Abstract
The design of a simple approach enabling the detection of bisphenol A (BPA) in water samples without the need for large amounts of solvents is of utmost importance. This paper reports a simple method for the separation, concentration, and quantification of BPA in water samples using high-performance liquid chromatography with fluorescence detection (HPLC-FLD) after its microextraction into an in situ formed organic ion-associate (IA) liquid phase (LP). Novel IA phase components without conjugated double bonds, such as benzene rings, were investigated. Ethylhexyloxypropylamine hydrochloride and sodium dodecyl sulfate solutions were added to the water samples to form IAs. The aqueous phase and ion-associate liquid phase (IALP) were separated by centrifugation. The aqueous phase was removed, and the liquid phase was recovered and measured using HPLC-FLD or HPLC-electrochemical detection (ECD). The concentrated phase (IALP) had a relatively low viscosity and could be injected directly into the chromatograph without dissolving it in organic solvents. The detection limits for BPA by HPLC-FLD and HPLC-ECD were 0.009 and 0.3 µg L−1, respectively.
1. Introduction
In the analysis of trace constituents in environmental water samples, a sample pretreatment step, such as concentration and separation, is often necessary to improve the sensitivity and selectivity of analytical techniques [1,2,3,4].
Solvent extraction is one of the most commonly used methods for the separation and concentration of environmental pollutants. However, the major drawbacks of conventional extraction methods include the (1) difficulty to increase the extractant concentration because of its partition between the two solvents, (2) toxicity of some extraction solvents such as chloroform, dichloromethane, and benzene [5,6] (whose concentrations in wastewater were regulated in 1993) [7], and (3) need for shaking and mixing to promote partitioning between the two phases [8,9].
Recently, the employment of green analytical approaches such as microextraction, which enables the extraction of a high concentration of a target substance in small amounts, has been gaining attention. For example, dispersive liquid–liquid microextraction [10,11] involves the use of a vortex or ultrasound to promote partitioning between the two phases [11] or the addition of 1/8 of the sample water volume of the dispersing solvent [3]. In this technique, a very small amount of a mixture of hydrophobic extraction and dispersing solvents is injected into the sample solution to extract the target component in a microvolume phase. Furthermore, some microextraction methods, such as homogeneous liquid–liquid extraction (HLLE) [12], cloud point (CP) extraction [13,14,15], ionic liquid [16], deep eutectic solvent [9,10,17,18], and our proposed organic ion-associate liquid phase (IALP) extraction, can enable in situ solvent formation. HLLE employs organohalogen compounds such as perfluorooctanoic acid in which the analyte is extracted from a homogeneous solution into a very small sedimented phase by pH modification, heating, salt addition (salting out), and constituent mixing/replacement [12]. CP extraction uses the cloud point of nonionic surfactants to separate the surfactant micellar phase from the aqueous phase to concentrate hydrophobic substances [13,14,15] upon heating.
In IALP microextraction [19,20,21,22,23,24], organic cations and anions are added to a water sample to form the IALP. The IA liquid and aqueous phases are then separated by centrifugation to extract the target component into the liquid phase. The target component is then dissolved in an organic solvent in the IALP or back-extracted from the IALP into an acid for measurement. This approach features several advantages, including the high capacity of the extractant, rapid extraction, avoiding harmful organic solvents, and minimization of contamination because it can be performed in a single container. Several studies have reported the application of this method for the separation, enrichment, and detection of ammonium ion, di(2-ethylhexyl) phthalate, cadmium, etc. [19,20,21,22,23,24].
Bisphenol A (BPA) is widely used as a raw material in the production of epoxy resin and polycarbonate plastics. In 2019, the total domestic production of BPA in Japan amounted to approximately 460,000 t [25]. However, leaching of BPA can occur through the hydrolysis of the polymer. High temperatures can increase this leaching [25,26,27,28,29,30,31]. Furthermore, it has been included in the Candidate List of Substances of Very High Concern according to the European Registration, Evaluation, Authorization, and Restriction of Chemicals regulations [26]. Japan currently requires a BPA content in water lower than 0.1 mg L−1 [27]. The effects of BPA on human health have already been reported [30]. Li et al. have also reported the detection of BPA in human urine [32]. Moreover, there is an increasing concern regarding the effects of BPA in environmental water on aquatic organisms [31]. Therefore, it is important to monitor BPA in environmental water. Consequently, the quantification of BPA is of great significance.
The analytical approach employed by the Ministry of the Environment for BPA detection [29] involves the acidification of a water sample (or filtrate) followed by extraction with dichloromethane (or performing solid-phase extraction and subsequent elution with dichloromethane). The extract is then dehydrated and concentrated for trimethylsilylation prior to its measurement by GC/MS-selected ion monitoring (SIM) mode. This approach not only requires large volumes of the water sample and extraction solvent, but also involves complex procedures, requiring derivatization for measurement.
Based on the Japanese and WHO guidelines, the maximum acceptable concentration of dichloromethane is 0.02 mg L−1 [5,6]; however, the solubility of dichloromethane in water [33] is one million times higher than this value. Consequently, care must be taken when handling the effluents.
IALP extraction methods often utilize certain reagents (dye-forming reagents [19], chelating reagents [22,23], pH buffers [21]) to convert the target component into an appropriate form (dye [19], chelate [22,23]), which is then added to the IALP with the counter ions. Hata et al. reported the extraction of polyaromatic hydrocarbons (PAHs) by an IALP composed of a combination of benzethonium (Ben+) chloride and ethylbenzenesulfonate sodium salt without conversion prior to their detection by high performance liquid chromatography with fluorescence detection (HPLC-FLD) [24]. However, the influence of organic reagents and their impurities on the IALP rendered the detection of light PAHs with three or fewer rings difficult. Although the combination of organic ions constituting a conventional IALP allowed for a high enrichment factor, their retention times in HPLC/FLD were relatively close to that of the hydrophilic analyte species (BPA), which interfered with the measurements.
