Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method
Abstract
:1. Introduction
2. Results and Discussion
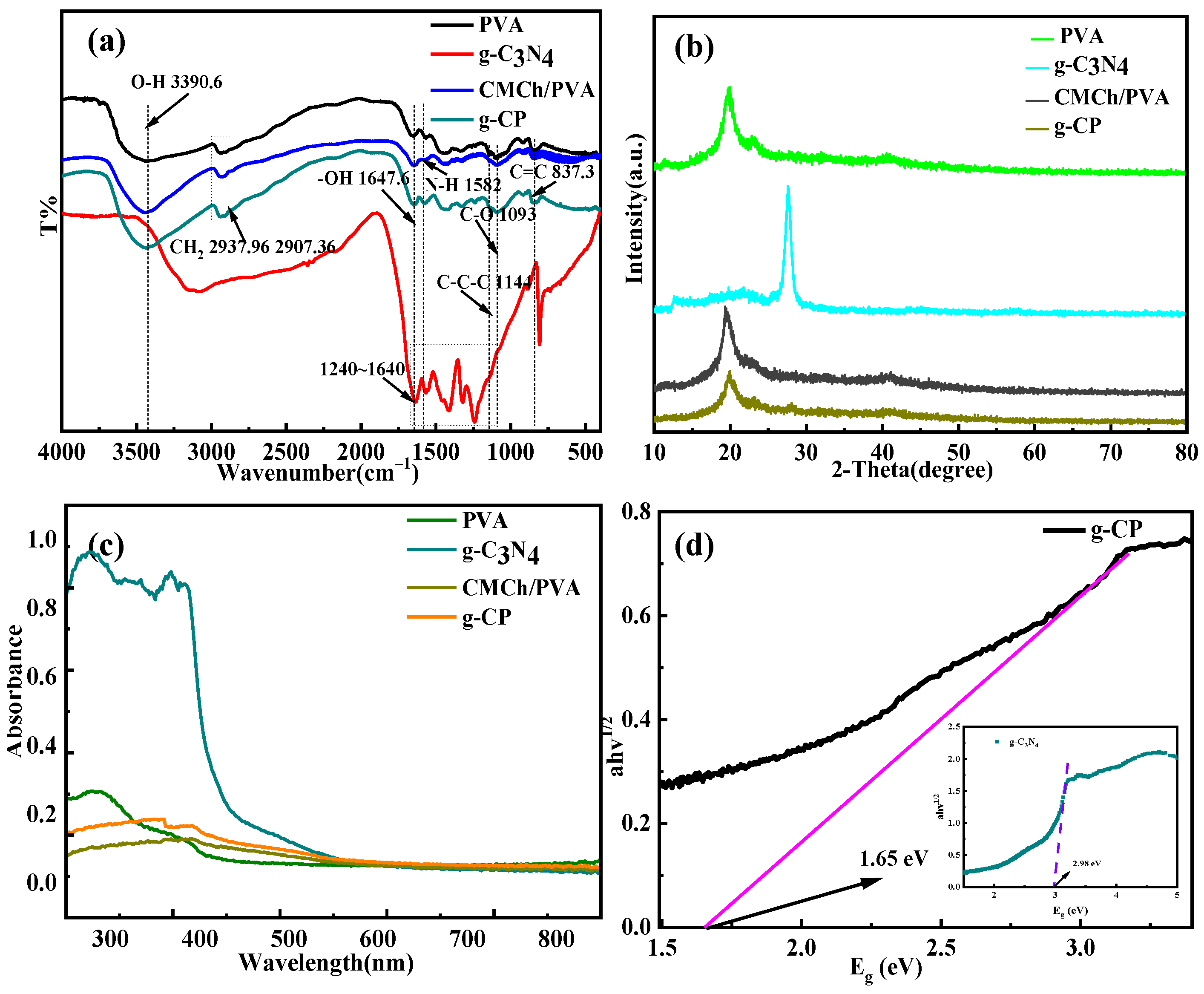
2.1. FTIR Analysis
2.2. XRD Analysis
2.3. UV-Vis Analysis
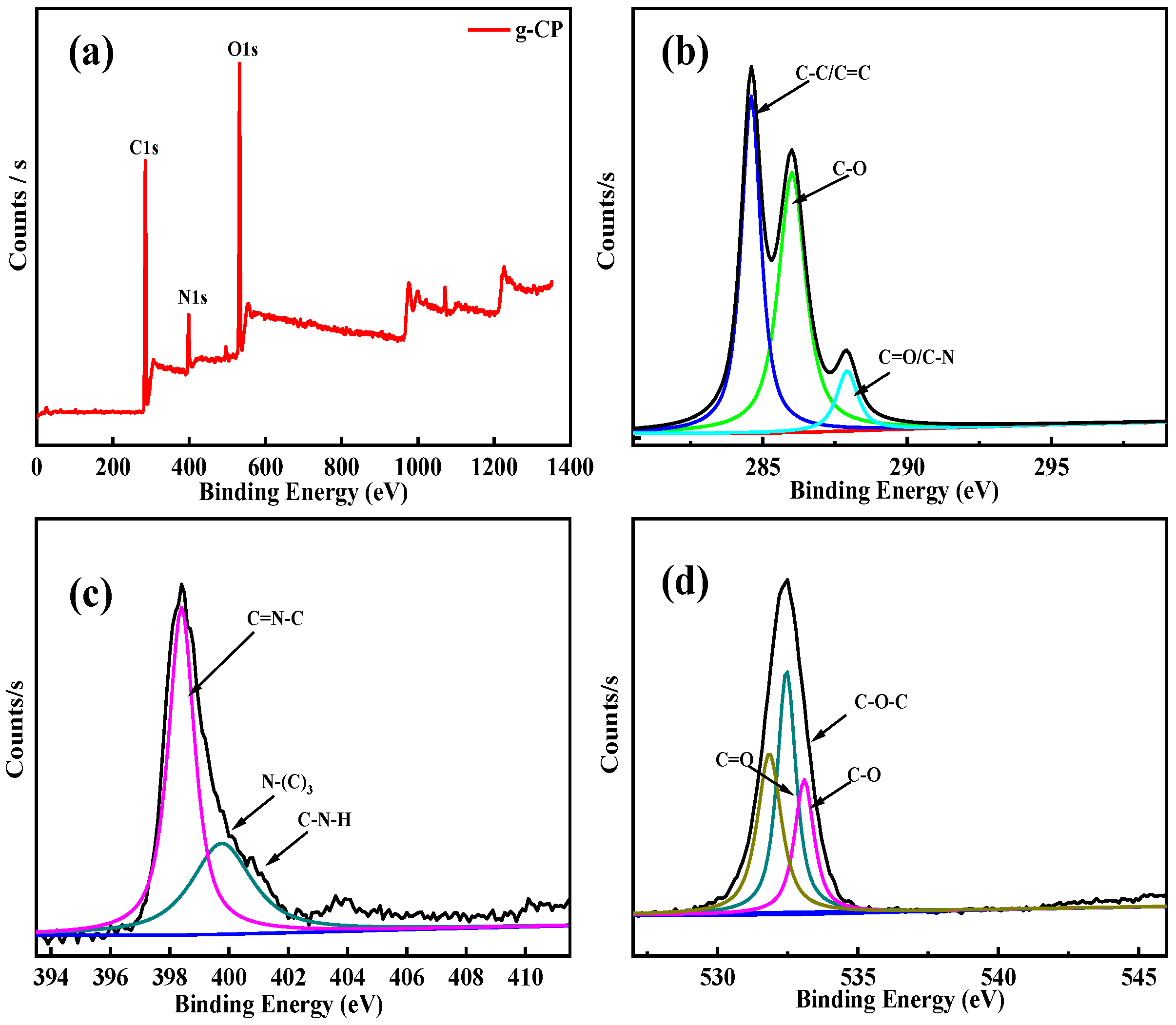
2.4. XPS Analysis
2.5. SEM Analysis
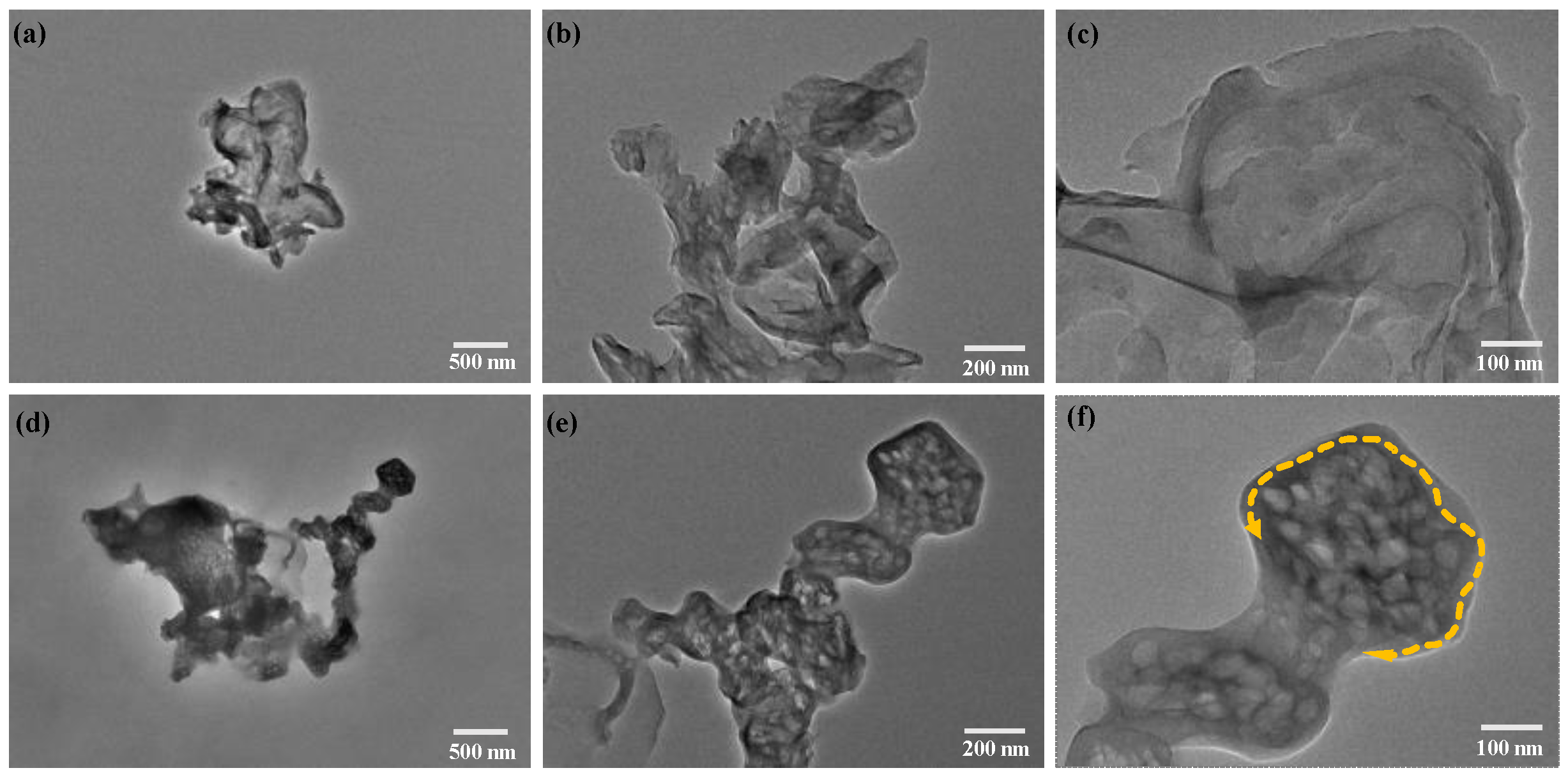
2.6. TEM Analysis
2.7. BET Analysis
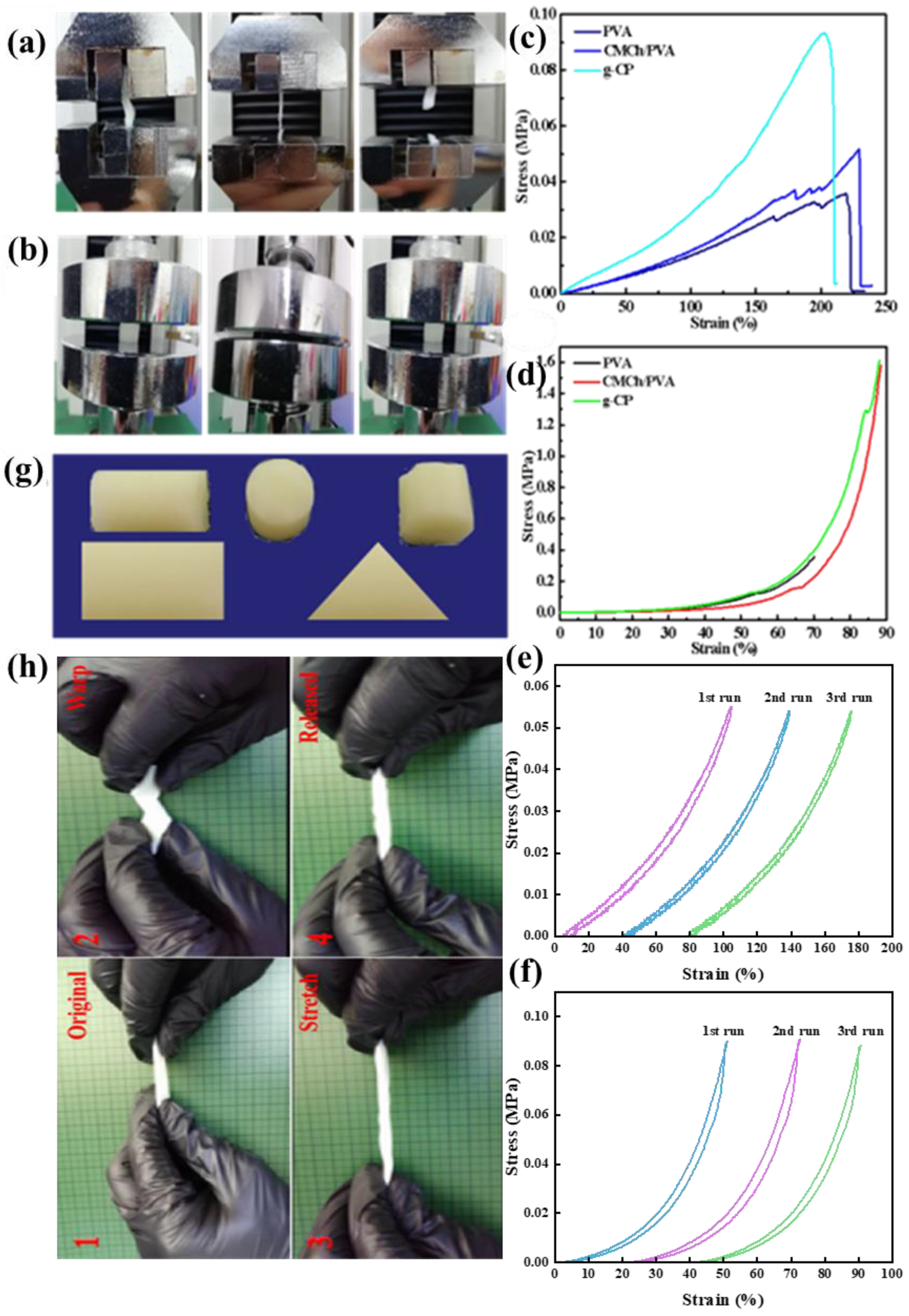
2.8. Mechanical Performance Analysis
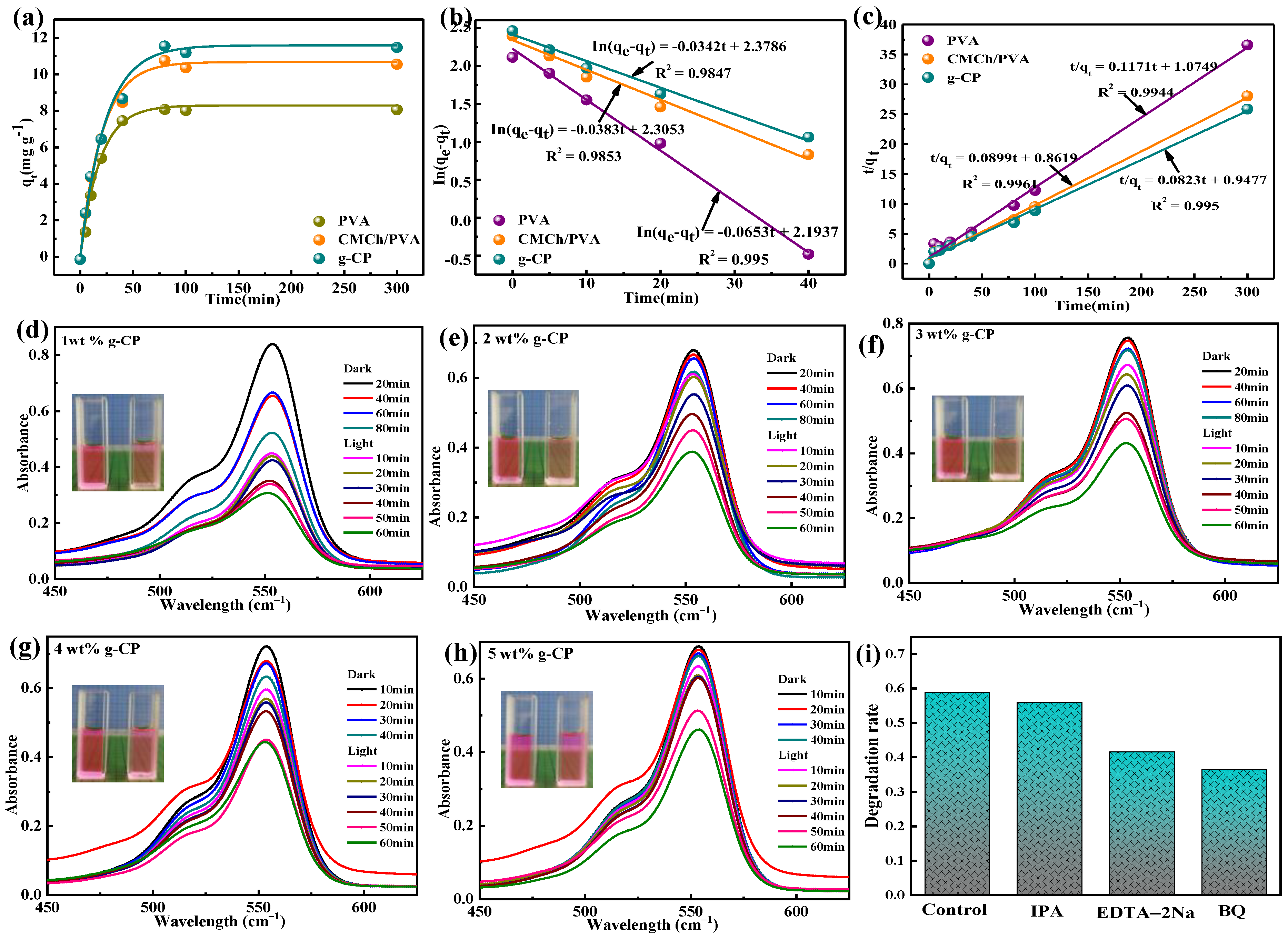
2.9. Adsorption–Photocatalytic Degradation of RhB
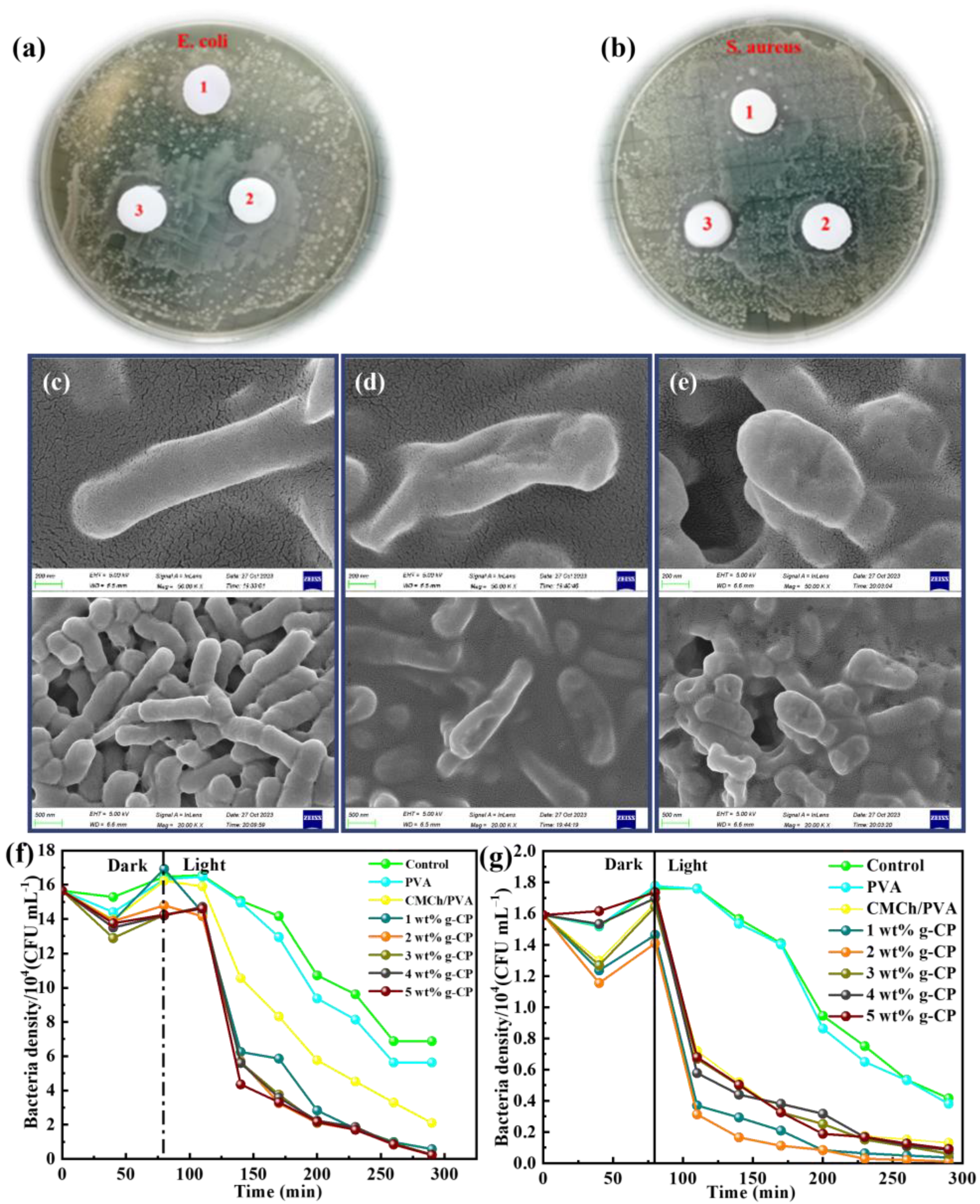
2.10. Zone of Inhibition and Photocatalytic Antibacterial Experiments
2.11. Cytotoxicity Analysis
2.12. Photocatalytic Degradation and Antibacterial Mechanism of g-CP
3. Experimental Section
3.1. Materials
3.2. Preparation of Samples
3.2.1. Preparation of g-C3N4
3.2.2. Radiation Construction of g-CP
3.3. Material Characterization
3.4. Photocatalytic Test
3.4.1. Photocatalytic Degradation of Dye
3.4.2. Zone of Inhibition Test
3.4.3. Photocatalytic Antibacterial Experiment
3.4.4. Cell Counting Kit-8 (CCK-8) Test
3.4.5. Live and Dead Cell Staining Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sudhaik, A.; Raizada, P.; Ahamad, T.; Alshehri, S.M.; Nguyen, V.; Le, Q.V.; Thakur, S.; Thakur, V.K.; Selvasembian, R.; Singh, P. Recent advances in cellulose supported photocatalysis for pollutant mitigation: A review. Int. J. Biol. Macromol. 2022, 226, 1284–1308. [Google Scholar] [CrossRef]
