Abstract
The Ethiopian potato (Plectranthus edulis) is an annual tuber crop indigenous to Ethiopia. The crop is underutilized and not much studied despite its high yield of starch, which has a good potential to contribute to the effort in meeting the quickly growing demand for starch. In this study, the effects of the ecotype and isolation methods on the physicochemical, functional, structural, and crystalline properties of starches were evaluated. Starches were isolated from two Ethiopian potato ecotypes (Loffo and Chanqua) using distilled water (DW), 0.01% sodium metabisulphite (SMS), and 1M sodium chloride (NaCl) in the isolation media. The results showed that the lowest starch yield was obtained from Chanqua using DW (97.4%), while the maximum was from Loffo using SMS (99.3%). The L* (lightness) and whiteness values of the starches obtained from Loffo were higher than those of Chanqua starches, with NaCl and SMS extractants yielding the highest values. The bulk density, water activity (aw), pH, proximate composition (moisture content, protein, ash, fat, crude fiber, and carbohydrate contents), and techno-functional properties were established. The majority of these parameters varied depending on both the isolation method and the ecotype. The crystallinity pattern of all starches showed B-type diffraction, with differences in diffraction peak intensities between all starches. FTIR tests showed structural changes as a function of the ecotype and isolation procedure used. The Loffo ecotype exhibited considerably better results, and the SMS isolation method was found to be the most effective way to acquire the highest starch quality in most of the characteristics evaluated.
1. Introduction
Starch is the abundant carbohydrate resource in plants which serves as a source of carbon and energy in a variety of natural plants [1]. The global industrial starch market is currently expanding quickly due to major factors such as expanding food and beverage, pharmaceutical, and textile industries, improving global economic conditions, rising demand for processed and convenience foods and beverages in developing countries, rising per capita income, and population growth [2]. The food and beverage industry takes the largest portion (>55%) and applies starches or their derivatives as a major ingredient or as an additive to optimize the processing efficiency, product quality, or shelf life [3,4].
The majority of the world’s starch supply comes from corn, potatoes, wheat, cassava, and sweet potatoes [2]. However, these conventional sources are being over-exploited, necessitating the exploration of new botanical sources of starches. Although it is currently receiving little attention, Ethiopian potato (Plectranthus edulis) (EP) is one of the extensively grown traditional root crops in Ethiopia, with a high starch content that has a promising potential in contributing to the effort for meeting the quickly growing starch demand [2,5,6]. EP is an annual crop indigenous to Ethiopia which belongs to the Lamiaceae family and the genus plectranthus. It has various local names such as Dincha Oromo, Wolaita donuwa, Gurage dinch, Hadiya dinch, or Agew dinch. It is grown for its edible tubers [5,7,8] in large hectares in mid and high altitudes of Ethiopia, particularly in the southern, northern, and western parts. The tubers are boiled before consumption [9,10]. EP has a high starch yield that reaches 80.4%, with amylose content ranging from 15 to 23.9% on a dry basis [11], making it an additional rich source of starch that might serve as an alternative to the current starch sources being utilized in the food industry [6].
The application of starches from underutilized tubers like EP requires an in-depth characterization of their physicochemical properties and the selection of a suitable starch extraction method. Studies have shown that variations in the tuber variety or ecotype [12,13,14] and the isolation methods used [14,15,16,17,18] to separate starch from tubers can have an impact on the starch yield and physicochemical, functional, and structural characteristics of starches. The protein, ash, and lipid contents of starches obtained from eight Korean sweet potato varieties varied between 0.01 and 0.28%, 0.10 and 0.12%, and 0.04 and 0.16%, respectively, with the Jeungmi and Shinwhangmi starches having significantly higher protein contents but Shinwhangimi and Shinyulmi having lower lipid contents than other sweet potato varieties [12]. Phogat et al. [14] reported that starch isolated from the potato variety Kufri Chipsona-4 showed the highest yield, water absorption capacity (WAC) (258%), swelling power (SP) (38.4 g/g), and solubility (35.8%) compared to other varieties. Tessema and Admassu [17] reported that the starch yield isolated by the sodium metabisulphite (SMS) (0.075% w/v) method was significantly the highest (75.56%, db) compared with the sodium chloride (NaCl) and distilled water (DW) methods, with different concentrations from the anchote tuber. According to Xu et al. [18], SMS and sodium hydroxide (NaOH) effectively reduced tissue browning reactions and resulted in the highest starch whiteness value during starch isolation from root tubers of purple, yellow, and white sweet potatoes. Based on our knowledge, however, there is no information available on the effect of different isolation methods and the EP ecotype on the physicochemical, functional, and structural characteristics of EP starch.
In this study, the physicochemical, functional, and structural properties of starch of EP from different EP ecotypes were assessed using the NaCl, SMS, and DW isolation methods. The results of this study will help to choose the most suitable EP ecotype for starch extraction among two of the most mainly produced in Ethiopia and to identify the most appropriate solvent to carry out its isolation process.
2. Results and Discussion
2.1. Starch Yield
The starch yield ranged from 97.39% to 99.26% and varied significantly (p ≤ 0.05) due to differences in the EP ecotype and starch isolation method (Table 1). The starch yield from the Loffo ecotype was significantly higher than that of Chanqua. The starch yield value obtained from the Loffo isolated by SMS (99.26%) was the highest value, similar to the result reported by Assefa et al. [11]. The SMS and DW media yielded a significantly higher starch isolation efficiency than NaCl, without significant differences between them. This corroborated the finding of Tessema and Admassu [17], who obtained a higher Anchote starch yield from SMS-isolated starch when comparing the effect of different concentrations of SMS and NaCl. SMS, when dissolved in an acidic medium, releases sulfur dioxide. The action of sulfur dioxide is very important because, as a reducing agent, it is capable of breaking the disulfide bonds that wrap matrix protein starch granules to free the starch granules that can help in the separation of starch and increase the amount of starch produced [19].

Table 1.
The EP starches yield and physical properties.
