Mechanochemical Synthesis of PdO2 Nanoparticles Immobilized over Silica Gel for Catalytic Suzuki–Miyaura Cross-Coupling Reactions Leading to the C-3 Modification of 1H-Indazole with Phenylboronic Acids
Abstract
1. Introduction
2. Results and Discussion
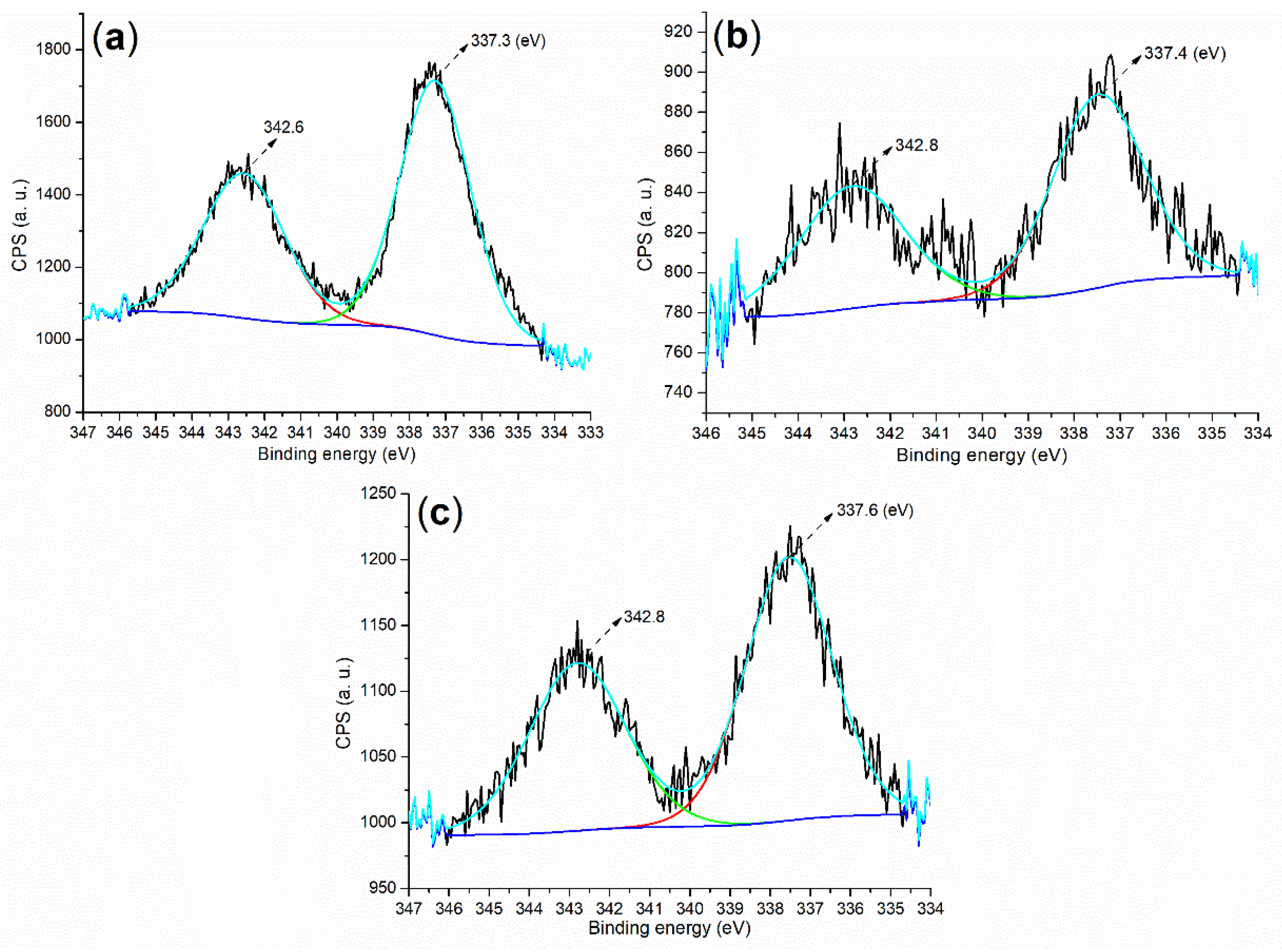
2.1. Elemental Composition and Chemical State on Surface of Catalyst
2.2. Textural and Other Properties of Synthesized Catalyst
2.3. Functional Group and Thermal Stability of Synthesized Catalyst
2.4. Morphology and Internal Structure of Synthesized Catalyst
2.5. Catalytic Suzuki–Miyaura Cross-Coupling Reaction
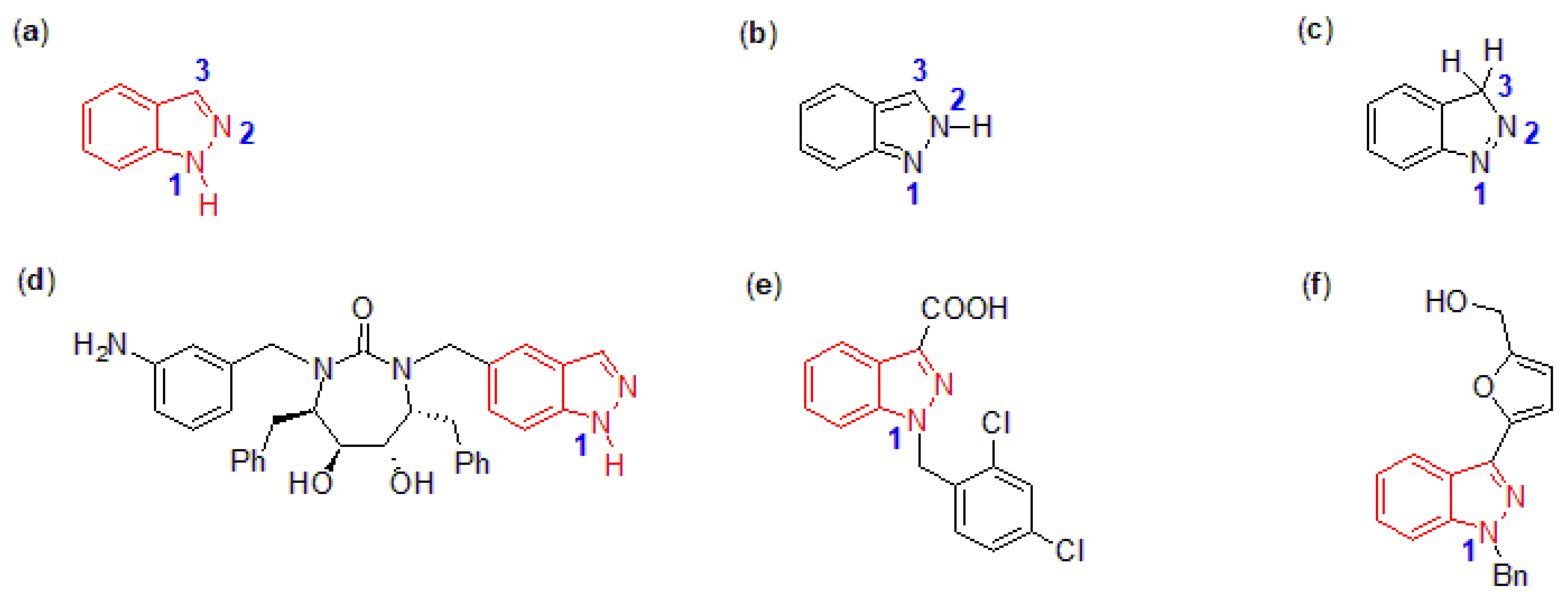
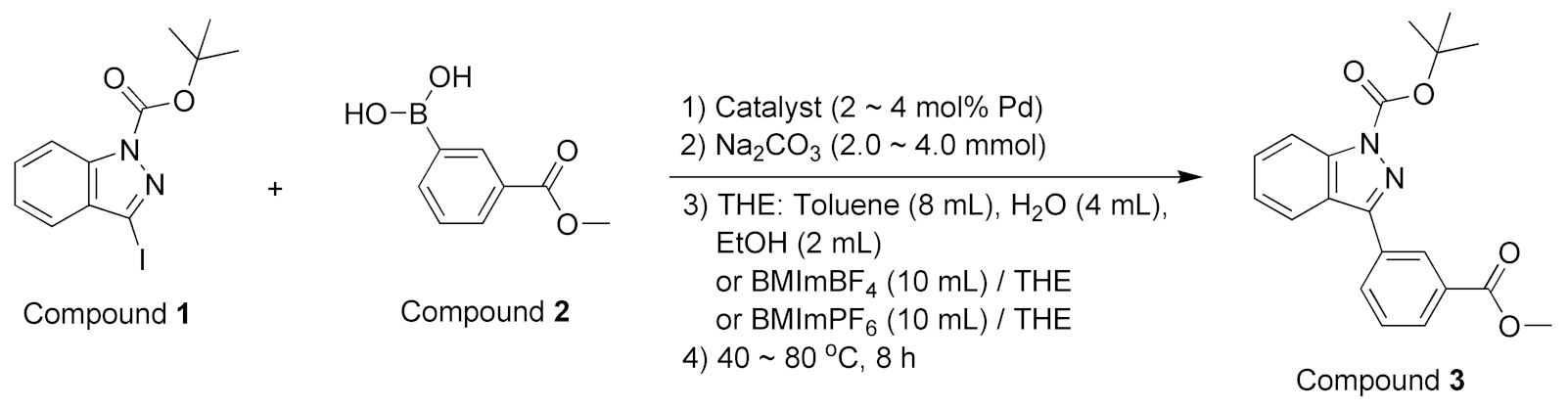
2.5.1. Preparation of Iodinated 1H-indazole Substrate
2.5.2. Reactions Using Two Substrates
2.5.3. Screening of Catalysts
2.5.4. Effects of Loading Amounts of Catalyst and Base
2.5.5. Effect of Temperature
2.5.6. Effect of Ionic Liquid
2.5.7. Effect of Catalyst Recycling
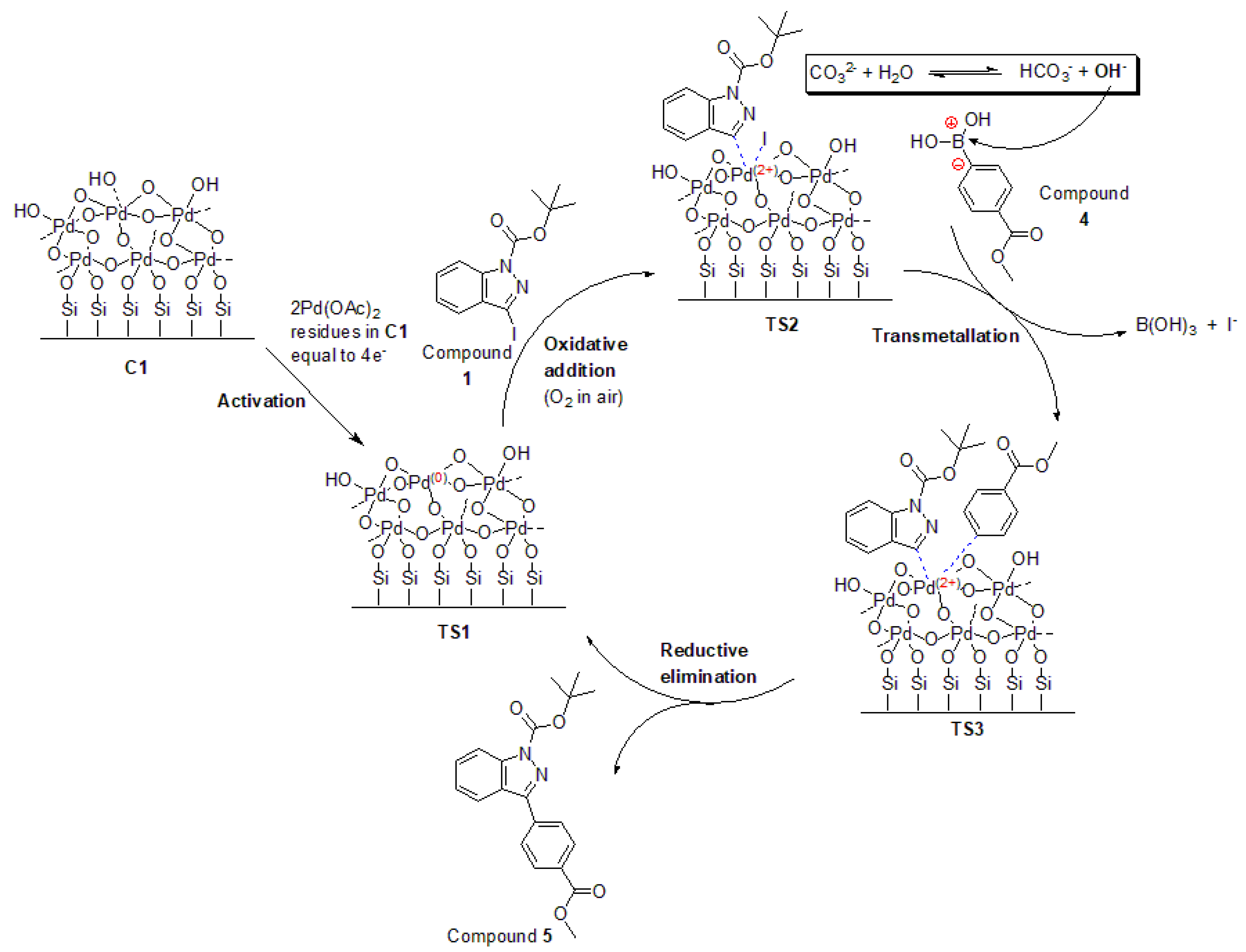
2.6. Proposed Mechanism for Heterogeneous Suzuki–Miyaura Cross-Coupling
3. Experimental Section
3.1. Starting Materials
3.2. Synthetic and Analytical Instruments
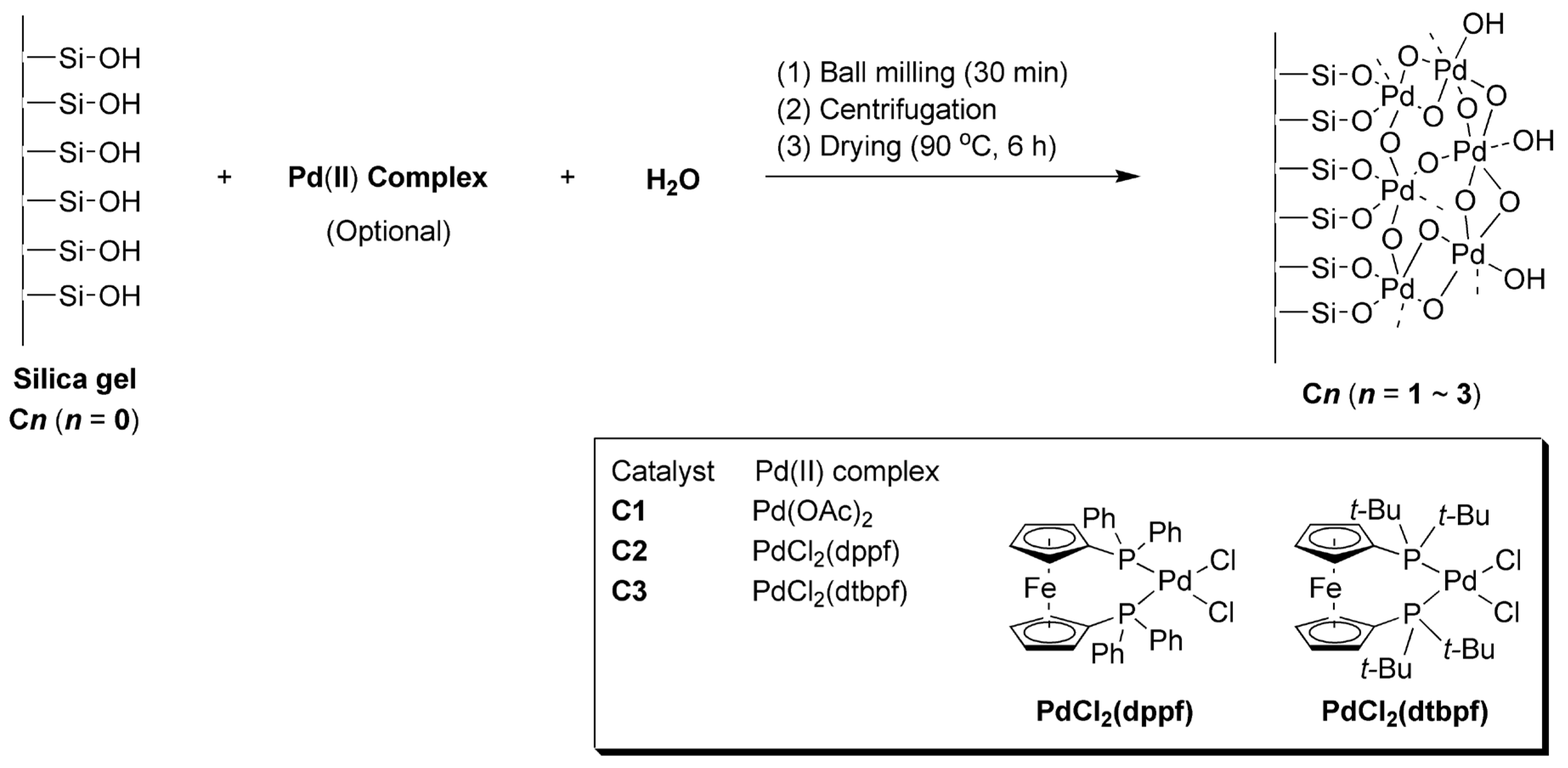
3.3. Synthesis of Catalysts
3.4. Synthesis of Tert-butyl-3-iodo-1H-indazole-1-carboxylate (Compound 1)
3.5. Catalytic Suzuki–Miyaura Cross-Coupling Reaction
3.5.1. Non-Ionic Liquid-Facilitated Reaction and Catalyst Recycling
3.5.2. Ionic Liquid-Facilitated Reaction and Catalyst Recycling
3.6. Characterization of Tert-butyl-3-(3-(methoxycarbonyl)phenyl)-1H-indazole-1-carboxylate (Compound 3)