So far, all organic ions selected for the IALP have benzene rings and exhibit fluorescence [19,20,21,22,23,24]. Consequently, it is necessary to find IALP components without benzene rings or imidazole groups among organic ions to develop an IALP that can be used for the separation and concentration of BPA.
This study reports a rapid, simple, and environmentally friendly analytical method for monitoring BPA in water by HPLC-FLD and HPLC with electrochemical detection (HPLC-ECD) following IALP extraction.
The IALP extraction method has the following advantages: (1) various types of organic cations and anions can be selected according to the application and can be monitored using various types of equipment; (2) a high enrichment factor can be obtained; (3) the target component can be extracted quickly because the extractive phase is precipitated from the aqueous phase; and (4) the method can be performed in a single vessel, thus minimizing contamination.
2. Results
2.1. Organic Ions Constituting the Ion-Associate Liquid Phase (IALP)
2.1.1. HPLC-FLD
The individual extraction constants for the organic anions (log Kanion, [34]) and octanol–water partition coefficients (log Kowwin, [35]) of the organic substances considered in this investigation are listed in Table 1. The individual extraction constant for anions (log Kanion) was obtained by separating the extraction constant (log Kex) for ion-pair extraction into the respective contributions from cations, anions, and the extraction solvent.

Table 1.
Organic substances and their log Kanion and log Kowwin values.
HPLC-FLD chromatograms of BPA after microextraction of IALP by EHOPA+/DS− or IALP (Ben+/ EBS−) alone are shown in Figure 1.
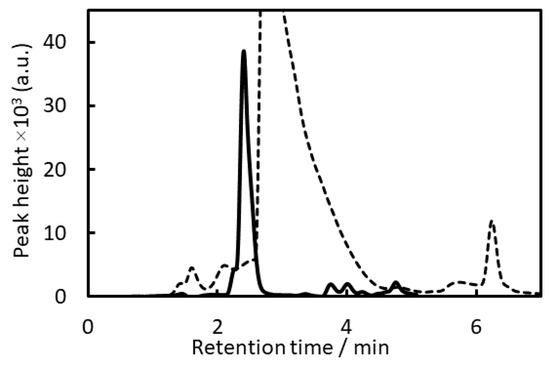
Figure 1.
HPLC-FLD chromatograms. Solid line: bisphenol A (BPA) after microextraction of IALP by (ethylhexyloxypropylamine (EHOPA+)/dodecylsulfate (DS−)); BPA concentration: 2 µg L−1; dotted line: IALP (benzethonium ions (Ben+)/Ethylbenzenesulfonate ion (EBS−)).
2.1.2. HPLC-ECD
Electrochemical detection is a method for studying compounds that undergo redox reactions; examples include compounds with phenolic hydroxyl groups. Four pairs of organic cations and organic anions forming the IALP were combined: 4-trifluoromethylanilium ion and dodecylbenzenesulfonate ion (DBS−), benzyldimethyldodecylammonium ion and phenol sulfonate ion (PS−), Ben+ and PS−, and Ben+ and ethylbenzenesulsonate (EBS−). The peaks of the reagent and the impurities that generated the IALP overlapped with the BPA peak, which was not desirable. Since EBS− and Ben+ should not be detectable by ECD under the conditions of this experiment, they were considered to be derived from the impurities. Therefore, when EBS− and Ben+ were recrystallized, the impurities in EBS were considerably reduced, but the impurities derived from Ben+ could not be removed. The combination of IALPs developed for FLD, EHOPA+, and DS− was also investigated for use in the ECD of BPA. As a result, the concentrates produced by the IALPs (EHOPA+ and DS−) could be used to detect BPA by HPLC-ECD.
2.2. Regions of Ion-Associate (IA) and Liquid Phase (LP) Formation
The concentrations of EHOPA+ and sodium dodecyl sulfate (SDS) during the formation of IAs and solvents were investigated by varying their concentrations in two dimensions (Figure 2).
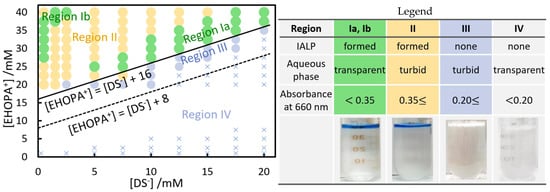
Figure 2.
Effect of EHOPA+ and DS− concentrations on IALP formation. Experimental condition: [EHOPA+] = 0.5 × 10−3 to 40 × 10−3 mol L−1, [DS−] = 0.5 × 10−3 to 20 × 10−3 mol L−1. After centrifugation, the standing time was 1 h; temperature during IA formation: 25 °C. Photographs of regions I and II represent IALP colored by adding bromophenol blue dye in base type.
2.3. Effect of Organic Ions on Ion-Associate Liquid Phase (IALP) Volume, Percent Extraction (%E), and Distribution Constant (Kd)
Figure 3a shows the case where EHOPA+ concentration was held constant (35 mM) while DS− concentration varied, and Figure 3b shows the case where [DS−] was 10 mM while EHOPA+ concentration varied.
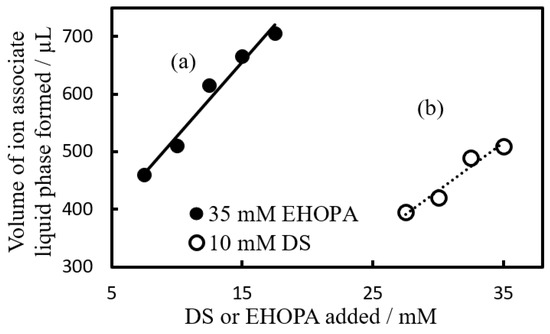
Figure 3.