2.2. Physical Properties of EP Starches
The EP ecotype and starch isolation method had a significant effect (p ≤ 0.05) on the bulk density (BD), water activity (aw), pH, and color of the starches obtained (Table 1). The BD of the isolated EP starches ranged from 0.75 g/cm3 (starch isolated from Chanqua with distilled water, CDW) to 0.85 g/cm3 (starch isolated from Loffo with NaCl, LNaCl). Starches obtained from Loffo had significantly higher (p ≤ 0.05) BDs than those of Chanqua. The variation in BD could be due to the variation in the particle size of starch granules, the compaction profile, and the particle packing arrangement [17,20]. The BD of EP starches also significantly varied with the isolation method, following the order: NaCl (0.84 g/cm3) > SMS (0.81 g/cm3) > DW (0.76 g/cm3). The BD of EP starch was higher than that of the anchote (0.51 g/cm3) and potato (0.71 g/cm3) starches reported by Tessema and Admassu, regardless of the ecotype and the solvent used in the isolation process [17]. The aw values ranged from 0.38 (LNaCl) to 0.61 (CDW), which is comparable with the aw of the native cassava starch (0.5) reported by Ocieczek et al. [21]. The mean aw of the starch isolates from Loffo had lower values than Chanqua. The mean aw scores of the starch isolated from the three isolation methods also varied significantly (p < 0.05), following the order: NaCl (0.40) ˂ SMS (0.51) ˂ DW (0.57). The lower aw value found in starch isolated with NaCl can be attributed to the known decreasing power of this salt on the vapor pressure and water activity of the unbound/available water of the starch, in which it remains dissolved [22]. On the other hand, starch isolates from Loffo had a slightly higher mean pH than those from Chanqua. The isolation methods also affected the mean pH of starch isolates obtained from EP and varied as: DW (5.94) ˂ SMS (7.12) ˂ NaCl (7.34).
2.3. Color
Whiteness is one of the physical properties of starch that is important in determining starch quality [18,20]. There were significant differences (p ≤ 0.05) between all color parameters due to the EP ecotypes and isolation methods (Table 1). The lightness (L*) and whiteness varied from 95.6 and 94.37, in CDW, to 97.6 and 96.92, in LNaCl, respectively. These whiteness values resulted to be higher than those found in anchote (89.16) and potato starch (93.28) reported by Tessema & Admassu [17]. The L* values measured in EP starches were also above those reported for eight Korean sweet potato varieties (89.7–93.9) [12]. Starch isolation methods significantly (p < 0.05) affected L* and whiteness, and the average values of these two parameters respectively varied as: DW (96.3, 95.3) < NaCl (96.5, 95.6) < SMS (97.1, 96.4). The lowest L* and whiteness values were obtained in DW-isolated starches, which could be due to the expected browning reaction during the isolation procedure [12]. The SMS isolation method showed the highest effect in restricting this darkening effect and leading to a significant (p ≤ 0.05) increase in L* and whiteness values for the same EP ecotypes. Different studies mentioned that SMS and NaCl could decrease the browning reactions of tissue during starch isolation and, as a result, could give whiter starches [18]. The starch of Loffo EP was found to have higher whiteness than Chanqua and had a significant difference (p < 0.05) in all color parameters. Such difference in the whiteness and lightness of the starch extracts could be attributed to the presence of some co-pigmented substances, and variations in their ash and protein contents [12,17,20]. The reddish/greenish (a*/−a*) values ranged from 0.71 (CDW) to −0.38 (LNaCl), whereas the yellowish (b*) values ranged from 1.19 to 3.43. The NaCl and SMS methods had no significant effect on the a* and b* values, while the water isolation method had the lowest (p ≤ 0.05) a* and b* values.
2.4. Starch Proximate Composition
Except for the fat content, starches isolated from the two EP ecotypes had significant differences (p ≤ 0.05) in their moisture, protein, fiber, ash, and carbohydrate contents (Table 2). The isolation method also had a significant (p < 0.05) effect on all the compositional parameters measured.

Table 2.
Chemical composition of EP starches (in % dry basis, except in moisture content).
Moisture content is one of the important factors affecting the physical and functional properties of starch. It has a considerable impact on the flow and other mechanical properties [23,24]. The moisture content varied between 10.23 and 14.80%, where the highest value was obtained in CDW starch and the lowest in LNaCl starch. The EP starch moisture content levels found in this study were comparable with those reported by Hellemans et al. [6] (14.1–17.5%,). In addition, the results were within the range (9.1–17.5%) reported for various tuber starches [2,20] and in the range (10–20%) suggested for commercial starches [25]. The starch isolation method had a significant impact on the moisture content of the resulting starches, and the values varied as follows: NaCl (11.76%) < SMS (13.16%) < DW (13.48%). Based on the ecotype, Loffo EP starch had a lower moisture content (11.42%) than Chanqua starch (14.14%). Tsakama et al. [26] also reported that the moisture content of starches could be significantly affected by the differences in the ecotype.
The fat content of EP starches lied between 0.21% and 0.37%, which was in the range reported by Hellemans et al. (0.15–0.59%) [6] and was near the finding of Assefa et al. (0.21%) [11]. The SMS method-isolated starch had the highest amount of fat content. Similar results (0.50–0.68%) were reported by Julianti et al. [27], who evaluated the effects of the SMS, DW, and citric acid methods on purple-fleshed sweet potato and found that the SMS technique-isolated starches had the highest fat content. Kale et al. [20] reported a 0.09–0.11% fat content in the isolation of sweet potatoes using the NaCl and SMS methods, respectively.