3.7. Characterization of Tert-butyl-3-(4-(methoxycarbonyl)phenyl)-1H-indazole-1- carboxylate (Compound 5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Collot, V.; Dallemagne, P.; Bovy, P.R.; Rault, S. Suzuki-type cross-coupling reaction of 3-iodoindazoles with aryl boronic acids: A general and flexible route to 3-arylindazoles. Tetrahedron 1999, 50, 6917–6922. [Google Scholar] [CrossRef]
- Büchel, G.E.; Kossatz, S.; Sadique, A.; Rapta, P.; Zalibera, M.; Bucinsky, L.; Komorovsky, S.; Telser, J.; Eppinger, J.; Reiner, T.; et al. cis-Tetrachlorido-bis(indazole)osmium(IV) and its osmium(III) analogues: Paving the way towards the cis-isomer of the ruthenium anticancer drugs KP1019 and/or NKP1339. Dalton Trans. 2017, 46, 11925–11941. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, J.-T.; Pan, C. Recent advances in C–H functionalization of 2H-indazoles. Org. Biomol. Chem. 2022, 20, 7746–7764. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Rodgers, J.D.; McHugh, R.J., Jr.; Johnson, B.L.; Cordova, B.C.; Klabe, R.M.; Bacheler, L.T.; Erickson-Viitanen, S.; Ko, S.S. Unsymmetrical cyclic ureas as HIV-1 protease inhibitors: Novel biaryl indazoles as P2/P2’ substituents. Bioorg. Med. Chem. Lett. 1999, 9, 3217–3220. [Google Scholar] [CrossRef]
- Sharma, R.; Yadav, L.; Yadav, R.K.; Chaudhary, S. Oxidative cross-dehydrogenative coupling (CDC) via C(sp2)–H bond functionalization: Tert-butyl peroxybenzoate (TBPB)-promoted regioselective direct C-3 acylation/benzoylation of 2H-indazoles with aldehydes/benzyl alcohols/styrenes. RSC Adv. 2021, 11, 14178–14192. [Google Scholar] [CrossRef]
