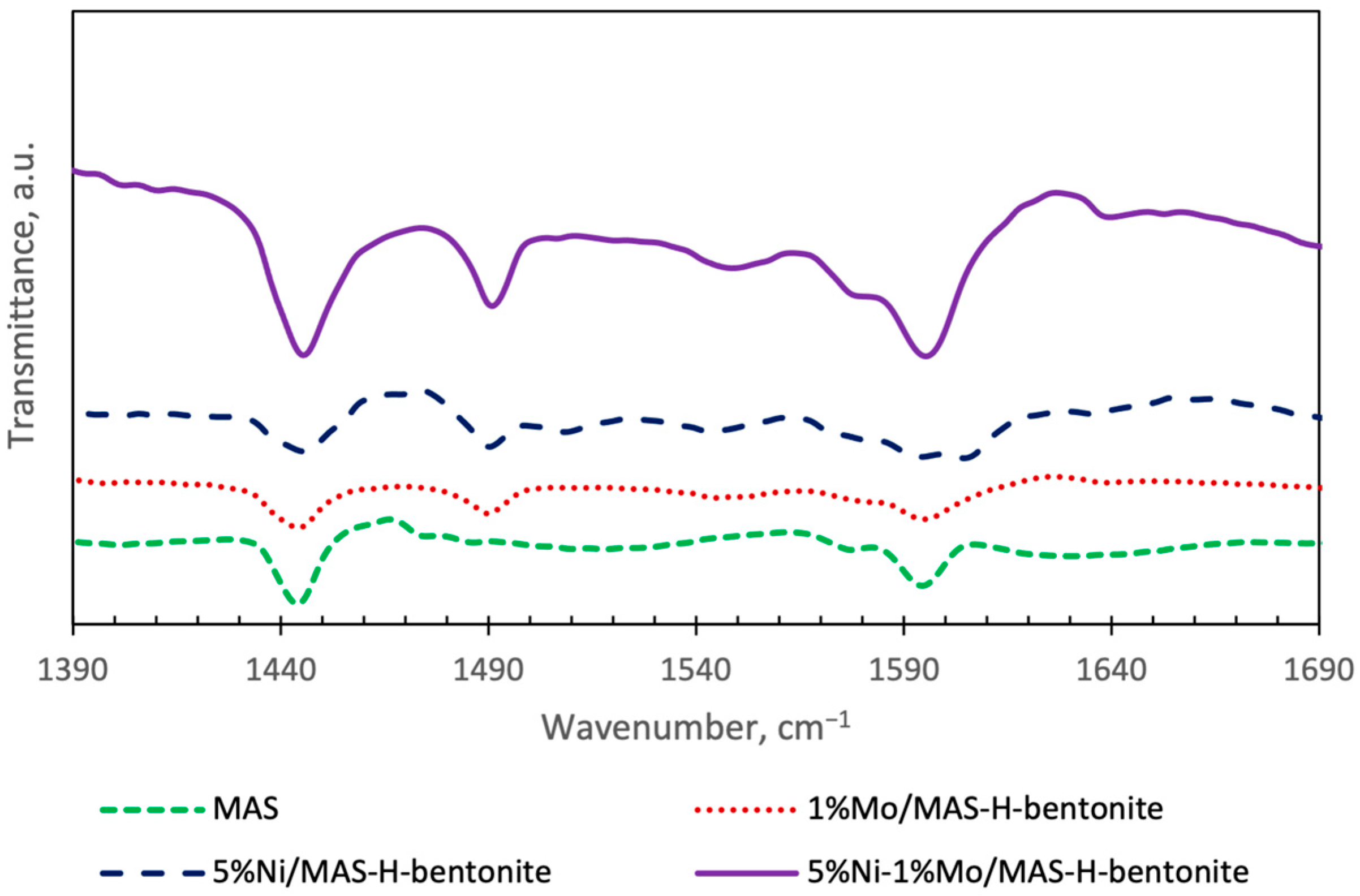

| Catalyst | SSA, m2/g | Pore Volume, cm3/g | Pore Average Size, nm |
|---|---|---|---|
| MAS-H-bentonite | 375 | 0.3523 | 3.5 |
| 1% Mo/MAS-H-bentonite | 284 | 0.2166 | 2.17 |
| 5% Ni/MAS-H-bentonite | 152 | 0.3565 | 1.67 |
| 5% Ni-1% Mo/MAS-H-bentonite | 265 | 0.2567 | 3.8 |
| Sample | Amount of the Acid Sites (Amount of Adsorbed NH3, µmol/g) | |||
|---|---|---|---|---|
| Weak Tmax <300 °C | Medium Tmax = 300–500 °C | Strong Tmax >500 °C | Total | |
| MAS | 1144 | 27 | - | 1171 |
| MAS-H-bentonite | 1193 | - | - | 1193 |
| Sample | Amount of the Acid Sites (Amount of Adsorbed NH3, µmol/g) | |||
|---|---|---|---|---|
| Weak Tmax < 300 °C | Medium Tmax = 300–500 °C | Strong Tmax > 500 °C | Total | |
| 1% Mo/MAS-H-bentonite | 419 | 81 | 120 | 620 |
| 2% Mo/MAS-H-bentonite | 440 | 116 | 128 | 684 |
| 1% Ni/MAS-H-bentonite | 255 | 162 | 29 | 446 |
| 2% Ni/MAS-H-bentonite | 252 | 174 | 6 | 432 |
| 5% Ni/MAS-H-bentonite | 231 | 196 | 94 | 521 |
| 5% Ni-1% Mo/MAS-H-bentonite | 491 | 122 | 130 | 743 |
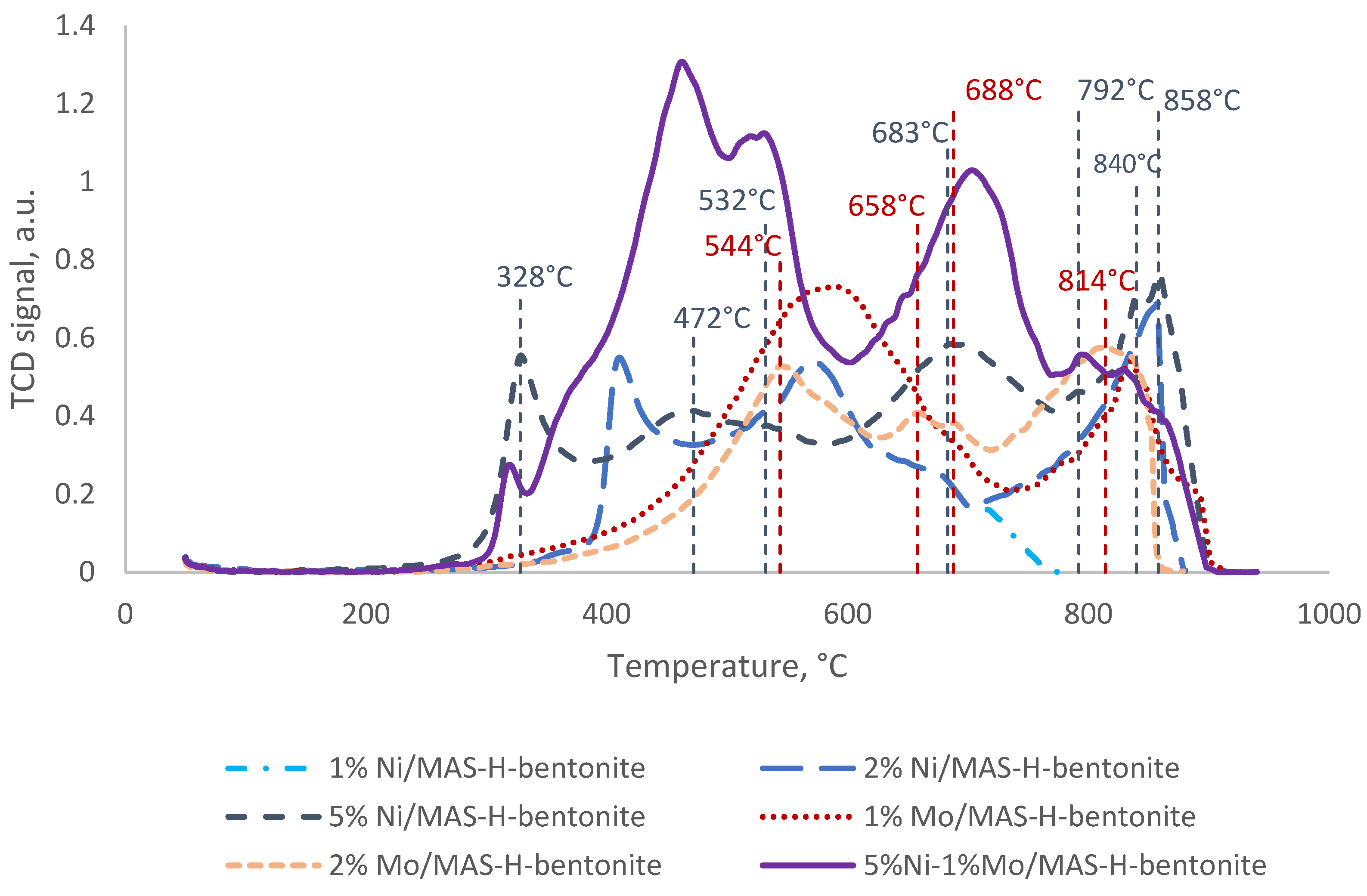
| Sample | Temperature, °C | Amounts of H2 Consumed, µmol/g |
|---|---|---|
| 1% Mo/MAS-H-bentonite | 544 | 170 |
| 658 | 15 | |
| 688 | 14 | |
| 814 | 1 | |
| 2% Mo/MAS-H-bentonite | 589 | 651 |
| 834 | 142 | |
| 1% Ni/MAS-H-bentonite | 328 | 127 |