The protein content ranged between 0.65% (CSMS) and 0.95% (LDW), which is more or less in the range reported by Hellemans et al. [6] (0.70–1.76%). The mean protein content of the starch isolated from Chanqua (0.71%) was significantly lower than that of Loffo (0.85%). This shows that the protein content of starches can vary depending on the type of ecotype [12]. The mean protein contents of the EP starches isolated by DW (0.87%) were significantly higher than those isolated by NaCl (0.70%) and SMS (0.76%). NaCl-isolated starch had the lowest (p ≤ 0.05) protein content. This could be explained by the fact that NaCl eliminated the protein component by dissolving the starch–protein agglomerates and caused the removal of floating proteins during isolation [16,20]. The protein contents of the EP starches measured were higher than those of the starches isolated from tubers like anchote [2] and purple-fleshed sweet potato [27]
The values of the fiber contents of the isolated starches ranged between 0.54 (LDW) and 0.89% (CSMS), which were below the values reported by Hellemans et al. [6] (1.2–5.3%). The mean crude fiber and ash in the starches from Chanqua (0.80%, 0.62%) were significantly higher than those of Loffo (0.66%, 0.55%), respectively. The ash content varied between 0.51 and 0.64%, where the highest value was obtained in CSMS starch and the lowest in LDW starch. The variations in the fiber or ash content might be influenced by growth conditions and/or genetic diversity [6]. The isolation method also notably varied the mean ash contents of the resulting starches as NaCl (0.61) > SMS (0.60) > DW (0.55). The reason why EP starches isolated with NaCl had SMS scored relatively higher ash contents could be attributed to the fact that SMS and NaCl contain minerals that are not burned in combustion and increase the accumulation of the mineral content [28]. Kale et al. [20] also mentioned that variations in starches’ ash content could be due to the isolation methods and the degree of homogenization for isolation. The difference in the fiber contents of the starch isolates due to the isolation method was SMS (0.83%) > NaCl (0.73%) > DW (0.64%). The carbohydrate content EP significantly varied between 83.51% (CDW) and 88.12% (LNaCl). The carbohydrate content was influenced by the EP ecotype and isolation method, with the highest values being found in the Loffo ecotype and NaCl isolation method.
2.5. X-ray Diffraction of EP Starches
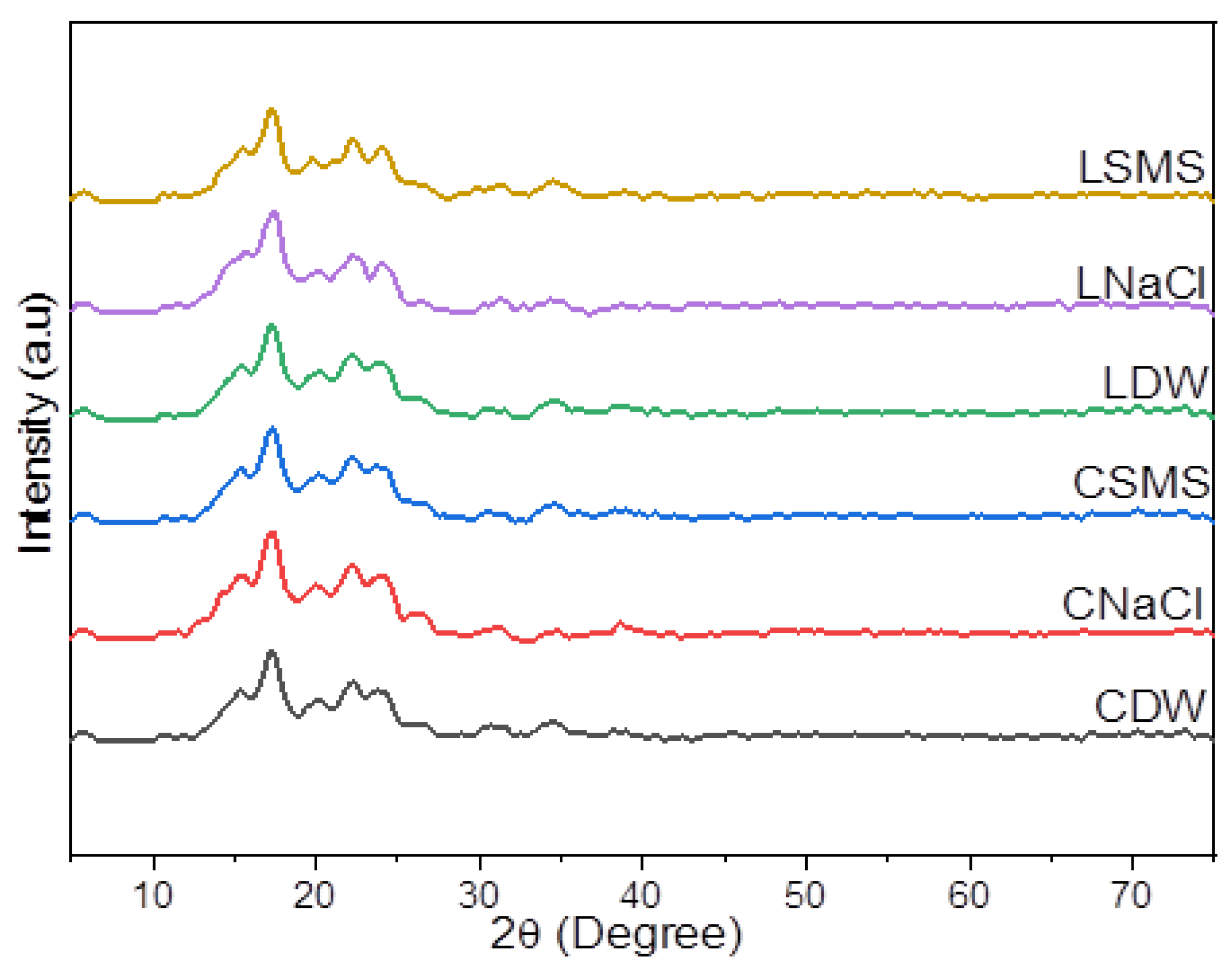
The crystallinity pattern of the EP starches showed a B-type XRD pattern (Figure 1), with the characteristic peaks around 5.6°, 15°, 17°, 22°, and 24° 2θ. All starches, however, showed different diffraction peak intensities, which could be attributed to the differences in the ecotype and/or isolation method. According to Wang et al. [29], the environment conditions, especially the growth temperature and moisture, could have a significant impact on the starch’s crystalline structure. Previous researchers found that EP starches exhibited B-type [11] and A- and B-type diffraction [6], whereas potato and cassava starches exhibited B-type and A-type diffraction, respectively [30].