- Hattori, K.; Yamaguchi, K.; Yamaguchi, J.; Itami, K. Pd- and Cu-catalyzed C–H arylation of indazole. Tetrahedron 2012, 68, 7605–7612. [Google Scholar] [CrossRef]
- Cheng, B.; Bao, B.; Zu, B.; Duan, X.; Duan, S.; Li, Y.; Zhai, H. Synthesis of spiro-3H-indazoles via 1,3-dipolar cycloaddition of arynes with 6-diazocyclohex-2-en-1-one derivatives and fused-2H-indazoles by subsequent rearrangement. RSC Adv. 2017, 7, 54087–54090. [Google Scholar] [CrossRef]
- Chevalier, A.; Ouahrouch, A.; Arnaud, A.; Gallavardin, T.; Franck, X. An optimized procedure for direct access to 1H-indazole-3-carboxaldehyde derivatives by nitrosation of indoles. RSC Adv. 2018, 8, 13121–13128. [Google Scholar] [CrossRef]
- Alaime, T.; Daniel, M.; Hiebel, M.-A.; Pasquinet, E.; Suzenet, F.; Guillaumet, G. Access to 1H-indazoles, 1H-benzoindazoles and 1H-azaindazoles from (het)aryl azides: A one-pot Staudinger-aza-Wittig reaction leading to N–N bond formation? Chem. Commun. 2018, 54, 8411–8414. [Google Scholar] [CrossRef]
- Chen, C.; Tang, G.; He, F.; Wang, Z.; Jing, H.; Faessler, R. A synthesis of 1H-indazoles via a Cu(OAc)2-catalyzed N–N bond formation. Org. Lett. 2016, 18, 1690–1693. [Google Scholar] [CrossRef]
- Ben-Yahia, A.; Naas, M.; Kazzouli, S.E.; Essassi, E.M.; Guillaumet, G. Direct C-3-arylations of 1H-indazoles. Eur. J. Org. Chem. 2012, 2012, 7075–7081. [Google Scholar] [CrossRef]
- Collot, V.; Varlet, D.; Rault, S. Heck cross-coupling reaction of 3-iodoindazoles with methyl acrylate: A mild and flexible strategy to design 2-azatryptamines. Tetrahedron Lett. 2000, 41, 4363–4366. [Google Scholar] [CrossRef]
