Isochlorogenic Acid Glucosides from the Arabian Medicinal Plant Artemisia sieberi and Their Antimicrobial Activities
Abstract
1. Introduction
2. Results
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
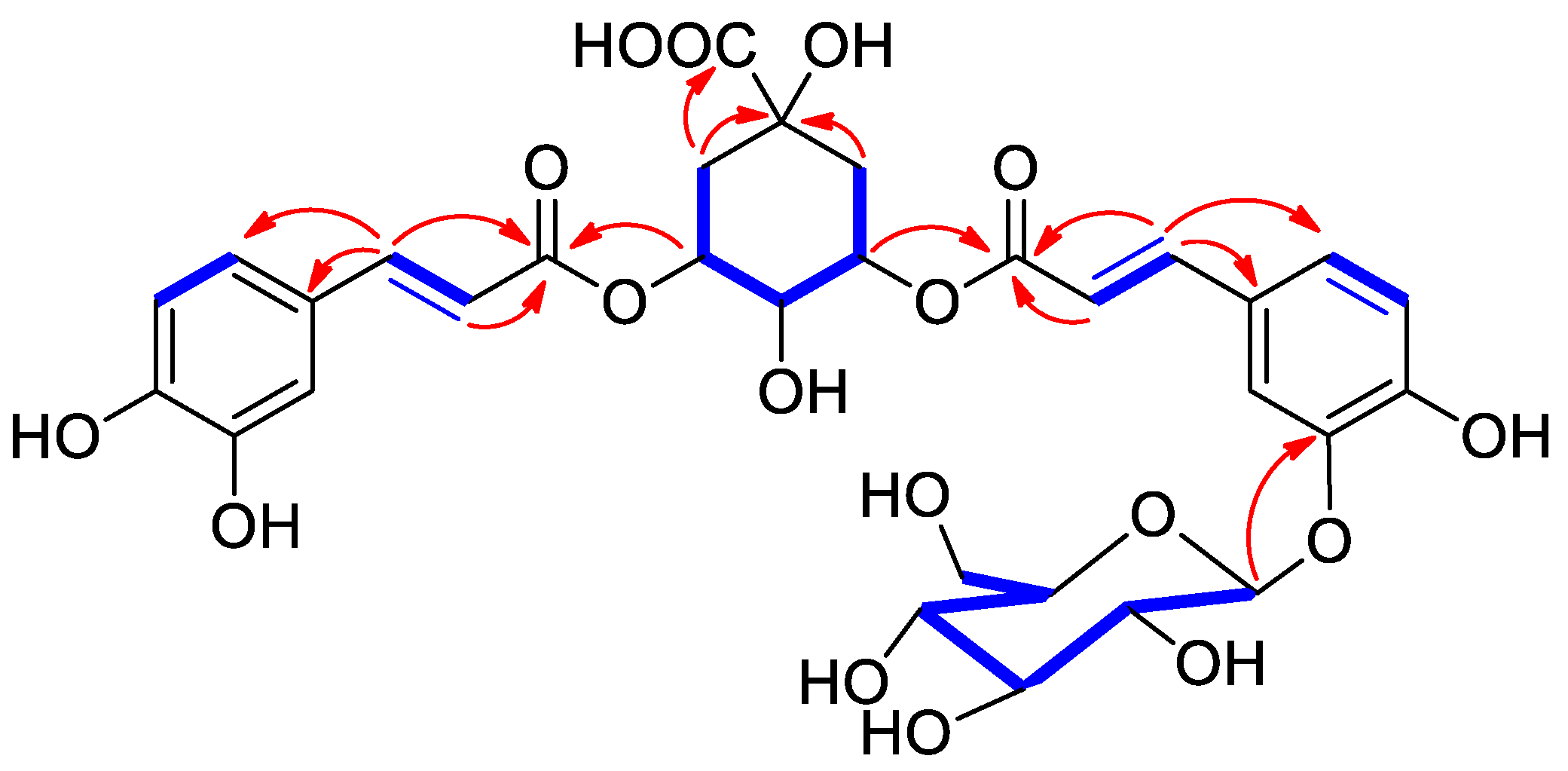
3.3.1. 3-O-(3′-O-caffeoyl glucosyl)-5-O-caffeoylquinic Acid (1)
3.3.2. 3-O-(3′-O-caffeoyl glucosyl)-5-O-caffeoylquinic Acid Methyl Ester (2)
3.3.3. 4-O-(3′-O-caffeoyl glucosyl)-5-O-caffeoylquinic Acid (3)
3.4. Antimicrobial Tests
3.4.1. Strains and Growth Condition
3.4.2. Agar Well Diffusion Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erel, S.B.; Reznicek, G.; Senol, S.G.; Yavasoglu, N.U.K.; Konyalioglu, S.; Zeybek, A.U. Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turk. J. Biol. 2012, 36, 75–84. [Google Scholar]
- Mahboubi, M. Artemisia sieberi Besser essential oil and treatment of fungal infections. Biomed. Pharmacother. 2017, 89, 1422–1430. [Google Scholar]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharif-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1–17. [Google Scholar]
- Mohamed, T.A.; Hegazy, M.E.; El Aty, A.A.; Ghabbour, H.A.; Alsaid, M.S.; Shahat, A.A.; Paré, P.W. Antimicrobial sesquiterpene lactones from Artemisia sieberi. J. Asian Nat. Prod. Res. 2017, 19, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Valian, M.; Kazempour, N. Chemical composition, antioxidant, and antimicrobial activity of Artemisia sieberi oils from different parts of Iran and France. J. Essent. Oil Res. 2015, 27, 140–147. [Google Scholar] [CrossRef]
- Abdolmaleki, Z.; Arab, A.; Amanpour, S.; Muhammadnejad, S. Anti-angiogenic effects of ethanolic extract of Artemisia sieberi compared to its active substance, artemisinin. Rev. Bras. Farmacogn. 2016, 26, 326–333. [Google Scholar] [CrossRef][Green Version]
- Mohamed, T.A.; Albadry, H.A.; Elshamy, A.I.; Younes, S.H.H.; Shahat, A.A.; El-Wassimy, M.T.; Moustafa, M.F.; Hegazy, M.E. A new Tetrahydrofuran sesquiterpene skeleton from Artemisia sieberi. J. Chin. Chem. Soc. 2021, 68, 338–342. [Google Scholar] [CrossRef]
- Adhikari, B.; Devkota, H.P.; Joshi, K.R.; Watanabe, T.; Yahara, S. Two new diacetylene glycosides: Bhutkesoside A and B from the roots of Ligusticopsis wallichiana. Nat. Prod. Res. 2016, 30, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Attoumbre, J.; Hano, C.; Mesnard, F.; Lamblin, F.; Bensaddek, L.; Raynaud-Le Grandic, S.; Laine, E.; Fliniaux, M.A.; Baltora-Rosset, S. Identification by NMR and accumulation of a neolignan, the dehydrodiconiferyl alcohol-4-β-d-glucoside, in Linum usitatissimum cell cultures. C. R. Chim. 2006, 9, 420–425. [Google Scholar] [CrossRef]