- Li, Y.F.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on g-C3N4-based S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, T.; Zhao, X.; Wang, K.; Zhao, L.; Tao, A.; Jiang, J.; Yuan, M.; Wang, J.; Tu, Q. Double network hydrogel based on curdlan and flaxseed gum with photothermal antibacterial properties for accelerating infectious wound healing. Int. J. Biol. Macromol. 2023, 242, 124715. [Google Scholar] [CrossRef] [PubMed]
- Budgell, E.; Davies, T.J.; Donker, T.; Hopkins, S.; Wyllie, D.H.; Peto, T.E.A.; Gill, M.J.; Llewelyn, M.J.; Walker, A.S. Impact of antibiotic use on patient-level risk of death in 36 million hospital admissions in England. J. Infect. 2021, 84, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.F.; Lei, H.; Fan, D. Antibacterial hydrogel with pH-responsive microcarriers of slow-release VEGF for bacterial infected wounds repair. J. Mater. Sci. Technol. 2023, 144, 198–212. [Google Scholar] [CrossRef]
- Gu, X.Y.; Hu, L.M.; Fu, Z.A.; Wang, H.T.; Li, Y. Reactive TiO2 nanoparticles compatibilized PLLA/PBSU blends: Fully biodegradable polymer composites with improved physical, antibacterial and degradable properties. Chin. J. Polym. Sci. 2021, 39, 1645–1656. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Tan, Y.; Xu, B.; Cai, J.; Zhang, Y.; Wang, Q.; Wu, Q.; Yang, B.; Huang, J. Recent Advances in Metal-Organic Framework (MOF)-Based Photocatalysts: Design Strategies and Applications in Heavy Metal Control. Molecules 2023, 28, 6681. [Google Scholar] [CrossRef]
- Rao, X.; Du, L.; Zhao, J.J.; Tan, X.D.; Fang, Y.X.; Xu, L.Q.; Zhang, Y.P. Hybrid TiO2/AgNPs/g-C3N4 nanocomposite coatings on TC4 titanium alloy for enhanced synergistic antibacterial effect under full spectrum light. J. Mater. Sci. Technol. 2022, 118, 35–43. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, S.; Yu, X.; Dong, C.; Ji, J.; Zhang, D.; Xing, M. Research progress of photocatalytic sterilization over semiconductors. RSC Adv. 2019, 9, 19278–19284. [Google Scholar] [CrossRef]