Effect of constituent organic ions on the volume of IALP. (a) Black circle: [EHOPA+] = 35 × 10−3 mol L−1, [DS−] = 7.5 to 17.5 × 10−3 mol L−1. (b) White circle: [EHOPA+] = 27.5 to 35 × 10−3 mol L−1, [DS−] = 10 × 10−3 mol L−1. After centrifugation, the standing time was 1 h.
Figure 4 shows the volume of the IALP formed, %E of BPA, and the partition constant for each concentration. The absorbance (turbidity) at 660 nm is shown in a grayscale contour plot (Figure 4A). As the concentration of EHOPA or DS increased, IAs were formed, resulting in a cloudy sample. Further increasing the concentration resulted in the formation of a liquid phase which became transparent in the appropriate concentration range, while IAs were colloidally dispersed and become cloudy in other regions. As shown in Figure 4A, the volume of the liquid phase increased upon increasing the concentrations of EHOPA and DS.
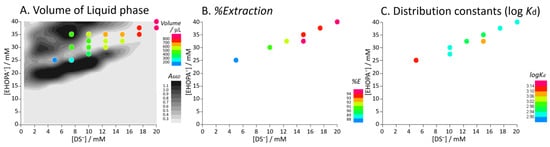
Figure 4.
Relationship between EHOPA and DS concentrations on the volume of ion-associate liquid phase (IALP), %E of BPA, and distribution coefficient (log Kd) of BPA. Experimental conditions: [EHOPA+] = 25 × 10−3 to 40 × 10−3 mol L−1, [DS−] = 5 × 10−3 to 20 × 10−3 mol L−1, [BPA] = 5.0 µg L−1.
2.4. Analytical Figure of Merit
The detection limit 3σb (n = 3), enrichment factor, %E, and log Kd were determined for different concentrations of EHOPA+ and DS−. The results are summarized in Table 2. At an EHOPA+ concentration of 27.5 mM and DS− concentration of 10 mM, the detection limit of BPA was 0.049 µg L−1, %E was 89%, and log Kd was 2.9. At an EHOPA+ concentration of 25 mM and DS− concentration of 5 mM, the detection limit of BPA was 0.009 µg L−1, %E was 86 %, and log Kd was 3.1. For HPLC-ECD, at an EHOPA+ concentration of 31 mM and DS− concentration of 12 mM, the detection limit of BPA was 0.2 µg L−1, %E was 93%, and log Kd was 3.0. Enrichment factors were calculated by comparing the slope of the calibration curve of the standard solution with and without microextraction; because the volumes of IALP were different, the enrichment factor by IALP microextraction was 150–300-fold. As shown in Figure 4B,C, %E and log Kd obtained by HPLC-FLD ranged from 86% to 95% and 2.9 to 3.1, respectively, and %E and log Kd obtained by HPLC-ECD were also within these ranges and could be considered reasonable values.

Table 2.
Limit of detection (LOD), %E, log Kd, and enrichment factor for different EHOPA+ and DS− concentrations.
Table 3 lists the limits of detection of some previously reported methods. Our proposed method has the lowest limit of detection.

Table 3.
Liquid and solid phase extraction procedure for bisphenol A.
2.5. Application to River Water Samples
To validate this method, quantification and recovery experiments were conducted on river water samples (Jinzu River (Toyama Prefecture), Hagiura Bridge; pH 6.54, EC 45.8 mS m−1) using EHOPA+/DS− = 25 mM/5 mM. For recovery experiments, river water samples containing 0.5, 1, 2, 3, and 5 µg L−1 BPA were also prepared (Figure 5).
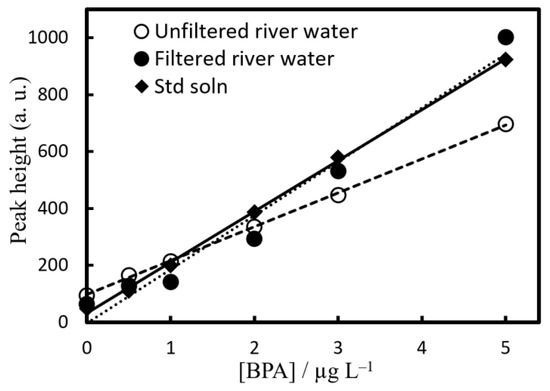
Figure 5.
Calibration curves of BPA in pure water (◆) and river water sample (○,●). Concentration of BPA was 0–5 µg L−1. Experimental conditions: [EHOPA+] = 25 × 10−3 mol L−1, [DS−] = 5 × 10−3 mol L−1, pH = 1.9. (○) Nonfiltered water and (●) filtered water.
3. Discussion
All previously reported organic ions used for IALP extraction have benzene rings, and their retention times and those of their impurities overlap with those of BPA. Thus, if an IALP is composed of Ben+Cl− and EBS−, BPA cannot be detected because the peaks of the organic ions forming the IALP and the impurities will overlap with those of BPA (Figure 1). Therefore, in this study, we investigated organic ions without benzene rings but with low fluorescence intensities for the HPLC-FLD of BPA after IALP extraction.
In ion-pair extraction, the extraction constant, Kex, includes the formation of ion pairs (Kass) and the distribution of the ion pairs formed (Kd); Kow (Kowwin), often used as a measure of the hydrophobicity of a substance, is only related to its distribution and not to the formation of ion pairs. Individual extraction constants for anions (log Kanion) [34] are used as references to select the optimum organic anions. Individual extraction constants are the extraction constants for ion-pair extraction (log Kex) separated into log Kcation, log Kanion, and log Ksolvent. Extraction constants represent the ease of association of cations and anions and the ease of distribution between the aqueous phase and extraction solvent and are considered helpful in selecting organic anions for the IALP. DBS− [20] is the only anion studied so far for which log Kanion has been determined among the anions constituting the IALP. Therefore, we selected organic anions with log Kanion similar to that of DBS, and their log Kanion and Kowwin are listed in Table 1. Of the three organic anions without benzene rings, SS−, DS−, and tetradecyl sulfonate (C14S−), DS− has the lowest Kowwin but the same log Kanion as that of DBS−. Furthermore, DS− was chosen as the organic anion because it is often used in the field of biochemistry and can be obtained in high purity.