Figure 1.
The XRD pattern of starches of Chanqua and Loffo starches. CDW, CNaCl, and CSMS = Distilled water-, Sodium Chloride- and Sodium metabisulfite-isolated Chanqua starch, whereas LDW, LNaCl, and LSMS = Distilled water-, Sodium Chloride- and Sodium metabisulfite-isolated Loffo starch.
The crystallinity indexes (CI) of EP starches are given in Table 3. The CI values ranged between 30.26 and 38.49%, where the lowest value was recorded for CSMS starch and the highest value was recorded for CSMS. These results showed that the DW, NaCl, and SMS isolation methods had an effect on the CI of starches. The CI values obtained for EP starches in this study were lower than the CI values reported by Tessema & Admassu [17] for anchote (39.15%) and potato (38.60%) and the values stated in Wolde et al. [30] for anchote, cassava, and potato starches, which ranged between 45.7 and 54.4%. Vanier et al. [31] underlined that differences in the crystallinity of starches could be influenced by the crystallite size, the number of crystallites, and the moisture content. They also noted that a decrease in the amorphous area results in an increase in the CI. The relative crystallinity had a significant positive correlation with the moisture content. Wolde et al. [30] also mentioned that crystallinity is directly proportional to the moisture content.

Table 3.
Characteristic peaks, CI, and average crystallite/grain size of the EP starches.
2.6. Fourier Transform Infrared (FTIR) Analysis
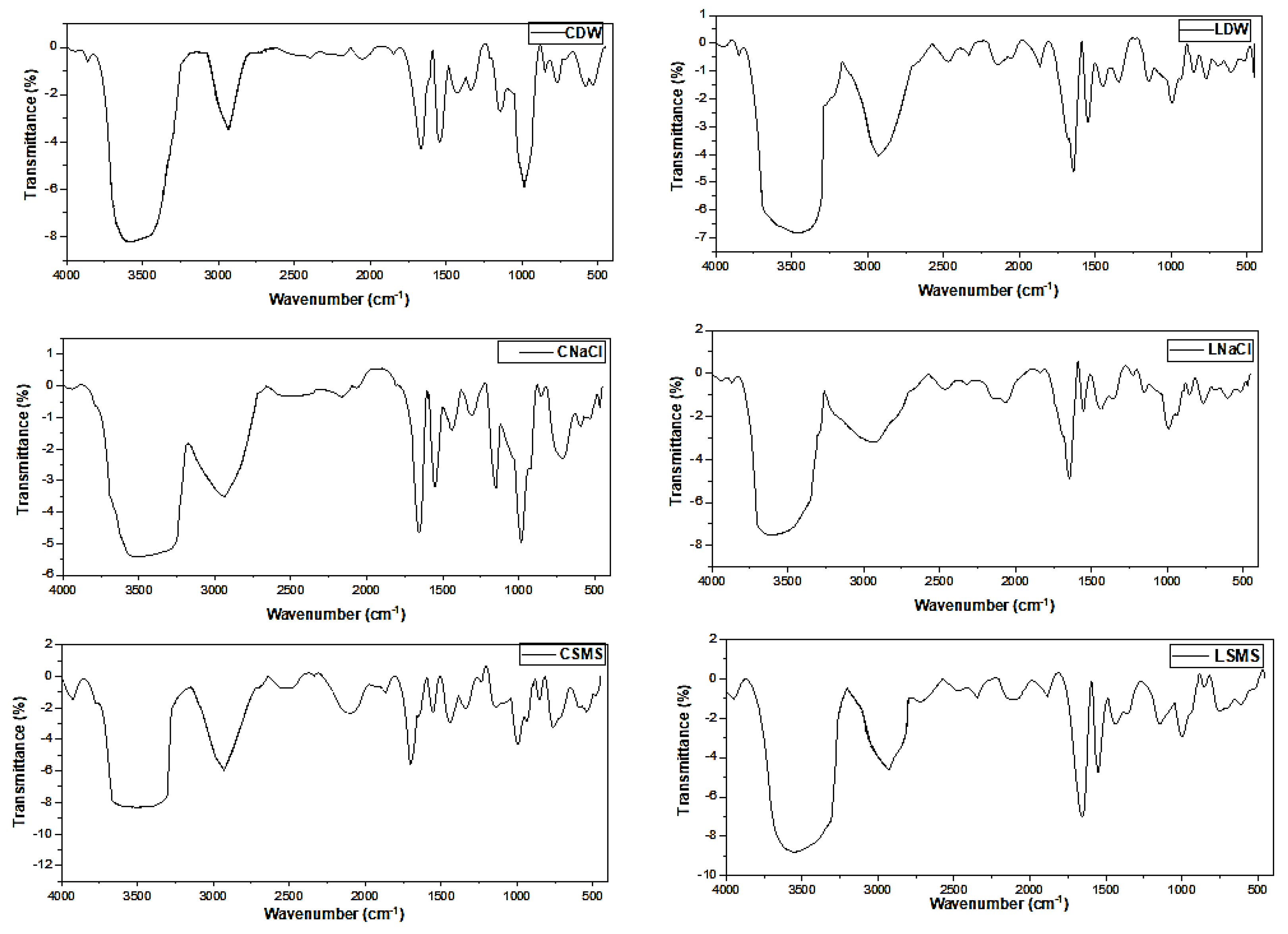
The FTIR bands obtained for different EP starches are presented in Table 4 and Figure 2. FTIR is the best technique for analyzing and spotting differences in the sample composition, ligand complexation, and structural make-up [32]. All EP starches had broad transmittance bands that fell between 3579 and 3466 cm−1, attributed to O-H stretching, and bands between 2929 and 2935 cm−1 that show the symmetric/asymmetric stretching modes of the C-H, which agreed with previous reports [33,34,35,36]. There were intense peaks between 1665 and 1647 cm−1 (Table 4 and Figure 3), which could be attributed to the C-O bending associated with the OH group and also with H-O-H vibration due to water because the presence of the water molecules bound to the starch tends to create the distinctive peak around 1648 cm−1 [36]. The bands that fell between 1362 and 1306 cm−1, 1151 and 1141 cm−1, and 998 and 983 cm−1 were probably due to C-H symmetric bending, C-O-C asymmetric stretching, and C-O stretching, respectively, and there are bands created due to the C-O-C vibration of carbohydrates [37]. The location of the infrared absorption peaks of the six samples had differences in the peak shift to a lower/higher wavenumber or intensity, especially in sensitive regions, because of the effect of the isolation methods and the difference in the EP type (Figure 2).