- Abegaz, B.; Camps, F.; Coll, J.; Feliz, M.; Jacobsson, U.; Miravitlles, C.; Molins, E.; Torramilans, J. The structures of vulgarin and its isomers: A reinvestigation. Tetrahedron 1986, 42, 6003–6009. [Google Scholar] [CrossRef]
- Sunnerheim-Sjöberg, K. (1S,2R,4S,5S)-angelicoidenol-2-O-β-D-glucopyranoside. A moose deterrent compound in Scots pine (Pinus sylvestris L.). J. Chem. Ecol. 1992, 18, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lin, Z.; Wang, D.; Sun, H. A sesquiterpene and other constituents from Erigeron breviscapus. Phytochem. 1994, 36, 717–719. [Google Scholar] [CrossRef]
- Morais, L.C.S.L.; Almeida, R.N.; da-Cunha, E.V.N.; da-Silva, M.S.; Barbosa-Filho, J.M.; Gray, A.I. Further lignans from Ocotea duckei. Pharm. Biol. 1999, 37, 144–147. [Google Scholar] [CrossRef]
- Flores-Parra, A.; Guiterrez-Avella, D.M.; Contreras, R.; Khuong-Huu, F. 13C and 1H NMR investigations of quinic acid derivatives: Complete spectral assignment and elucidation of preferred conformations. Magn. Res. Chem. 1989, 27, 544–545. [Google Scholar] [CrossRef]
- Forino, M.; Tenore, G.C.; Tartaglione, L.; Dell’Aversano, C.; Novellino, E.; Ciminiello, P. (1S,3R,4S,5R)5-O-Caffeoylquinic acid: Isolation, stereo-structure characterization and biological activity. Food Chem. 2015, 178, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Peng, C.; Chi, F.; Yu, C.; Yang, Q.; Li, Z. Antibacterial and antibiofilm activities of chlorogenic acid against Yersinia enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.A.; Denning, D.W.; Warn, P. Candida tropicalis in human disease. Crit. Rev. Microbiol. 2010, 36, 282–298. [Google Scholar] [CrossRef] [PubMed]

| No. | 1 | 3 | ||
|---|---|---|---|---|
| δH (Mult., J in Hz) | δc | δH (Mult., J in Hz) | δc | |
| 1 | 75.3 | 75.7 | ||
| 2a | 1.84 (dd, 12.1, 3.2) | 37.8 | 2.01 (dd, 10.5, 3.2) | 37.0 |
| 2b | 2.15 (d, 12.1, 4.9) | 2.09 (dd, 10.5, 5.0) | ||
| 3 | 5.19 (dt, 4.9, 3.2) | 73.8 | 4.30 (bt, 8.5) | 68.8 |
| 4 | 3.62 (dd, 8.5, 3.2) | 70.5 | 4.98 (dd, 8.5, 3.2) | 75.7 |
| 5 | 5.29 (bt, 8.5) | 71.6 | 5.52 (dt, 4.9, 3.2) | 68.0 |
| 6a | 1.84 (dd, 12.1, 8.5) | 39.2 | 2.07 (dd, 12.1, 8.5) | 39.4 |
| 6b | 1.97 (bd, 12.1) | 2.14 (bd, 12.1) | ||
| 7 | 177.9 | 176.9 | ||
| 1′ | 129.1 | 128.8 | ||
| 2′ | 7.12 (bs) | 116.4 | 7.12 (m) | 116.4 |
| 3′ | 147.7 | 147.7 | ||
| 4′ | 148.9 | 148.9 | ||
| 5′ | 7.09 (d, 7.1) | 121.1 | 7.05 (d, 7.1) | 121.9 |
| 6′ | 7.14 (bd, 7.1) | 115.0 | 7.11 (bd, 7.1) | 115.4 |
| 7′ | 7.52 (d, 16.1) | 144.4 | 7.53 (d, 15.0) | 145.3 |
| 8′ | 6.37 (d, 16.1) | 117.4 | 6.37 (d, 15.0) | 116.3 |
| 9′ | 166.8 | 166.5 | ||
| 1″ | 125.9 | 125.8 | ||