Dodecyltrimethylammonium bromide, tetradecyltrimethylammonium bromide, and Zephiramine (Zeph+, benzyldimethyltetradecylammonium chloride) were examined as counter ions for detecting indothymol, which is more hydrophobic than BPA. However, only Zeph+ could quantitatively recover indothymol in the case of collection on a glass fiber filter [37]. Cetyltrimethylammonium chloride is not very soluble in water. Therefore, these alkyltrimethylammonium were not considered. In this study, amines were protonated to ammonium salts, which were considered as organic cations for IALP formation. Many peaks due to impurities were observed in previous IALPs. In general, commercially available reagents, including analytical grade reagents, contain a small amount (a few percent) of impurities. Amines were chosen because they can usually be purified easily by changing the pH and can be used for back-extraction.
The following reagents were thus selected as candidates for organic cations and attempts were made to prepare aqueous solutions for each reagent: Bis[3-(trimethoxysilyl)propyl]amine instantly solidified when mixed with water, while 3-butoxypropylamine (BOPA) and tris[2-(2-methoxyethoxy)ethyl]amine (TMOEEA) were soluble in water. N-methyldidodecylamine (MDDA) and EHOPA+ did not dissolve in water. EHOPA+ dissolved in hydrochloric acid, while MDDA did not. Consequently, TMOEEA, BOPA, and EHOPA were chosen as the organic cationic reagents, while DS−, which is soluble in water, was considered as the organic anionic reagent. The TMOEEA, BOPA, and EHOPA HCl (EHOPA+) solutions, in addition to DS−, were used to obtain samples with varying cation and anion concentrations. When the obtained samples were cloudy, they were centrifuged and subsequently observed for the formation of IALP. In the combination of TMOEEA and DS−, their respective concentrations varied from 2.5 to 20 mM, while they varied from 2.5 to 25 mM in the combination of BOPA and DS−. No IALP was obtained with these two combinations. The aqueous phase could be separated from the IALP only when EHOPA+ was used as an organic cation. Based on these results, the EHOPA+/DS− combination was selected as the organic ion constituting the IALP in this study. The formed IALP floated above the aqueous phase.
In the analysis of BPA, it is common to make water samples acidic to prevent BPA dissociation; however, in this method, the pH is set to 2 by adding EHOPA hydrochloric acid solution [29]. Thus, it is not necessary to make the water particularly acidic.
In Region I (Figure 2), an IALP was formed at the top and the aqueous phase was clear (absorbance at 660 nm was less than 0.35). In Region II, an IALP was also formed, but the aqueous phase was cloudy (absorbance at 660 nm was greater than 0.35). In Region III, the aqueous phase was cloudy (absorbance at 660 nm was greater than 0.35), but no IALP was formed. In Region IV, the aqueous phase was clear (absorbance at 660 nm was less than 0.15). The IALP formed from Ben+Cl− and EBS− had a single Region I [24], while for the EHOPA+/DS− combination, Region I was divided into two Regions, Ia and Ib (Figure 2). The volume of the IALP formed in Region Ib was smaller than that in Region Ia. The equations are:
[EHOPA+] = [DS−] + 8 mmol L−1,
[EHOPA+] = [DS−] + 16 mmol L−1,
The critical micelle concentration (CMC) of SDS is 16.9 mmol L−1 [38]. For an IALP to form, there must be an EHOPA+ concentration higher than the concentration of DS− plus 16 mmol L−1.
The results (Figure 3 and Figure 4A) revealed that the IALP increased as the amount of added organic ions increased.
As seen in Figure 3, the effect of the organic anions on the volume of the IALP formed was approximately 1.5 times greater than that of the organic cations. The volume of the IALP was more influenced by the concentration of DS− than by the concentration of EHOPA+.
In Figure 4A, the volume of the IALP increases with increasing concentrations of EHOPA and DS, i.e., the volume of the IALP is affected by EHOPA and DS concentrations. In the grayscale contour plot in Figure 4A, at [DS] = 5 mM, as the concentration of EHOPA increases, IAs form and the solution becomes turbid (Region IV →Region III). After centrifugation, at [EHOPA] = 25 mM, an LP is formed and turbidity decreases (Region Ia). Further, as EHOPA increases, some of the IAs formed aggregate to form the LP, while some disperse and become turbid (Region II).
In order to evaluate the ability of the IALP (EHOPA+/DS−) to extract IAs, the distribution constant of BPA was determined: the Kow of BPA is log Kowwin = 3.64 [35]. The Kd of BPA in the IA phase can be expressed as follows:
Equation (3) was transformed to obtain the Kd.
Here, m is the amount of the substance, v is the volume, ialp is the IALP, and aq is the aqueous phase. The amount of the substance was calculated by determining the percentage extraction of BPA at each concentration. The volume of the aqueous phase was calculated by subtracting the volume of the IALP.
As described in a previous study [24], a calibration curve was constructed from the peak heights of the chromatograms of the first and second extractions, and %E was calculated from the slope of the curve. If the slope at 100% recovery is α, %E can be obtained from the slopes of the calibration curves of the first and second extractions. The calibration curves for BPA from the IALP (EHOPA+/DS−) extraction (first extraction) and the calibration curve obtained by extracting its aqueous phase again with IALP (second extraction) are shown in Figure S1.