Table 4.
FTIR Frequencies region and absorbing features.
Figure 2.
FTIR spectra of the EP starches. CDW, CNaCl, and CSMS = Distilled water-, Sodium Chloride-, and Sodium metabisulfite-isolated Chanqua starch, whereas LDW, LNaCl, and LSMS = Distilled water-, Sodium Chloride-, and Sodium metabisulfite-isolated Loffo starch.

Figure 3.
The two types of EP ecotype included in the study: (a) Chanqua and (b) Loffo.
2.7. Functional Properties of EP Starches
The EP ecotype had significant (p ≤ 0.05) effect on the starch water absorption capacity (WAC) and oil absorption capacity (OAC), while the isolation methods had a significant effect on all four of the functional properties evaluated (Table 5). WAC is an important starch processing parameter that is associated with interacting forces inside the starch component, with a higher WAC resulting from lower interaction forces [20,40]. The WAC values ranged from 0.69 g/g (LSMS) to 1.03 g/g (SDW), and closer value ranges were also reported by Hellemans et al. [6]. The variation in WAC could be related to the difference in the ratio of amylose and amylopectin, where a higher amylose content generally results in a lower WAC, while a higher amylopectin content leads to a higher WAC [6]. Starches extracted from the Loffo EP ecotype (0.83 g/g and 0.80 g/g) had a significantly lower mean WAC and OAC than those from Chanqua (0.96 g/g and 1.00 g/g). EP starch isolation by salts (NaCl and SMS) led to a lower starch WAC and OAC in the starches from both EP ecotypes, and depending on the isolation methods, the mean WAC and OAC scores of the starches varied as follows: DW (1.03 g/g) > NaCl (0.85 g/g) > SMS (0.80 g/g) and DW (1.00 g/g) > SMS (0.90 g/g) > NaCl (0.80 g/g), respectively. Comparable WAC and OAC values, (0.88 g/g to 0.95 g/g) and (0.96 g/g to1.15 g/g), were recorded on starches isolated from cassava and sweet potato [41]. The starch swelling power (SP) indicates its capacity to retain water after undergoing heating, cooling, and centrifugation, while water solubility measures the extent to which starch dissolves when it swells in water [18]. The amount of water that is absorbed during gelatinization is what determines the capacity of starch swelling [42]. The effect of the EP ecotype SP and solubility of the isolated starches was not significant. However, the influence of the starch isolation method was important in varying the SP and solubility scores of the isolated starches, respectively, as DW (6.6 g/g) ≥ SMS (6.4 g/g) > NaCl (5.6/g) and DW (6.72%) ≤ SMS (6.53%) < NaCl (7.21%). The starches isolated by NaCl had the lowest SP compared to the others, and this could be attributed to the fact that the salt could impede the swelling and gelatinization of starch [43]. Such significant effect of the isolation media has been reported in starches isolated from root tubers of purple, yellow, and white sweet potatoes [18]. The SP values obtained are negatively correlated (r = −0.78) with the solubility percent, corroborating the earlier findings of Singh Sandhu and Singh [44].

Table 5.
Functional properties of EP starches.
3. Materials and Methods
3.1. Materials
Two types of EP ecotypes, Chanqua and Loffo (Figure 3), were included in this study. The Chanqua and Loffo were collected from Ezzo and Gembelagesha, respectively, in the Southern Nations and Nationalities Peoples Region, Ethiopia, where the EP ecotypes are commonly grown. Analytical grade SMS, NaOH, NaCl, and ethanol were purchased from local chemical markets in Addis Ababa Ethiopia.
3.2. Starch Isolation and Yield
Material preparation was conducted following the method described by Hellemnas et al. [6]. Fresh and mature EP tubers were first washed and peeled, and the edible portion of the tuber was cut into smaller pieces/slices; then, the starches were isolated by three different methods depending on the solvent used to perform the extraction. Distilled water (DW), 0.01% sodium metabisulphite (SMS), and 1M sodium chloride (NaCl) were used as solvents following the methods described in Kale et al. [20], Surendra Babu & Parimalavalli [16], and Xu et al. [18], with some modifications. The slices of EP tubers were blended with the different solvents at a ratio of 1:10 (v/v) using a laboratory-grade blender (Universal fritter QS806, Chana, IL, USA) until a smooth slurry was formed. Then, the slurries were filtered with double-layered cheesecloth and centrifuged (TGL-16, Sichuan Shoke, Leshan, China) for 20 min at 5000× g and 20 °C. The supernatant was decanted, and the starch settled at the bottom of the centrifuge tube was washed with distilled water to remove impurities. Finally, the starch was allowed to dry overnight at room temperature, ground with a mortar and pestle into fine powder, and packed in polyethylene bags for further investigations.
The starch yield was determined, as described in Awolu et al. [45], by calculating the starch obtained as the percentage ratio of starch recovered after isolation to the EP tuber sample utilized.
3.3. Starch Bulk Density
The starch bulk density (BD) was determined following the method described in Stasiak et al. [46]. A total of 20 g of the starch sample was gently loaded into a 100 mL graduated cylinder. The measured volume was used to calculate the bulk density according to the mass/volume ratio.