- Han, F.-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Ohtaka, A.; Teratani, T.; Fujii, R.; Ikeshita, K.; Shimomura, O.; Nomura, R. Facile preparation of linear polystyrene-stabilized Pd nanoparticles in water. Chem. Commun. 2009, 14, 7188–7190. [Google Scholar] [CrossRef]
- Borkowski, T.; Zawartka, W.; Pospiech, P.; Mizerska, U.; Trzeciak, A.M.; Cypryk, M.; Tylus, W. Reusable functionalized polysiloxane-supported palladium catalyst for Suzuki-Miyaura cross-coupling. J. Catal. 2011, 282, 270–277. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura cross-coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Z.; Liu, T.; Lü, J.; Lin, Z.; Li, H.; Cao, R. Palladium nanoparticles supported on amino functionalized metal-organic frameworks as highly active catalysts for the Suzuki-Miyaura cross-coupling reaction. Catal. Commun. 2011, 14, 27–31. [Google Scholar] [CrossRef]
- Soomro, S.S.; Röhlich, C.; Köhler, K. Suzuki coupling reactions in pure water catalyzed by supported palladium–relevance of the surface polarity of the support. Adv. Synth. Catal. 2011, 353, 767–775. [Google Scholar] [CrossRef]
- Muratsugu, S.; Maity, N.; Baba, H.; Tasaki, M.; Tada, M. Preparation and catalytic performance of a molecularly imprinted Pd complex catalyst for Suzuki cross-coupling reactions. Dalton Trans. 2017, 46, 3125–3134. [Google Scholar] [CrossRef]
- Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Solid-state Suzuki–Miyaura cross-coupling reactions: Olefin-accelerated C–C coupling using mechanochemistry. Chem. Sci. 2019, 10, 8202–8210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Shi, S.; He, Q.; He, X.; Gan, T.; Ji, H. Highly efficient fabrication of kilogram-scale palladium single-atom catalysts for the Suzuki–Miyaura cross-coupling reaction. ACS Appl. Mater. Interfaces 2022, 14, 53755–53760. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Siamaki, A.R.; Lin, Y.; Woodberry, K.; Connell, J.W.; Gupton, B.F. Palladium nanoparticles supported on carbon nanotubes from solventless preparations: Versatile catalysts for ligand-free Suzuki cross coupling reactions. J. Mater. Chem. A 2013, 1, 12909–12918. [Google Scholar] [CrossRef]
- Yang, Y.; Gilliland III, S.E.; Ghobadi, S.; Burkholder, M.; Smith, S.E.; Gupton, B.F.; Castano, C.E. Three dimensional composites of graphene as supports in Pd-catalyzed synthetic applications. React. Chem. Eng. 2019, 4, 90–99. [Google Scholar] [CrossRef]
- Ji, S.; Lu, X.; Zhang, M.; Leng, L.; Liu, H.; Yin, K.; Xu, C.; He, C.; Horton, J.H.; Zhang, J.; et al. Construction of a single-atom palladium catalyst by electronic metal-support interaction and interface confinement effect with remarkable performance in Suzuki coupling reaction. Chem. Eng. J. 2023, 452, 139205. [Google Scholar] [CrossRef]
- Seo, T.; Kubota, K.; Ito, H. Mechanochemistry-directed ligand design: Development of a high-performance phosphine ligand for palladium-catalyzed mechanochemical organoboron cross-coupling. J. Am. Chem. Soc. 2023, 145, 6823–6837. [Google Scholar] [CrossRef]