- Hu, X.L.; Li, H.G.; Tan, B.E. COFs-based Porous Materials for Photocatalytic Applications. Chin. J. Polym. Sci. 2020, 38, 673–684. [Google Scholar] [CrossRef]
- Wang, H.; Yin, R.; Chen, X.; Wu, T.; Bu, Y.; Yan, H.; Lin, Q. Construction and Evaluation of Alginate Dialdehyde Grafted RGD Derivatives/Polyvinyl Alcohol/Cellulose Nanocrystals IPN Composite Hydrogels. Molecules 2023, 28, 6692. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, A.; Kumari, R.; Sinha, K.K.; Singh, M.K.; Das, P. Carbon dots assisted formation of DNA hydrogel for sustained release of drug. Carbon 2017, 114, 169–176. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, W.; Li, X.; Ling, G.; Zhang, P. Tunicate-mimetic antibacterial hydrogel based on metal ion crosslinking and chitosan functionalization for wound healing. Int. J. Biol. Macromol. 2023, 244, 125062. [Google Scholar] [CrossRef]
- Rosa, E.; Gallo, E.; Sibillano, T.; Giannini, C.; Rizzuti, S.; Gianolio, E.; Scognamiglio, P.L.; Morelli, G.; Accardo, A.; Diaferia, C. Incorporation of PEG Diacrylates (PEGDA) Generates Hybrid Fmoc-FF Hydrogel Matrices. Gels 2022, 8, 831. [Google Scholar] [CrossRef] [PubMed]
- Echalier, C.; Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun. 2019, 20, 100536. [Google Scholar] [CrossRef]
- Glass, S.; Kühnert, M.; Abel, B.; Schulze, A. Controlled Electron-Beam Synthesis of Transparent Hydrogels for Drug Delivery Applications. Polymers 2019, 11, 501. [Google Scholar] [CrossRef]