3.4. Color
The color measurements of the starches were carried out using a Color Measuring System (Hunter Lab colorimeter, Minolta). L*, a*, and b* coordinates were obtained with the D65 standard illuminant and 10° standard observer. The whiteness of the starches was determined as per the method described by Kale et al. [20], where the whiteness of starch was calculated using Equation (1).
where: L* = lightness, b* = yellowness, and a*= greenness.
Whiteness = 100 − [(100 − L*)2 + a*2 + b*2]½
3.5. Starch Proximate Compositions
The moisture, protein, fat, crude fiber, and total ash contents of the extracted EP starches were determined according to method given by AACC, 2001 [47]. The carbohydrate content was determined by subtracting the sum of the percentages of moisture, ash, protein, and lipid contents from 100 [48].
3.6. Functional Properties
The water absorption capacity (WAC) was measured according to the method described in Surendra Babu & Parimalavalli [16]. A total of 1 g of the EP starch sample was weighed into a pre-weighed centrifuge tube, and 10 mL of distilled water was added. The mixture was allowed to stand for 30 min and centrifuged at 3500× g for 15 min, and the supernatant was discarded. The tube was allowed to drain for 10 min at a 45° angle. Subsequently, the sample tube was weighed, and the gain in weight was used to calculate the WAC, which was expressed as g H2O/g starch.
The oil absorption capacity (OAC) was determined according to the method used by Bikila et al. [49]. A total of 1 g of the starch sample (m1) was immersed in 10 mL of soybean oil at room temperature, shaken to mix well, and then left for 30 min to reach the maximal absorption. Then, the mixture was centrifuged at 1811× g for 30 min, and the clear supernatant was decanted. Additionally, the sediment was weighed (m2). Finally, the OAC was calculated using Equation (2):
The starch swelling power (SP) and solubility were studied by the method used by Shimels et al. [50] and Kaur et al. [51], with a slight modification. A total of 1 g (dry basis) of the EP starch sample was mixed with 10 mL of distilled water in a centrifuge tube and heated in a water bath at 90 °C for 30 min. After heating, the suspension was centrifuged at 2200× g for 15 min. The supernatant was drawn off by suction and dried in an oven at 120 °C for 4 h. The solutes that remained after evaporation were weighed, and the solubility, expressed as a percentage, was calculated using Equation (3). The SP was calculated as the weight of the sediment paste per gram of starch, as stated in Equation (4).
3.7. Crystalline Structure and Average Crystallite Size of EP Starches
The crystalline structure of the starches was analyzed as described by Singh et al. [39] using an X-ray powder diffractometer (XRD-7000, Drawel, Drawel scientific instrument Co., Ltd., Shangai, China) equipped with a copper tube operating at 35 kV (25 mA), with CuKα radiation of a 0.154 nm wavelength. The samples were scanned from 5° to 75 (2θ) at a rate of 10°/min.
The crystallinity index (CI) was calculated as described by Singh et al. [39] from the ratio of the integrated area of all crystalline peaks to the total (crystalline plus amorphous) integrated area under the XRD peaks (Equation (5)).
3.8. Fourier Transform Infrared Analysis
The FTIR analysis was conducted following the procedure used by Awolu et al. [45]. The starch samples were treated with equal quantities of potassium bromide (KBr) salt, and each sample was pressed in KBr Salt Plates and transferred to the Fourier transform infrared spectrometer (Perkin-Elmer spectrometer, Liantrisant, UK) with Spectrum 10TM software, V.10.4.3. The range of the scanning wavenumber was 4000–450 cm−1.
3.9. Data Analysis
Analysis of Variance (ANOVA) was performed using the statistical software OriginPro 2019b, SAS version 9.0, and MS Excel 2016. Tukey’s multiple comparison test was used to compare the individual difference in the physicochemical properties of the starches. A 95% confidence interval (p ≤ 0.05) was considered.
4. Conclusions and Recommendation
In this study, two EP (Chanqua and Loffo ) ecotypes and three different starch isolation methods were evaluated to determine their effect on the physicochemical, functional, and structural properties of EP starches. The isolation method used had a significant (p < 0.05) impact on the yield, physicochemical properties (bulk density, aw, pH, color, moisture, protein, ash, and fiber), and functional properties (WAC, OAC, SP, and solubility). Similarly, the ecotype had a significant impact on the characteristics, with the exception of fat, SP, and solubility. The XRD pattern of all starches showed B-type diffraction, with a difference in the diffraction peak intensities between all starches, which could be attributed to the differences in the ecotype and/or isolation methods. The results from the FTIR tests confirmed differences in the location (wavenumber) and intensity of the infrared absorption peaks of all the samples, especially in sensitive regions, depending on the ecotype and isolation method of EP starches. Between the two EP ecotypes, Loffo exhibited considerably better results in terms of the starch yield, color/whiteness, and some other physicochemical properties. The SMS isolation method was the most effective in acquiring better EP starch characteristics in most of the parameters evaluated.
Author Contributions
Conceptualization, M.M., S.A.E., W.A. and F.R.; data curation, M.M. and S.A.E.; formal analysis, M.M. and W.A.; funding acquisition, M.M. and S.A.E.; investigation, M.M., W.A. and F.R.; methodology, M.M., W.A. and F.R.; resources, S.A.E.; supervision, S.A.E., W.A. and F.R.; validation, S.A.E.; visualization, W.A.; writing—original draft, M.M.; writing—review and editing, W.A. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Authors will avail data upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Alcázar-alay, S.C.; Angela, M.; Meireles, A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Dereje, B. Composition, morphology and physicochemical properties of starches derived from indigenous Ethiopian tuber crops: A review. Int. J. Biol. Macromol. 2021, 187, 911–921. [Google Scholar] [CrossRef]
- Egharevba, H.O. Chemical Properties of Starch and Its Application in the Food Industry; IntechOpen: Rijeka, Croatia, 2016; pp. 225–240. Available online: https://www.intechopen.com/chapters/68437 (accessed on 12 September 2023).