| 2″ | 7.15 (bs) | 115.3 | 7.06 (m) | 116.3 |
| 3″ | 146.1 | 146.1 | ||
| 4″ | 147.2 | 147.2 | ||
| 5″ | 6.98 (d, 7.0) | 121.8 | 6.99 (d, 7.0) | 121.4 |
| 6″ | 6.77 (d, 7.0) | 116.2 | 6.77 (d, 7.0) | 116.3 |
| 7″ | 7.45 (d, 15.5) | 145.3 | 7.41 (d, 15.0) | 145.6 |
| 8″ | 6.23 (d, 15.5) | 114.8 | 6.17 (d, 15.0) | 114.3 |
| 9″ | 166.6 | 166.5 | ||
| 1‴ | 4.81 (d, 8.1) | 101.7 | 4.79 (d, 8.1) | 101.7 |
| 2‴ | 3.31 (m) | 73.6 | 3.30 (m) | 73.6 |
| 3‴ | 3.31 (m) | 76.2 | 3.30 (m) | 76.2 |
| 4‴ | 3.19 (m) | 70.1 | 3.18 (m) | 70.1 |
| 5‴ | 3.36 (m) | 77.5 | 3.35 (m) | 77.5 |
| 6‴a | 3.72 (m) | 61.0 | 3.70 (m) | 61.0 |
| 6‴b | 3.47 (m) | 3.47 (m) |
| Compound | S. aureus ATCC 29213 | B. subtilis ATCC 6633 | E. coli ATCC 29213 | P. aeruginosa ATCC 27853 | C. tropicalis ATCC 66019 |
|---|---|---|---|---|---|
| 1 | - | 24 | - | - | - |
| 2 | 13 | - | - | - | - |
| 3 | - | 22 | - | - | - |
| 4 | - | - | - | 13 | - |
| 5 | - | 23 | - | 9 | 9 |
| 6 | - | - | - | 13 | 30 |
| 7 | - | - | - | 11 | - |
| 9 | - | - | - | 15 | 30 |
| 10 | - | 24 | - | - | - |
| 15 | 18 | - | - | - | - |
| 16 | 13 | - | - | - | - |
| Ampicillin | 6 | 8 | 8 | 24 | - |
| Fluconazole | - | - | - | - | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, K.; Al-Taweel, A.; Bukhari, S.I.; Orfali, R.; Moubayed, N.M.S.; Al-Qahtani, J.; Aati, H.; Taglialatela-Scafati, O.; Peng, J.; Perveen, S. Isochlorogenic Acid Glucosides from the Arabian Medicinal Plant Artemisia sieberi and Their Antimicrobial Activities. Molecules 2023, 28, 7460. https://doi.org/10.3390/molecules28227460
Jamal K, Al-Taweel A, Bukhari SI, Orfali R, Moubayed NMS, Al-Qahtani J, Aati H, Taglialatela-Scafati O, Peng J, Perveen S. Isochlorogenic Acid Glucosides from the Arabian Medicinal Plant Artemisia sieberi and Their Antimicrobial Activities. Molecules. 2023; 28(22):7460. https://doi.org/10.3390/molecules28227460
Chicago/Turabian StyleJamal, Khlood, Areej Al-Taweel, Sarah I. Bukhari, Raha Orfali, Nadine M. S. Moubayed, Jawaher Al-Qahtani, Hanan Aati, Orazio Taglialatela-Scafati, Jiangnan Peng, and Shagufta Perveen. 2023. "Isochlorogenic Acid Glucosides from the Arabian Medicinal Plant Artemisia sieberi and Their Antimicrobial Activities" Molecules 28, no. 22: 7460. https://doi.org/10.3390/molecules28227460
APA StyleJamal, K., Al-Taweel, A., Bukhari, S. I., Orfali, R., Moubayed, N. M. S., Al-Qahtani, J., Aati, H., Taglialatela-Scafati, O., Peng, J., & Perveen, S. (2023). Isochlorogenic Acid Glucosides from the Arabian Medicinal Plant Artemisia sieberi and Their Antimicrobial Activities. Molecules, 28(22), 7460. https://doi.org/10.3390/molecules28227460