In Figure 4B, %E of the IALP increases with increasing concentrations of EHOPA and DS, i.e., %E of the IALP is affected by the concentrations of EHOPA and DS. The IALP/water log Kd was not significantly affected by the concentrations of EHOPA and DS (Figure 4C). The IALP/water log Kd ranged from 2.9 to 3.1 which was slightly smaller than the octanol–water partition constant, log Kow = 3.32 [35], indicating that the IALP (EHOPA+/DS−) had a slightly smaller extraction ability for BPA than octanol. For each concentration condition, %E ranged from 87.6% to 94.7%, where the %E increased as IALP volume increased. When EHOPA and DS were added, the pH of the aqueous phase was approximately 2 since EHOPA was dissolved in HCl (2 M).
The fact that our proposed method showed the lowest detection limit among the methods listed in Table 3 may be due to its high concentration factor and good reagent purity as well as owing to the fact that the concentration step was performed in a single vessel.
When a recovery test was performed using unfiltered river water samples, the slope was slightly lower than that of the calibration curve of ultrapure water. Therefore, an additive recovery test was performed using a river water sample filtered through a glass fiber filter. The results were in agreement with the slope of the calibration curve of the standard solution, thus indicating that the slope was reduced because of the influence of suspended solids in the river water sample.
The BPA content in the water of the Furu River, a small tributary of the Jinzu River, measured by HPLC-ECD was below the detection limit. Therefore, we conducted an additive recovery experiment to verify whether BPA could be recovered quantitatively. In the addition recovery experiment, BPA was added to water at concentrations of 5, 10, and 15 nM for 40 mL. The recovery obtained from the slope was 90%, indicating that quantitative addition and recovery were possible for river water samples. In this IALP combination, a good linear calibration curve was obtained in the range of 5 to 15 nM, and the detection limit (3σ) was 0.3 μg L−1. In addition, when the recovery experiment was applied to environmental water samples, the recovery was as high as 90%.
4. Materials and Methods
4.1. Reagents and Chemicals
The tris[2-(2-methoxyethoxy)ethyl]amine [organic cation] solution (1 M) was prepared by dissolving tris[2-(2-methoxyethoxy)ethyl]amine (Tokyo Chemical Industry, Tokyo, Japan) (32 g) in water (100 mL). The 3-butoxypropylamine [organic cation] solution (0.5 M) was obtained by dissolving 3-butoxypropylamine (6.6 g, Tokyo Chemical Industry, Japan) in water (100 mL). The EHOPA+Cl− solution was prepared by dissolving ethylhexyloxypropylamine (18.7 g, Tokyo Chemical Industry, Japan) with a HCl solution (2 M, 100 mL). The sodium dodecyl sulfate [organic anion] solution (0.1 M) was prepared by dissolving sodium lauryl sulfate (2.9 g, specially prepared reagent for biochemical research, Nacalai Tesque Inc., Kyoto, Japan, purity ≥ 99.5% (as C12, GC)) in water (100 mL). A standard BPA solution (100 mg L−1) was prepared by dissolving BPA (10 mg, Wako Pure Chemical, Osaka, Japan) in acetonitrile (100 mL). The HCl solution (2 M) was prepared by mixing HCl and water at a volume ratio of 1:5. The tetramethylammonium hydroxide (5%, TMAH) aqueous solution was prepared by diluting TAMAPURE AA100 TMAH (10 mL, 25 %, Tama Chemical, Kawasaki, Japan) up to 50 mL using water. The TMAH ethanol solution (5%) was prepared by diluting TAMAPURE AA100 TMAH (10 mL, 25%) to 50 mL using ethanol. Figure 6 shows the structure of BPA and the cation source, EHOPA.
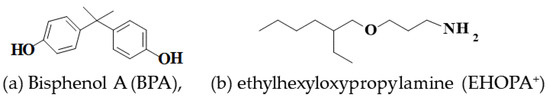
Figure 6.
Structure of bisphenol A (BPA) and EHOPA.
All other reagents were of analytical grade and used as received. In all experiments, ultrapure water (18.2 MΩ) made by an ultrapure water production system (Direct-Q3UV (Millipore, Burlington, MA, USA)) was used.
Water samples were stored either in brown glass bottles or polyethylene bottles and kept in a cool, dark place.
4.2. Apparatus
The UV-VIS spectra were recorded with a Shimadzu Model UV-2450 spectrophotometer using a 1 cm quartz cell or a 1 cm quartz cell with a 2 mm optical path width. A centrifuge (Type 5420, Kubota, Tokyo, Japan) was used for centrifugation. A thermominder system (SX-10N; Taitec, Koshigaya, Japan) was used as a thermostatic apparatus. pH measurements were carried out using a D-24 pH meter (Horiba, Kyoto, Japan).
4.3. Ion-Associate (IA) and Liquid Phase (IALP) Formation
Water (~30 mL) was added to a glass centrifuge tube (50 mL). The organic cation (EHOPA+, 1 M, 0.8 to 1.6 mL), HCl solution (2 M), and organic anion (DS−, 0.1 M, 0.2 to 8 mL) solution were then added, respectively. Finally, the volume was made up to 40 mL by adding water, and the solution was mixed. The aqueous phase was visually checked for the formation of IAs (turbidity or transparency) and centrifuged (3500 rpm for 30 min). After a standing time of 1 h, the formation of the IALP and the conditions of the aqueous phase were visually examined. The turbidity of the aqueous phase after centrifugation was determined by measuring absorbance at 660 nm using a UV-vis spectrophotometer. Temperature was maintained at 25 °C using a thermostatic device when studying the conditions for IALP formation since DS− precipitates at low temperatures.