- Yu, H.; Hu, J.; Fan, J.; Chang, J. One-pot conversion of sugars and lignin in ionic liquid and recycling of ionic liquid. Ind. Eng. Chem. Res. 2012, 51, 3452–3457. [Google Scholar] [CrossRef]
- Parker, H.L.; Dodson, J.R.; Budarin, V.L.; Clark, J.H.; Hunt, A.J. Direct synthesis of Pd nanoparticles on alginic acid and seaweed supports. Green Chem. 2015, 17, 2200–2207. [Google Scholar] [CrossRef]
- Hu, L.; Huang, X.; Nie, Q.; Wang, T.; Liu, P.; Liu, J.; Tan, Z.; Yu, H. Single Pd atoms supported on ultra-thin bismuth tungstate nanosheets with oxygen vacancies as an efficient photocatalyst. J. Mater. Chem. A 2023, 11, 1723–1733. [Google Scholar] [CrossRef]
- Wang, H.; Gregorczyk, K.E.; Lee, S.B.; Rubloff, G.W.; Lin, C.-F. Li-containing organic thin film-structure of lithium propane dioxide via molecular layer deposition. J. Phys. Chem. C 2020, 124, 6830–6837. [Google Scholar] [CrossRef]
- Hess, C.; Tzolova-Mu1ller, G.; Herbert, R. The Influence of water on the dispersion of vanadia supported on silica SBA-15: A combined XPS and Raman study. J. Phys. Chem. C 2007, 111, 9471–9479. [Google Scholar] [CrossRef]
- Ma, J.; Wu, Y.; Pan, Q.; Wang, X.; Li, X.; Li, Q.; Xu, X.; Yao, Y.; Sun, Y. The Al-containing silicates modified with organic ligands and SnO2 nanoparticles for catalytic Baeyer-Villiger oxidation and aerobic carboxylation of carbonyl compounds. Nanomaterials 2023, 13, 433. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, X.; Liu, D.; Hao, S.; Du, G.; Ma, Y.; Asiri, A.M.; Sun, X.; Chen, L. Mn Doping of CoP Nanosheets Array: An Efficient Electrocatalyst for Hydrogen Evolution Reaction with Enhanced Activity at All pH Values. ACS Catal. 2017, 7, 98–102. [Google Scholar] [CrossRef]