- Niu, Y.; Shen, J.; Guo, W.; Zhu, X.; Guo, L.; Wang, Y.; Li, F. The Synergistic Effect in CdS/g-C3N4 Nanoheterojunctions Improves Visible Light Photocatalytic Performance for Hydrogen Evolution Reactions. Molecules 2023, 28, 6412. [Google Scholar] [CrossRef]
- Chen, X.; Weng, M.; Lan, M.; Weng, Z.; Wang, J.; Guo, L.; Lin, Z.; Qiu, B. Superior antibacterial activity of sulfur-doped g-C3N4 nanosheets dispersed by Tetrastigma hemsleyanum Diels & Gilg’s polysaccharides-3 solution. Int. J. Biol. Macromol. 2021, 168, 453–463. [Google Scholar] [CrossRef]
- Altan, O.; Altintas, E.; Alemdar, S.; Metin, Ö. The rational design of a graphitic carbon nitride-based dual S-scheme heterojunction with energy storage ability as a day/night photocatalyst for formic acid dehydrogenation. Chem. Eng. J. 2022, 441, 136047. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Xu, T.; Zhang, X. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Song, L.; Qiu, Y.; Zhang, X.; Liu, F.; Li, A. Green strategy with high iron utilization for Cr(VI) removal via sodium polyacrylate-based hydrogel. Chem. Eng. J. 2022, 442, 136162. [Google Scholar] [CrossRef]
- Miao, R.; Liu, H.; Lei, Q.; Zhong, L.; Zhang, L.; He, J.; Ma, Z.; Yao, Y. Single-organic component g-C3.6N4 achieves superior photoactivity antibacterial. Chem. Eng. J. 2022, 440, 135873. [Google Scholar] [CrossRef]
- Hao, D.; Huang, Q.; Wei, W.; Bai, X.; Ni, B.J. A reusable, separation-free and biodegradable calcium alginate/g-C3N4 microsphere for sustainable photocatalytic wastewater treatment. J. Clean. Prod. 2021, 314, 128033. [Google Scholar] [CrossRef]