4.4. Ion-Associate Liquid Phase (IALP) Microextraction
The water sample (40 mL) along with EHOPA+ HCl (1 M) and Na+DS− (0.1 M) solutions were centrifuged for 30 min to separate the IA liquid and aqueous phases. The aqueous phase was then removed. After centrifugation for 1 min to remove the water droplets adhering to the vessel wall, the IALP was directly collected and measured by HPLC-FLD or HPLC-ECD).
BPA was detected by reverse-phase HPLC, followed by two different detectors: FLD and ECD. The FLD of BPA was performed using a Prominance simple system (LC-20AT, RF-10AXL, DGU-20 A3, LC workstation, Shimadzu, Japan), an injection valve with a 20 μL loop (Rheodyne, Rohnert Park, CA, USA), and an Inertsil ODS-3 chromatography column (4.0 mm I.D. × 150 mm, 3 μm, GLSciences, Japan; column temperature: 40 °C, flow rate 0.7 mL min−1). The eluent was prepared by mixing acetonitrile and a potassium chloride solution (0.05 M) at a ratio of 75:25. For the ECD of BPA, an HPLC system (system controller: SCL-10Avp, data processing, column oven, Shimadzu damper, DGU-12A, Shimadzu, Kyoto, Japan) with a C18 reverse-phase column (CAPCELL PAK C18 MG II HPLC column (150 × 4.6 mm, 3 μm, Shiseido, Kyoto, Japan) or Inertsil ODS-3 chromatography column (see above)) and an injection valve (Rheodyne, USA) with a 20 μL loop was used. BPA was detected using a coulometric detection system (ECD, Guard Cell Model 5020, Analytical Cell Model 5010, ESA, Memphis, TN, USA) consisting of two porous carbon working electrodes controlled using a Coulochem III (ESA, USA) potentiostat. The eluent consisted of phosphate buffer (50 mM, pH 2.1)/acetonitrile (50:50) mixture. The rate of the mobile phase flow through the system was 0.5 mL min−1. The initial potential was 650 mV and the measured voltage was 800 mV.
A schematic of the microextraction of an IALP generated in situ from the aqueous phase by adding the EHOPA+, HCl, and Na+DS− solutions is presented in Figure 7. For the preparation of the IALP, EHOPA (1 M) and DS− (0.1M) were added to the water sample (40 mL) in a glass centrifuge tube (50 mL) to form an IA emulsion to extract the BPA into the IALP. After centrifugation at 3500 rpm for 30 min, the IALP and aqueous phase were separated. The IALP from which BPA was extracted was taken with a syringe, injected, and analyzed using HPLC-ECD.
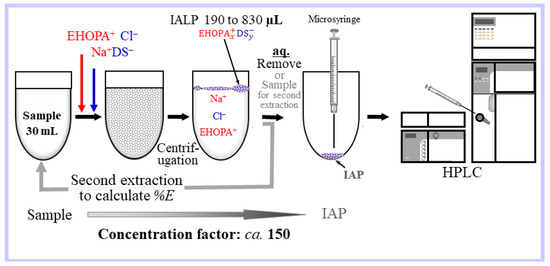
Figure 7.
Schematic of the microextraction of an IALP formed in situ from the aqueous phase by adding EHOPA, HCl, and SDS.
For the second extraction, the aqueous phase was transferred to another centrifuge tube and DS− (0.1 M) was added to form another IALP. The solution was centrifuged to separate the (second) IALP from the aqueous phase. The concentrate (IALP) was injected directly into the HPLC system and analyzed by FLD or ECD, and the %E of BPA from the IALP consisting of EHOPA+DS− was determined.
4.5. Measurement of IALP Volume
Water (~30 mL) was added into a glass centrifuge tube (50 mL). EHOPA+ and DS− were then added, followed by the addition of water, to achieve a final volume of 40 mL. The mixture was then centrifuged (30 min, 3500 rpm) to separate the IA liquid and aqueous phases. The aqueous phase was removed. The mixture was centrifuged (3 min, 3000 rpm) again to collect the aqueous phase adhering to the vessel wall and remove the collected aqueous phase. Volume was measured by aspirating the IALP using a microsyringe and reading the scale.
5. Conclusions
This study employed fluorescent organic ions in an IALP extraction method. An IALP formed from EHOPA+DS− enabled the quantitative extraction of BPA, an endocrine-disrupting chemical with a predicted no-effect concentration in environmental water of 11 μg L−1 [27], prior to its successful detection by HPLC-FLD and HPLC-ECD. The developed method is environmentally friendly and can be used as a pretreatment for the separation and concentration of other fluorescent and electrochemically reactive substances such as PAHs and estrogens prior to HPLC.
This method enabled a reduction in sample volume to approximately 1/20 of that required using the method employed by the Ministry of the Environment. The analysis time was also reduced, from 3 h to less than 1 h. A sufficient quantitative range was obtained for the concentrations required for measurement. Furthermore, dichloromethane, a hazardous chemical, was not used in this study. The concentrate was prepared in a single centrifuge tube without changing the instrument and had a low viscosity. It was directly collected using a microsyringe and injected into the HPLC system. The concentrates were sufficiently clear for both FLD and ECD. Consequently, the proposed method can be considered an efficient and environmentally friendly detection method.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28227525/s1, Figure S1: Calibration curve for bisphenol A (BPA) from the first and second extractions from IALP (EHOPA+/DS−).
Author Contributions
Conceptualization, N.H. and S.T. (Seira Takahashi); methodology, N.H., S.T. (Seira Takahashi) and S.O.; investigation, N.H., S.T. (Seira Takahashi), S.O., S.K., M.N. and A.I.; resources, N.H., K.S., S.T. (Shigeru Taguchi) and H.K.; writing—original draft preparation, N.H., S.T. (Seira Takahashi) and S.O.; writing—review and editing, N.H., S.O., K.S., S.T. (Shigeru Taguchi) and H.K.; visualization, N.H., S.T. (Seira Takahashi) and S.O.; supervision, N.H., K.S., S.T. (Shigeru Taguchi) and H.K.; project administration, N.H.; funding acquisition, N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 19K05561 and 15K00579.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article and its supporting information.