| 472 | 144 | |
| 532 | 37 | |
| 683 | 126 | |
| 792 | 2 | |
| 840 | 33 | |
| 858 | 57 | |
| 2% Ni/MAS-H-bentonite | 415 | 127 |
| 603 | 930 | |
| 710 | 81 | |
| 763 | 13 | |
| 866 | 2 | |
| 5% Ni/MAS-H-bentonite | 411 | 124 |
| 571 | 164 | |
| 657 | 4 | |
| 5% Ni-1% Mo/MAS- H-bentonite | 317 | 29 |
| 460 | 670 | |
| 529 | 406 | |
| 702 | 472 | |
| 792 | 41 | |
| 827 | 15 |
| Indicators | Values |
|---|---|
| Sulfur content, ppm | 251 |
| Flashpoint in a closed cup, °C | 66 |
| CFPP, °C | −10 |
| Pour point, °C | −12 |
| Flow point, °C | −5 |
| Fractional composition: | |
| Boiling point, °C | 197 |
| Fraction yields up to a temperature of 350 °C, % vol. | 97 |
| Compounds | Content, wt.% |
|---|---|
| N-paraffins | 18.15 |
| Iso-paraffins | 16.08 |
| Naphthenes | 32.78 |
| Aromatic hydrocarbons, % | 26.31 |
| From them: | |
| monocyclic | 24.3 |
| polycyclic | 2.01 |
| Olefins | 1.72 |
| Heteroatomic compounds | 4.96 |
| Total | 100.0 |
| Catalyst | T, °C | CFPP, °C | Flash Point in a Closed Cup, °C | Pour Point, °C | Diesel Fraction Yield, % |
|---|---|---|---|---|---|
| 1% Mo/MAS-H-bentonite | 260 | −14 | 52 | −26 | 93.6 |
| 280 | −19 | 50 | −31 | 90.6 | |
| 300 | −27 | 45 | −36 | 87.5 | |
| 320 | −38 | 36 | −38 | 85.2 | |
| 340 | −42 | 20 | −41 | 72.4 | |
| 2% Mo/MAS-H-bentonite | 260 | −16 | 51 | −27 | 92.8 |