- Thurston, J.H.; Clifford, A.J.; Hendersonm, B.S.; Smith, T.R.; Quintana, D.; Cudworth, K.F.; Lujan, T.J.; Cornell, K.A. Development of Photoactive g-C3N4/Poly(vinyl alcohol) Composite Hydrogel Films with Antimicrobial and Antibiofilm Activity. ACS Appl. Bio. Mater. 2020, 3, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Li, C.; Xie, X.; Li, G.; Hu, Z.; Li, S. Research Progress of Chitosan-Based Biomimetic Materials. Mar. Drugs 2021, 19, 372. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Wang, H.; Lu, Y.; Zhong, C.; Chu, L. Preparation, characterization and antibacterial applications of carboxymethyl chitosan/CuO nanocomposite hydrogels. Int. J. Biol. Macromol. 2017, 101, 690. [Google Scholar] [CrossRef]
- Kang, W.; Liang, J.; Liu, T.; Long, H.; Huang, L.; Shi, Q.; Zhang, J.; Deng, S.; Tan, S. Preparation of silane-dispersed graphene crosslinked vinyl carboxymethyl chitosan temperature-responsive hydrogel with antibacterial properties. Int. J. Biol. Macromol. 2022, 200, 99–109. [Google Scholar] [CrossRef]
- Ahad, N.; Saion, E.; Gharibshahi, E. Structural, Thermal, and Electrical Properties of PVA-Sodium Salicylate Solid Composite Polymer Electrolyte. J. Nanomater. 2012, 2012, 857569. [Google Scholar] [CrossRef]
- Ladeira, N.M.B.L.; Donnici, C.L.; Mesquita, J.P.; Pereira, F.V. Preparation and characterization of hydrogels obtained from chitosan and carboxymethyl chitosan. J. Polym. Res. 2021, 28, 335. [Google Scholar] [CrossRef]
- Aliakbari, A.M.; Ghamsari, S.; Mozdianfard, M.R. β-Carbon nitride nanoflake with enhanced visible light emission. Opt. Mater. 2020, 107, 110036. [Google Scholar] [CrossRef]