Acknowledgments
We thank A. Izumi, a student in our laboratory, for conducting the preliminary experiments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sajid, M. Advances in on-site analytical sample preparation for analysis of environmental waters: A review. TrEAC Trends Env. Anal. Chem. 2022, 36, e00175. [Google Scholar] [CrossRef]
- Tazoe, H. Water quality monitoring. Anal. Sci. 2023, 39, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Lu, W.H.; Liu, H.T.; Wu, X.G.; Li, J.H.; Chen, L.X. Dispersive liquid-liquid microextraction for four phenolic environmental estrogens in water samples followed by determination using capillary electrophoresis. Electrophoresis 2016, 37, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Kuramitz, H.; Hata, N.; Taguchi, S.; Murai, K.; Okauchi, K. Visual colorimetry for determination of trace arsenic in groundwater based on improved molybdenum blue spectrophotometry. Anal. Methods 2015, 7, 2794–2799. [Google Scholar] [CrossRef]
- Ministry of the Environment, Japan. Environmental Quality Standards for Water Pollution. Available online: https://www.env.go.jp/en/water/wq/wp.pdf (accessed on 23 June 2023).
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st and 2nd addenda; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004506-4. [Google Scholar]
- Ministry of the Environment, Japan. National Effluent Standards. Available online: https://www.env.go.jp/en/water/wq/nes.html (accessed on 23 June 2023).
- Yang, X.; Diao, C.P.; Sun, A.L.; Liu, R.M. Rapid pretreatment and determination of bisphenol A in water samples based on vortex-assisted liquid–liquid microextraction followed by high-performance liquid chromatography with fluorescence detection. J. Sep. Sci. 2014, 37, 2745–2750. [Google Scholar] [CrossRef]
- Li, T.; Song, Y.; Dong, Z.; Shi, Y.; Fan, J. Hydrophobic deep eutectic solvents as extractants for the determination of bisphenols from food-contacted plastics by high performance liquid chromatography with fluorescence detection. J. Chromatogr. A 2020, 1621, 461087. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, M.; Qin, Y.; Zhou, Y. Hydrophobic deep eutectic solvent based dispersive liquid–liquid microextraction for the preconcentration and HPLC analysis of five rice paddy herbicides in water samples. Microchem. J. 2022, 181, 107790. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Elmansi, H.; Belal, F.; Magdy, G. Recent advances in dispersion strategies for dispersive liquid–liquid microextraction from green chemistry perspectives. Microchem. J. 2023, 191, 108807. [Google Scholar] [CrossRef]
- Manaka, A.; Sawada, M.; Tafu, M. Simple and High-sensitivity Analysis of Small Microplastics with Phase Separation by Using Water/isopropanol/chloroform. Chem. Lett. 2023, 52, 420–421. [Google Scholar] [CrossRef]
- Tani, H.; Kamidate, T.; Watanabe, H. Micelle-mediated extraction. J. Chromatogr. A 1997, 780, 229–241. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Ning, J.; Yang, Y. Cloud point extraction for the determination of bisphenol A, bisphenol AF and tetrabromobisphenol A in river water samples by high-performance liquid chromatography. Anal. Methods 2014, 6, 3285–3290. [Google Scholar] [CrossRef]
- Yıldırım, E.; Gürkan, R.; Altunay, N. A new ultrasonic thermostatic-assisted cloud point extraction/spectrophotometric method for the preconcentration and determination of bisphenol A in food, milk, and water samples in contact with plastic products. Food Anal. Methods 2017, 10, 1765–1776. [Google Scholar] [CrossRef]
- Haq, H.U.; Elik, A.; Durukan, H.; Sarac, H.; Ahmet Demirbas, A.; Boczkaj, G.; Gürsoy, N.; Altunay, N. Application of chemometric modeling for ionic liquid-based ultrasonic-assisted dispersive liquid-liquid microextraction: Analysis of fosetyl-aluminum in fruit and vegetable samples. J. Food Compos. Anal. 2023, 124, 105725. [Google Scholar] [CrossRef]
- Li, K.; Jin, Y.; Jung, D.; Park, K.; Kim, H.; Lee, J. In situ formation of thymol-based hydrophobic deep eutectic solvents: Application to antibiotics analysis in surface water based on liquid-liquid microextraction followed by liquid chromatography. J. Chromatogr. A 2020, 1614, 460730. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Jiang, Y.; Qin, Z.; Ma, P.; Sun, Y.; Wang, X.; Song, D.; Fei, Q. Application of an in-situ formulated magnetic deep eutectic solvent for the determination of triazine herbicides in rice. Talanta 2021, 222, 121527. [Google Scholar] [CrossRef]
- Hata, N.; Kasahara, I.; Taguchi, S. Micro-phase sorbent extraction for trace analysis via in situ sorbent formation: Application to the preconcentration and the spectrophotometric determination of trace ammonia. Anal. Sci. 2002, 18, 697–699. [Google Scholar] [CrossRef][Green Version]
- Hata, N.; Yuwatini, E.; Ando, K.; Yamada, M.; Kasahara, I.; Taguchi, S. Micro-organic ion-associate phase extraction via in situ fresh phase formation for the preconcentration and determination of di(2-ethylhexyl)phthalate in river water by HPLC. Anal. Sci. 2004, 20, 149–152. [Google Scholar] [CrossRef][Green Version]