- Ren, H.; Qi, F.; Labidi, A.; Allam, A.A.; Ajarem, J.S.; Bahnemann, D.W.; Wang, C. Turning Agroforestry Waste into Value-Added Fluorescent Carbon Quantum Dots for Effective Detection of Fe 3+ in an Aqueous Environment. ACS EST Eng. 2023, 3, 260–270. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Hiyoshi, N. Nanocrystalline sodalite: Preparation and application to epoxidation of 2-cyclohexen-1-one with hydrogen peroxide. Appl. Catal. A Gen. 2012, 419–420, 164–169. [Google Scholar] [CrossRef]
- Zenou, V.Y.; Bakardjieva, S. Microstructural analysis of undoped and moderately Sc-doped TiO 2 anatase nanoparticles using Scherrer equation and Debye function analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Gao, Z.R.; Balestra, S.R.G.; Gómez-Hortigüela, L.; Li, J.; Márquez-Alvarez, C.; Camblor, M.A. Dication containing three aromatic ring structure-directs toward a chiral zeolite, spans three cavities, and effectively traps water. Chem. Mater. 2022, 34, 3197–3205. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, A.; Pan, Q.; Wu, Y.; Wang, X.; Li, X.; Wang, W.; Gao, M.; Sun, Y. The synthesis of Sn-containing silicates coated with binaphthol and their specific application for catalytic synthesis of 6-hydroxyhexanoic acid and cyclohexylformate through Baeyer-Villiger oxidation. Catalysts 2023, 13, 805. [Google Scholar] [CrossRef]