| 280 | −18 | 47 | −28 | 89.2 | |
| 300 | −27 | 43 | −31 | 85.7 | |
| 320 | −33 | 35 | −36 | 84.0 | |
| 340 | −36 | 31 | −40 | 73.2 | |
| 1% Ni/MAS-H-bentonite | 260 | −12 | 53 | −19 | 97.0 |
| 280 | −16 | 50 | −21 | 96.7 | |
| 300 | −23 | 47 | −23 | 94.8 | |
| 320 | −27 | 41 | −26 | 92.3 | |
| 340 | −30 | 37 | −29 | 90.4 | |
| 2% Ni/MAS-H-bentonite | 260 | −12 | 55 | −18 | 97.0 |
| 280 | −15 | 52 | −19 | 95.9 | |
| 300 | −21 | 48 | −22 | 94.3 | |
| 320 | −25 | 43 | −24 | 93.1 | |
| 340 | −28 | 38 | −27 | 90.0 | |
| 5% Ni/MAS-H-bentonite | 260 | −15 | 53 | −20 | 97.0 |
| 280 | −17 | 50 | −21 | 96.5 | |
| 300 | −26 | 46 | −25 | 96.3 | |
| 320 | −31 | 41 | −30 | 95.4 | |
| 340 | −35 | 33 | −33 | 93.3 | |
| 5% Ni-1% Mo/ MAS- H-bentonite | 260 | −12 | 52 | −19 | 97.2 |
| 280 | −16 | 50 | −22 | 97.5 | |
| 300 | −22 | 45 | −27 | 96.3 | |
| 320 | −33 | 39 | −36 | 97.4 | |
| 340 | −36 | 27 | −38 | 96.9 |
| Indicators | Raw Material | Reaction Products Obtained on Catalysts at 320 °C, 2 MPa, 1 h−1 | ||
|---|---|---|---|---|
| 1% Mo/MAS-H-Bentonite | 5% Ni/MAS- H-Bentonite | 5% Ni-1% Mo/ MAS- H-Bentonite | ||
| The content of n-paraffins C10-C27, wt.% | 18.15 | 9.6 | 12.6 | 10.6 |
| The content of iso-paraffins, wt.% | 16.08 | 24.3 | 22.4 | 25.3 |
| Naphthenes | 32.78 | 33.01 | 35.16 | 33.04 |