- Nanda, P.; De, S.K.; Manna, S.; De, U.; Tarafdar, S. Effect of gamma irradiation on a polymer electrolyte: Variation in crystallinity, viscosity and ion-conductivity with dose. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 73–78. [Google Scholar] [CrossRef]
- Xing, C.; Yu, G.; Chen, T.; Liu, S.; Sun, Q.; Liu, Q.; Hu, Y.; Liu, H.; Li, X. Perylenetetracarboxylic diimide covalently bonded with mesoporous g-C3N4 to construct direct Z-scheme heterojunctions for efficient photocatalytic oxidative coupling of amines. Appl. Catal. B Environ. 2021, 298, 120534. [Google Scholar] [CrossRef]
- Belmonte, G.K.; Charles, G.; Strumia, M.C.; Weibel, D.E. Permanent Hydrophilic Modification of Polypropylene and Poly(vinyl alcohol) films by Vacuum Ultraviolet Radiation. Appl. Surf. Sci. 2016, 382, 93–100. [Google Scholar] [CrossRef]
- Li, X.B.; Luo, Q.; Han, L.; Deng, F.; Yang, Y.; Dong, F. Enhanced photocatalytic degradation and H2 evolution performance of Nsingle bondCDs/S-C3N4 S-scheme heterojunction constructed by π-π conjugate self-assembly. J. Mater. Sci. Technol. 2022, 114, 222–232. [Google Scholar] [CrossRef]
- Mei, J.; Mo, S.; Zhang, H.; Zheng, X.; Li, Z. Removal of Sr(II) from water with highly-elastic carboxymethyl chitosan gel. Int. J. Biol. Macromol. 2020, 163, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Chang, N.; Sun, J.; Xiang, S.; Ayaz, T.; Zhang, H.; Wang, H. Modification of coal fly ash and its use as low-cost adsorbent for the removal of directive, acid and reactive dyes. J. Hazard. Mater. 2021, 422, 126778. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Qin, L.; Kang, W.; Ma, J.; Yang, Y.; Liu, X. Magnetic carbon nanospheres: Synthesis, characterization, and adsorbability towards quinoline from coking wastewater. Chem. Eng. J. 2020, 382, 122995. [Google Scholar] [CrossRef]