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Gulla, A.; Getachew, A.; Haile, T.G.; Molla, F. Evaluation of Acid-Modified Ethiopian Potato (Plectranthus edulis) Starch as Directly Compressible Tablet Excipient. BioMed Res. Int. 2020, 2020, 9325173. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, T.; Abera, G.; De Leyn, I.; Van der Meeren, P.; Dewettinck, K.; Eeckhout, M.; De Meulenaer, B.; Van Bockstaele, F. Composition, Granular Structure, and Pasting Properties of Native Starch Extracted from Plectranthus edulis (Oromo dinich) Tubers. J. Food Sci. 2017, 82, 2794–2804. [Google Scholar] [CrossRef]
- Taye, M.; Lommen, W.J.M.; Struik, P.C. Seasonal light interception, radiation use efficiency, growth and tuber production of the tuber crop Plectranthus edulis. Eur. J. Agron. 2013, 45, 153–164. [Google Scholar] [CrossRef]
- Gifty, A.G.; De Meulenaer, B.; Olango, T.M. Variation in tuber proximate composition, sugars, fatty acids and amino acids of eight Oromo dinich (Plectranthus edulis) landraces experimentally grown in Ethiopia. J. Food Compos. Anal. 2018, 67, 191–200. [Google Scholar] [CrossRef]
- Garedew, W.; Tsegaye, A.; Tesfaye, B.; Mohammed, H. Diversity analysis in Plectranthus edulis (Vatke) Agnew collection in Ethiopia. Int. J. Biodivers. Conserv. 2013, 5, 549–554. [Google Scholar]
- Geleta, G.A.; De Meulenaer, B. The effect of peeling and cooking processes on nutrient composition of Oromo dinich (Plectranthus edulis) tuber. Food Res. Int. 2019, 116, 387–396. [Google Scholar] [CrossRef]
- Assefa, A.; Belete, A.; Gebre-Mariam, T. Physicochemical Characterization of Starch Isolated From Ethiopian Potato (Plectranthus edulis). SINET Ethiop. J. Sci. 2016, 39, 11–20. [Google Scholar]
- Kim, J.; Ren, C.; Shin, M. Physicochemical properties of starch isolated from eight different varieties of Korean sweet potatoes. Starch-Stärke 2013, 65, 923–930. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Lin, L.; Bian, X.; Wei, C. Effects of variety and growing location on physicochemical properties of starch from sweet potato root tuber. Molecules 2021, 26, 7137. [Google Scholar] [CrossRef]
- Phogat, N.; Siddiqui, S.; Dalal, N.; Srivastva, A.; Bindu, B. Effects of varieties, curing of tubers and extraction methods on functional characteristics of potato starch. J. Food Meas. Charact. 2020, 14, 3434–3444. [Google Scholar] [CrossRef]
- Saleem, N.; Nidhi, S.; Anuradha, D.; Ashok, S.; Pathera, K. Physicochemical, morphological, functional, and pasting properties of potato starch as a function of extraction methods. J. Food Meas. Charact. 2021, 15, 2805–2820. [Google Scholar] [CrossRef]
- Babu, A.S.; Parimalavalli, R. Effect of Starch Isolation Method on Properties of Sweet Potato Starch. Ann. Univ. Dunarea Jos Galati. Fascicle VI Food Technol. 2014, 38, 48–63. [Google Scholar]
- Tessema, A.; Admassu, H. Extraction and characterization of starch from anchote (Coccinia abyssinica): Physico-chemical, functional, morphological and crystalline properties. J. Food Meas. Charact. 2021, 15, 3096–3110. [Google Scholar] [CrossRef]
- Xu, A.; Guo, K.; Liu, T.; Bian, X.; Zhang, L.; Wei, C. Effects of different isolation media on structural and functional properties of starches from root tubers of purple, yellow and white sweet potatoes. Molecules 2018, 23, 2135. [Google Scholar] [CrossRef]
- Chandra, R.A.I.; Hasanah, A.N.; Agustina, R. Optimization of Starch from Indonesian Local Corn with Concentration Variation of Sodium Metabisuphite and Drying Time. Int. J. Chem. Eng. Appl. 2016, 7, 89. [Google Scholar] [CrossRef]
- Kale, R.; Shere, D.M.; Sontakke, M.D.; Gadhe, K.S. Effect of isolation methods on physicochemical and functional properties of sweet potato (Ipomoea batatas L.) starch. J. Pharmacogn. Phytochem. 2017, 6, 37–41. [Google Scholar]
- Ocieczek, A.; Mesinger, D.; Toczek, H. Hygroscopic Properties of Three Cassava (Manihot esculenta Crantz) Starch Products: Application of BET and GAB Models. Foods 2022, 11, 1966. [Google Scholar] [CrossRef]
- Henney, J.E.; Taylor, C.L.; Boon, C.S. Taste and Flavor Roles of Sodium in Foods: A Unique Challenge to Reducing Sodium Intake. 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK50952/ (accessed on 20 May 2023).