- Hata, N.; Hieda, S.; Yamada, M.; Yasui, R.; Kuramitz, H.; Taguchi, S. Formation of a liquid organic ion associate in aqueous solution and its application to the GF-AAS determination of trace cadmium in environmental water as a complex with 2-(5-bromo-2-pyridylazo)5-(N-propyl-N-sulfopropylamino)phenol. Anal. Sci. 2008, 24, 925–928. [Google Scholar] [CrossRef][Green Version]
- Mizuna, K.; Murashima, R.; Okazaki, T.; Sazawa, K.; Kuramitz, H.; Taguchi, S.; Nakayama, K.; Yamamoto, T.; Takamura, Y.; Hata, N. Organic Ion-associate Phase Extraction/Back-microextraction for the Preconcentration and Determination of Lithium Using 2,2,6,6-Tetramethyl-3,5-heptanedione by Liquid Electrode Plasma Atomic Emission Spectrometry and GF-AAS in Environmental Water. Anal. Sci. 2020, 36, 595–600. [Google Scholar] [CrossRef]
- Kosugi, M.; Mizuna, K.; Sazawa, K.; Okazaki, T.; Kuramitz, H.; Taguchi, S.; Hata, N. Organic Ion-Associate Phase Microextraction/Back-Microextraction for Preconcentration: Determination of Nickel in Environmental Water Using 2-Thenoyltrifluoroacetone via GF-AAS. Appl. Chem. 2021, 1, 130–141. [Google Scholar] [CrossRef]
- Hata, N.; Igarashi, A.; Matsushita, M.; Kohama, N.; Komiyama, T.; Sazawa, K.; Kuramitz, H.; Taguchi, S. Evaluation of an Ion-Associate Phase Formed In Situ from the Aqueous Phase by Adding Benzethonium Chloride and Sodium Ethylbenzenesulfonate for Microextraction. Appl. Chem. 2023, 3, 32–44. [Google Scholar] [CrossRef]
- Ministry of Economy, Trade and Industry (METI), Japan. Yearbook of Current Production Statistics 2019: Chemical Industry. Available online: https://www.meti.go.jp/statistics/tyo/seidou/result/gaiyo/resourceData/02_kagaku/nenpo/h2dbb2019k.pdf (accessed on 31 August 2023).
- European Chemicals Agency (ECHA). All News: Four New Substances of Very High Concern Added to the Candidate List. Available online: https://echa.europa.eu/-/four-new-substances-of-very-high-concern-added-to-the-candidate-list (accessed on 31 August 2023).
- Ministry of the Environment, Japan. Environmental Risk Assessment of Chemical Substances, 15. Bisphenol A 2004, 20 Pages. Available online: https://www.env.go.jp/chemi/report/h16-01/pdf/chap01/02_2_15.pdf (accessed on 31 August 2023).
- Ministry of Health, Labour and Welfare, Japan. Drinking Water Quality Standards, Water Quality Control Target Setting Items (Pollutants). Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/topics/bukyoku/kenkou/suido/kijun/kijunchi.html#01 (accessed on 8 September 2023).
- Water Quality Management Division, Water Quality Protection Bureau, Environment Agency, Tentative Manual for Investigation of Exogenous Endocrine Disrupting Chemicals (Water Quality, Sediment, Aquatic Life), 1998. Available online: https://www.env.go.jp/chemi/end/sympo/manual199810/water.html (accessed on 12 September 2023).
- Hahladakis, J.N.; Iacovidou, E.; Gerassimidou, S. An overview of the occurrence, fate, and human risks of the bisphenol-A present in plastic materials, components, and products. Integr. Environ. Assess Manag. 2023, 19, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Czarny-Krzymińska, K.; Krawczyk, B.; Szczukocki, D. Bisphenol A and its substitutes in the aquatic environment: Occurrence and toxicity assessment. Chemosphere 2023, 315, 137763. [Google Scholar] [CrossRef]
- Yao, Y.; Shao, Y.; Zhan, M.; Zou, X.; Qu, W.; Zhou, Y. Rapid and sensitive determination of nine bisphenol analogues, three amphenicol antibiotics, and six phthalate metabolites in human urine samples using UHPLC-MS/MS. Anal. Bioanal. Chem. 2018, 410, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare, Japan. Safety Data Sheet. Dichloromethane. Available online: https://anzeninfo.mhlw.go.jp/anzen/gmsds/75-09-2.html (accessed on 12 September 2023).
- Kasahara, I.; Ohgaki, Y.; Matsui, K.; Kano, K.; Taguchi, S.; Goto, K. Assignment of Individual Ion-Pair Extraction Constants Based on the KPh4As+ = KPb4B-; Kbenzene = 1 Assumption. Nippon Kagaku Kaishi 1986, 1986, 894–900. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA, Washington, DC, USA). KOWWIN 1.68 in “Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11.” 2012. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 8 September 2023).
- Zhavoronok, M.F.; Vakh, C.; Bulatov, A. Automated primary amine-based supramolecular solvent microextraction with monoterpenoid as coacervation agent before high-performance liquid chromatography. J. Food Compos. Anal. 2023, 116, 10585. [Google Scholar] [CrossRef]
- Hata, N.; Teraguchi, K.; Yamaguchi, M.; Kasahara, I.; Taguchi, S.; Goto, K. Spectrophotometric Determination of Ammonia-Nitrogen After Preconcentration as Indothymol on a Glass-Fiber Filter in the Presence of a Cationic Surfactant. Mikrochim. Acta 1992, 106, 101–108. [Google Scholar] [CrossRef]
- Gabal, R.A.; Osama, S.; Hanafy, N.; Oraby, A. Micellization thermodynamics as a function of the temperature of a cationic zwitterionic dodecyl phosphocholine and anionic sodium dodecyl sulfate mixed micelles with fluorometry. Appl. Phys. A 2023, 129, 201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).