- Cui, Y.; Kang, W.; Qin, L.; Ma, J.; Liu, X.; Yang, Y. Ultrafast synthesis of magnetic hollow carbon nanospheres for the adsorpti on of quinoline from coking wastewater. New J. Chem. 2020, 44, 7490–7500. [Google Scholar] [CrossRef]
- Ming, K.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, J.L. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surf. B Biointerfaces 2008, 65, 197–202. [Google Scholar] [CrossRef]
- Huang, S.; Yu, Z.; Zhang, Y.; Qi, C.; Zhang, S. In situ green synthesis of antimicrobial carboxymethyl chitosan-nanosilver hybrids with controlled silver release. Int. J. Nanomed. 2017, 12, 3181–3191. [Google Scholar] [CrossRef]
- Liu, N.; Ming, J.; Sharma, A.; Sun, X.; Kawazoe, N.; Chen, G.; Yang, Y. Sustainable photocatalytic disinfection of four representative pathogenic bacteria isolated from real water environment by immobilized TiO2-based composite and its mechanism. Chem. Eng. J. 2021, 426, 131217. [Google Scholar] [CrossRef]
- Fouad, M.; Alalm, M.G.; El-Etriby, H.K.; Boffito, D.C.; Ookawara, S.; Ohno, T.; Fujii, M. Visible-light-driven photocatalytic disinfection of raw surface waters (300–5000 CFU/mL) using reusable coated Ru/WO3/ZrO2. J. Hazard. Mater. 2021, 402, 123514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohydr. Polym. 2021, 256, 117590. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, X.; Zheng, Y.; Chu, P.K.; Zhang, Y.; Wu, S. Graphitic carbon nitride-based materials for photocatalytic antibacterial application. Mater. Sci. Eng. R Rep. 2021, 145, 100610. [Google Scholar] [CrossRef]