- Awol, A.M.; Waghray, K.; Prabhakara, R.P.G.; Rudrayya, M.G. Characterizing Physicochemical Properties of Enset Starch. J. Text. Polym. 2020, 8, 43–52. [Google Scholar]
- Yang, Z.; Hao, H.; Wu, Y.; Liu, Y.; Ouyang, J. Influence of moisture and amylose on the physicochemical properties of rice starch during heat treatment. Int. J. Biol. Macromol. 2021, 168, 656–662. [Google Scholar] [CrossRef]
- Martínez, P.; Peña, F.; Bello-Pérez, L.A.; Núñez-Santiago, C.; Yee-Madeira, H.; Velezmoro, C. Physicochemical, functional and morphological characterization of starches isolated from three native potatoes of the Andean region. Food Chem. X 2019, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Tsakama, M.; Mwangwela, A.M.; Manani, T.A.; Mahungu, N.M. Physicochemical and pasting properties of starch extracted from eleven sweetpotato varieties. Afr. J. Food Sci. Technol. 2010, 1, 90–98. [Google Scholar]
- Julianti, E.; Rusmarilin, H.; Ridwansyah; Yusraini, E. Effect of Isolation Methods on Physicochemical Properties of Purple-fleshed Sweet Potato Starch. In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018)-Research in Industry, Medan, Indonesia, 30–31 August 2018; pp. 37–41. [Google Scholar] [CrossRef]
- Sembiring, A.T.B.; Nurminah, M.; Nainggolan, R.J. Effect of sodium metabisulphite concentration and salt concentration on the physicochemical properties of durian seed flour (Durio zibethinus Murr). IOP Conf. Ser. Earth Environ. Sci. 2020, 454, 012107. [Google Scholar] [CrossRef]
- Wang, J.; Guo, K.; Fan, X.; Feng, G.; Wei, C. Physicochemical properties of c-type starch from root tuber of apios fortunei in comparison with maize, potato, and pea starches. Molecules 2018, 23, 2132. [Google Scholar] [CrossRef] [PubMed]
- Wolde, Y.T.; Emire, S.A.; Abebe, W.; Ronda, F. Physicochemical, Morphological, Thermal, and Rheological Properties of Native Starches Isolated from Four Cultivars of Anchote (Coccinia abyssinica (Lam.) Cogn.) Tuber. Gels 2022, 8, 591. [Google Scholar] [CrossRef]
- Vanier, N.L.; Da Rosa Zavareze, E.; Pinto, V.Z.; Klein, B.; Botelho, F.T.; Dias, A.R.G.; Elias, M.C. Physicochemical, crystallinity, pasting and morphological properties of bean starch oxidised by different concentrations of sodium hypochlorite. Food Chem. 2012, 131, 1255–1262. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, H.; Shi, M. Effect of ethanol–water solution on the crystallization of short chain amylose from potato starch. Starch/Staerke 2016, 68, 683–690. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Pacia, M.Z.; Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Di Donato, F.; Di Cecco, V.; Torricelli, R.; D’Archivio, A.A.; Di Santo, M.; Albertini, E.; Veronesi, F.; Garramone, R.; Aversano, R.; Marcantonio, G.; et al. Discrimination of potato (Solanum tuberosum L.) accessions collected in majella national park (Abruzzo, italy) using mid-infrared spectroscopy and chemometrics combined with morphological and molecular analysis. Appl. Sci. 2020, 10, 1630. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Babu, A.S.; Parimalavalli, R.; Jagannadham, K.; Rao, J.S. Chemical and structural properties of sweet potato starch treated with organic and inorganic acid. J. Food Sci. Technol. 2015, 52, 5745–5753. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Singh, V.; Ali, S.Z.; Somashekar, R.; Mukherjee, P.S. Nature of crystallinity in native and acid modified starches. Int. J. Food Prop. 2006, 9, 845–854. [Google Scholar] [CrossRef]
- Oswal, M. Functional Characteristics of Starches from Indian Sweet Potato Cultivars. Int. J. Pure Appl. Biosci. 2019, 7, 80–85. [Google Scholar] [CrossRef]
- Azima, F.; Nazir, N.; Efendi, H.C. Characteristics of physico-chemical and functional properties of starch extracts from tubers. J. Phys. Conf. Ser. 2020, 1469, 012002. [Google Scholar] [CrossRef]
- Wijaya, C.; Do, Q.D.; Ju, Y.H.; Santoso, S.P.; Putro, J.N.; Laysandra, L.; Soetaredjo, F.E.; Ismadji, S. Isolation and characterization of starch from Limnophila aromatica. Heliyon 2019, 5, e01622. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, D.; Xue, J.; Yanniotis, S.; Mandala, I. The effect of salt concentration on swelling power, rheological properties and saltiness perception of waxy, normal and high amylose maize starch. Food Funct. 2017, 8, 3792–3802. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Singh, N. Relationships between selected properties of starches from different corn lines. Int. J. Food Prop. 2005, 8, 481–491. [Google Scholar] [CrossRef]
- Awolu, O.O.; Odoro, J.W.; Adeloye, J.B.; Lawal, O.M. Physicochemical evaluation and Fourier transform infrared spectroscopy characterization of quality protein maize starch subjected to different modifications. J. Food Sci. 2020, 85, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Molenda, M.; Horabik, J.; Mueller, P.; Opaliński, I. Mechanical properties of potato starch modified by moisture content and addition of lubricant Mechanical properties of potato starch modified by moisture content and addition of lubricant. Int. Agrophys. 2014, 28, 501–509. [Google Scholar] [CrossRef]
- Bitrus, J.; Amadi, O.C.; Nwagu, T.N.; Nnamchi, C.I.; Moneke, A.N. AACC Approved Methods of Analysis, Method 10-05.01. Guidelines for Measurement of Volume by Rapeseed Displacement. Food Nutr. Sci. 2001, 11, 1–4. Available online: http://methods.aaccnet.org/summaries/10-05-01.aspx (accessed on 5 June 2022).
- Dora, R.; Haron, H.; Shahar, S.; Phang, C.C.; Fauzi, M.F.M.; Noh, M.F.M. Macronutrients and sugar content in foods and beverages from three selected zones in Peninsular Malaysia. Sains Malays. 2018, 47, 1557–1562. [Google Scholar] [CrossRef]
- Bikila, A.M.; Tola, Y.B.; Esho, T.B.; Forsido, S.F.; Mijena, D.F. Starch composition and functional properties of raw and pretreated anchote (Coccinia abyssinica (Lam.) Cogn.) tuber flours dried at different temperatures. Food Sci. Nutr. 2022, 10, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Shimelis, E.A.; Meaza, M.; Rakshit, S.K.; Ababa, A. Physico-chemical properties, pasting behavior and functional characteristics of flours and starches from improved bean (Phaseolus vulgaris L.) varieties grown in East Africa. Agric. Eng. 2006, 8, 1–19. [Google Scholar]
- Kaur, M.; Oberoi, D.P.S.; Sogi, D.S.; Gill, B.S. Physicochemical, morphological and pasting properties of acid treated starches from different botanical sources. J. Food Sci. Technol. 2011, 48, 460–465. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).