| Aromatic | 26.31 | 25.85 | 23.81 | 25.16 |
| hydrocarbons, % | ||||
| From them: | ||||
| monocyclic | 24.3 | 24.7 | 23.64 | 24.26 |
| polycyclic | 2.01 | 1.15 | 0.17 | 0.90 |
| Hydrocracking products, wt.% | - | 8.5 | 0.7 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdildina, K.; Vassilina, G.; Abdrassilova, A.; Klassen, I.A.; Orynbassar, R.; Kanapiyeva, F. The Role of Catalyst Promotive Additives and Temperature in the Hydroisodewaxing Process. Molecules 2023, 28, 7598. https://doi.org/10.3390/molecules28227598
Abdildina K, Vassilina G, Abdrassilova A, Klassen IA, Orynbassar R, Kanapiyeva F. The Role of Catalyst Promotive Additives and Temperature in the Hydroisodewaxing Process. Molecules. 2023; 28(22):7598. https://doi.org/10.3390/molecules28227598
Chicago/Turabian StyleAbdildina, Kamilla, Gulzira Vassilina, Albina Abdrassilova, Ivan A. Klassen, Raigul Orynbassar, and Fatima Kanapiyeva. 2023. "The Role of Catalyst Promotive Additives and Temperature in the Hydroisodewaxing Process" Molecules 28, no. 22: 7598. https://doi.org/10.3390/molecules28227598
APA StyleAbdildina, K., Vassilina, G., Abdrassilova, A., Klassen, I. A., Orynbassar, R., & Kanapiyeva, F. (2023). The Role of Catalyst Promotive Additives and Temperature in the Hydroisodewaxing Process. Molecules, 28(22), 7598. https://doi.org/10.3390/molecules28227598