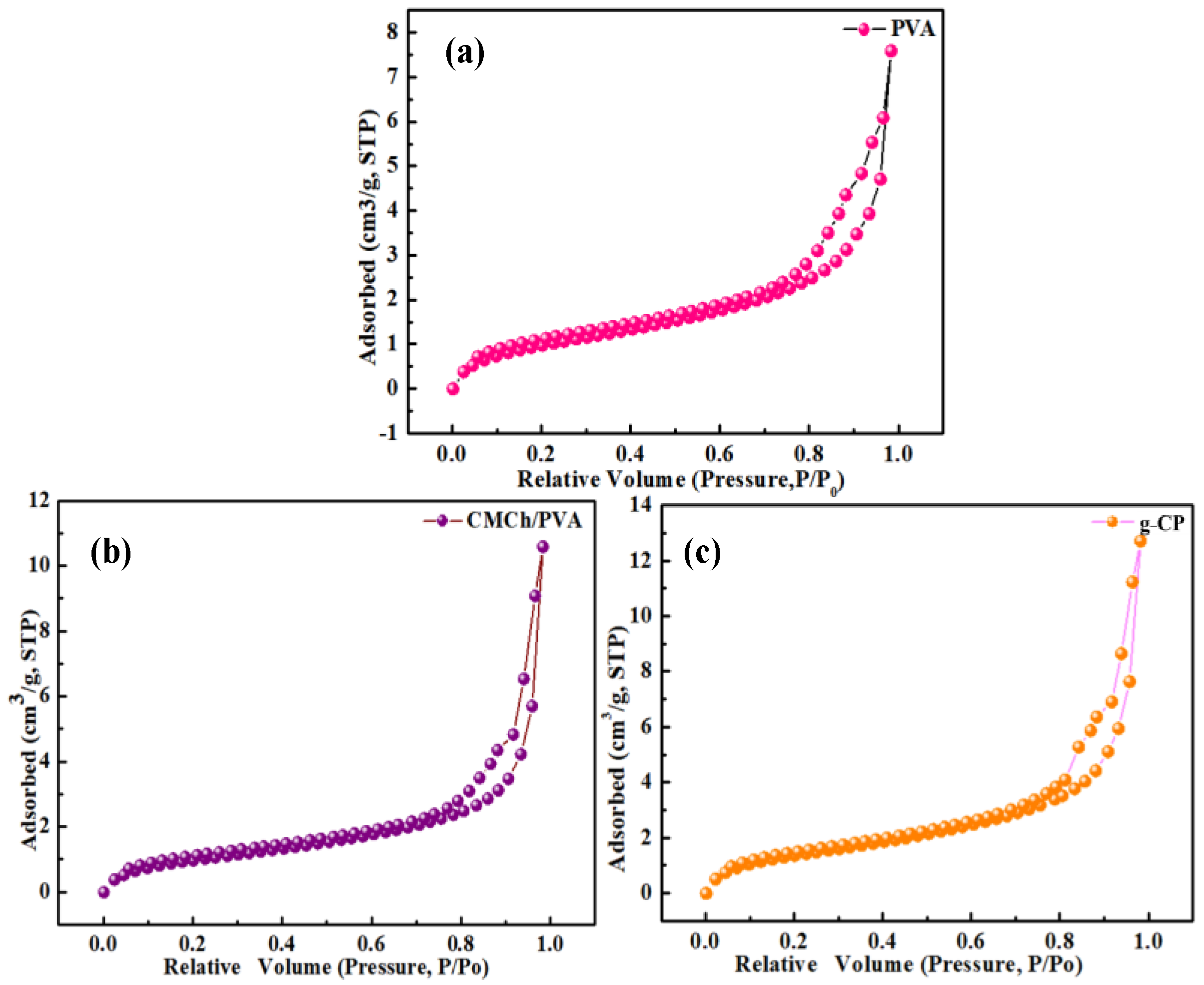


| Samples | Surface Area (m2 g−1) | Pore Volume (cc g−1) | Pore Diameter Dv (d) (nm) |
|---|---|---|---|
| PVA | 0.132494 | 0.000239772 | 5.9785 |
| CMCh/PVA | 0.208232 | 0.000320396 | 3.85686 |
| g-CP | 4.3618 | 0.0541498 | 3.10875 |
| Absorbent | T (K) | qe, Exp (mg g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| qe, Cal (mg g−1) | k1 (min−1) | R2 | qe, Cal (mg g−1) | k2 (g mg−1 min−1) | R2 | |||
| PVA | 298 | 8.23 | 8.97 | 0.0653 | 0.9847 | 8.54 | 0.0128 | 0.9944 |
| CMCh/PVA | 298 | 10.9 | 10.02 | 0.0383 | 0.9853 | 11.12 | 0.0094 | 0.9961 |
| g-CP | 298 | 11.69 | 10.79 | 0.0342 | 0.995 | 12.15 | 0.0072 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-Y.; Tang, D.-X.; Liu, D.-L.; Liu, K.; Yang, X.-J.; Li, Y.-S.; Liu, Y. Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method. Molecules 2023, 28, 7544. https://doi.org/10.3390/molecules28227544
Yang J-Y, Tang D-X, Liu D-L, Liu K, Yang X-J, Li Y-S, Liu Y. Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method. Molecules. 2023; 28(22):7544. https://doi.org/10.3390/molecules28227544
Chicago/Turabian StyleYang, Jin-Yu, Dong-Xu Tang, Dong-Liang Liu, Kun Liu, Xiao-Jie Yang, Yue-Sheng Li, and Yi Liu. 2023. "Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method" Molecules 28, no. 22: 7544. https://doi.org/10.3390/molecules28227544
APA StyleYang, J.-Y., Tang, D.-X., Liu, D.-L., Liu, K., Yang, X.-J., Li, Y.-S., & Liu, Y. (2023). Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method. Molecules, 28(22), 7544. https://doi.org/10.3390/molecules28227544