- Minaev, B.; Baryshnikov, G.; Agren, H. Principles of phosphorescent organic light emitting devices. Phys. Chem. Chem. Phys. 2014, 16, 1719–1758. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.; De, S.K.; Chen, L.-H.; Riel-Mehan, M.; Emdadi, A.; Cellitti, J.; Stebbins, J.L.; Rega, M.F.; Pellecchia, M. Development of paramagnetic probes for molecular recognition studies in protein kinases. J. Med. Chem. 2008, 51, 3460–3465. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Zheng, W.; Ding, L.; Wang, Y. Computational study on C–B homolytic bond dissociation enthalpies of organoboron compounds. New J. Chem. 2017, 41, 1346–1362. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, A.; Jin, L.; Wu, Y.; Pan, Q.; Wang, X.; Li, X.; Wang, W.; Gao, M.; Sun, Y. The C-3 Functionalization of 1H-indazole through Suzuki–Miyaura cross-coupling catalyzed by a ferrocene-based divalent palladium complex immobilized over ionic liquid, as well as theoretical insights into the reaction mechanism. Appl. Sci. 2023, 13, 4095. [Google Scholar] [CrossRef]
- Mathews, C.J.; Smith, P.J.; Welton, T. Palladium catalysed Suzuki cross-coupling reactions in ambient temperature ionic liquids. Chem. Commun. 2000, 1249–1250. [Google Scholar] [CrossRef]
- Shibata, K.; Kiyoura, T.; Kitagawa, J.; Sumiyoshi, T.; Tanabe, K. Acidic properties of binary metal oxides. Bull. Chem. Soc. Jpn. 1973, 46, 2985–2988. [Google Scholar] [CrossRef]
- Bocchi, V.; Palla, G. High yield selective bromination and iodination of indoles in N,N-dimethylformamide. Synthesis 1982, 1982, 1096–1097. [Google Scholar] [CrossRef]
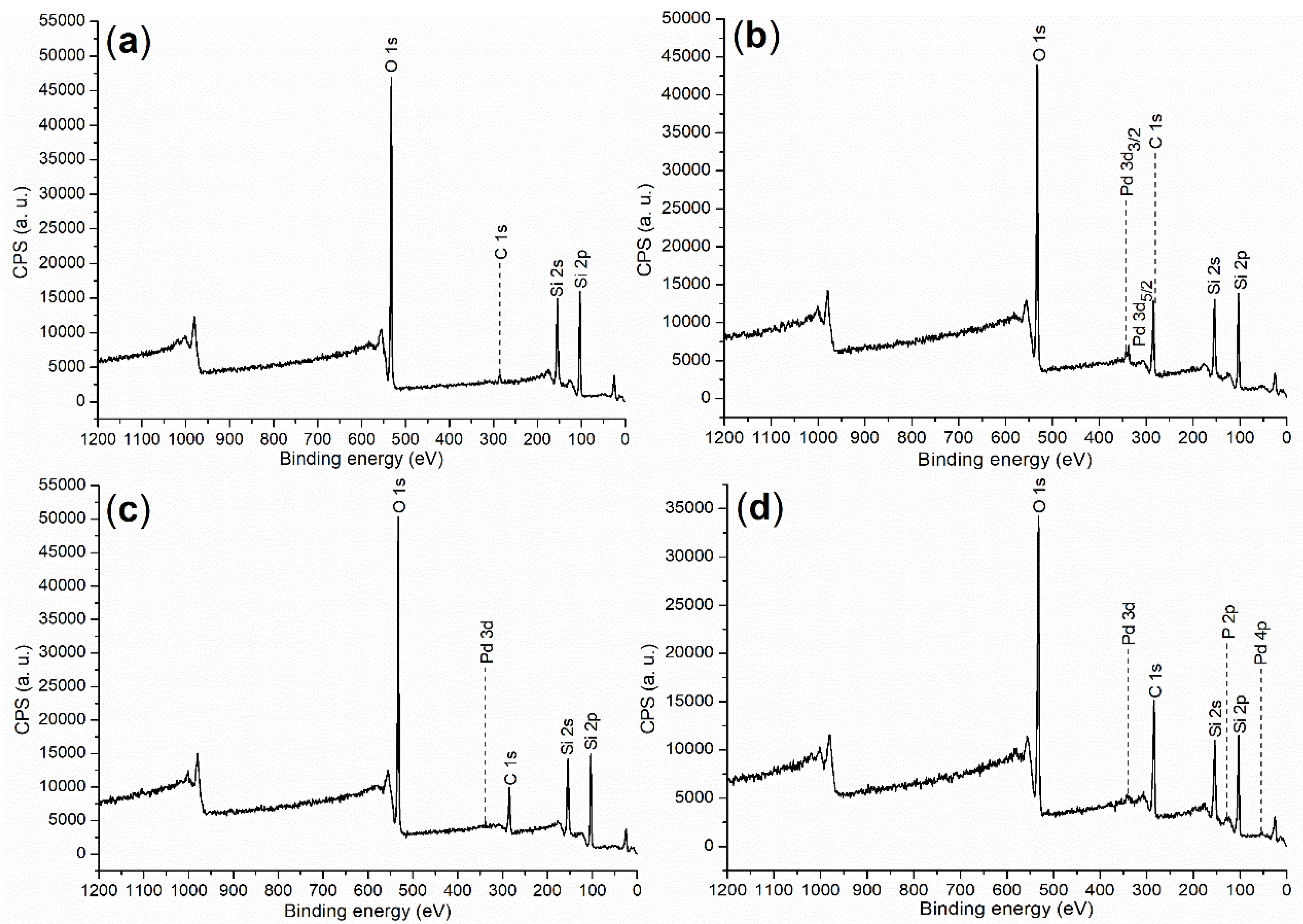
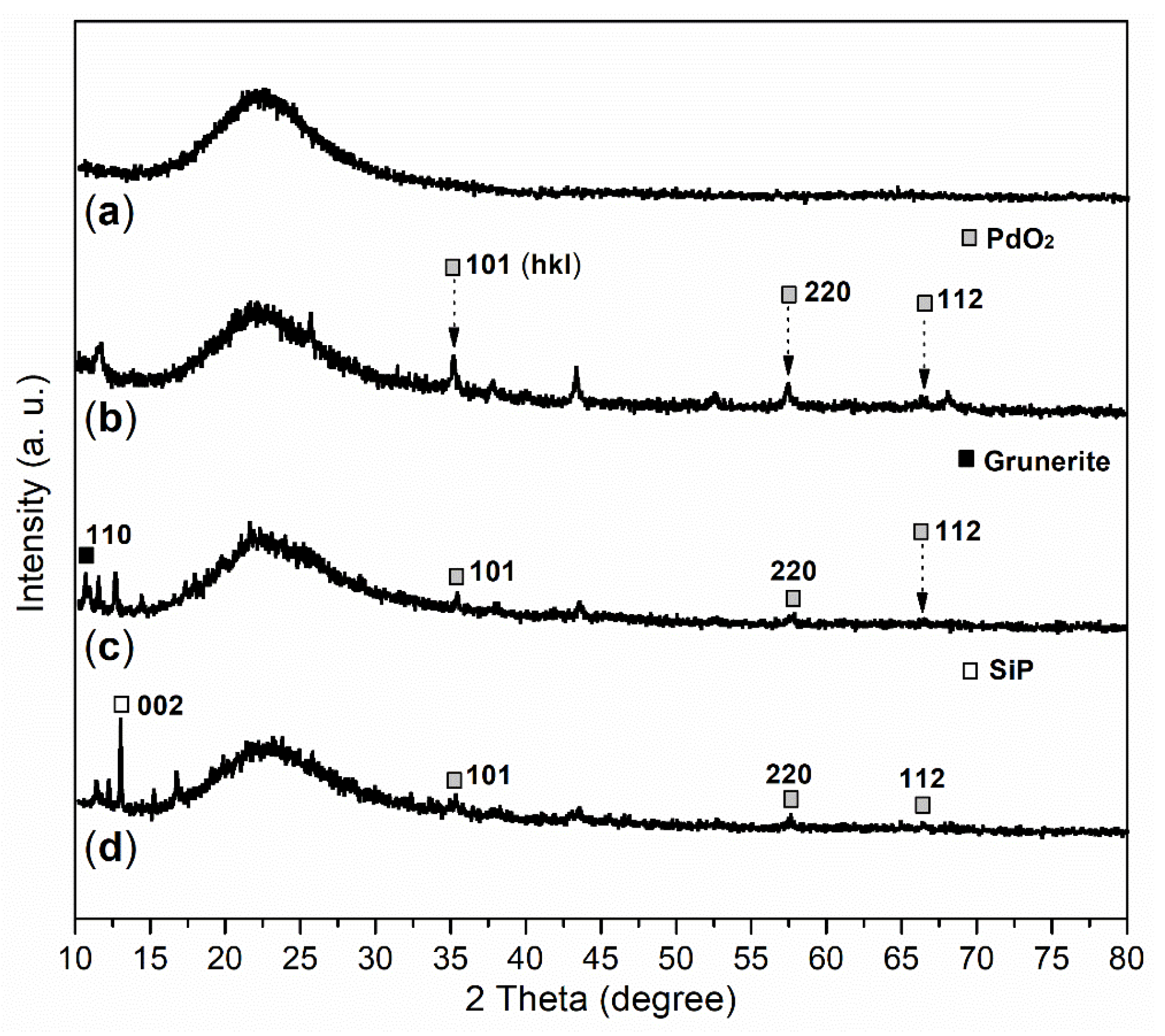
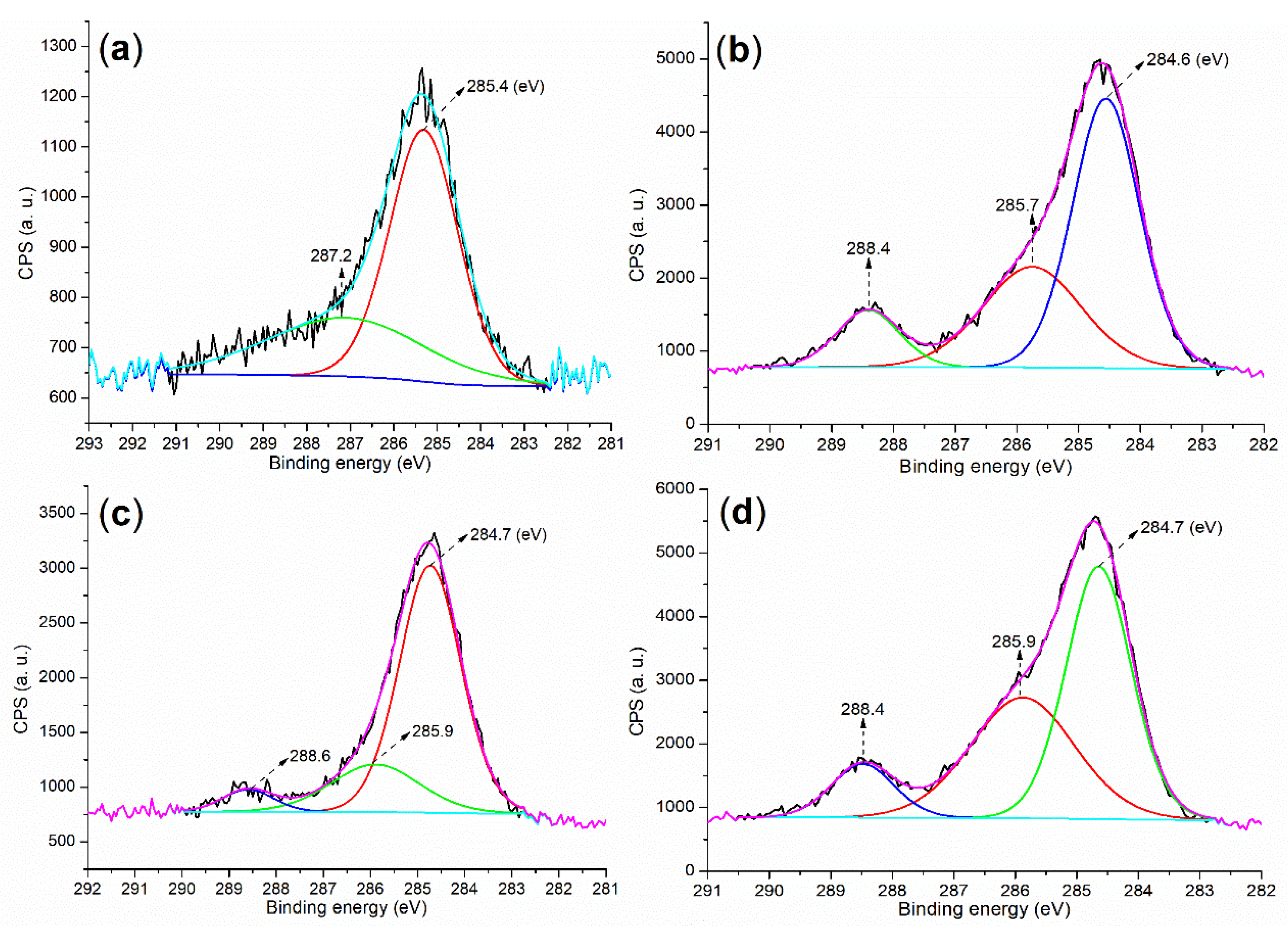
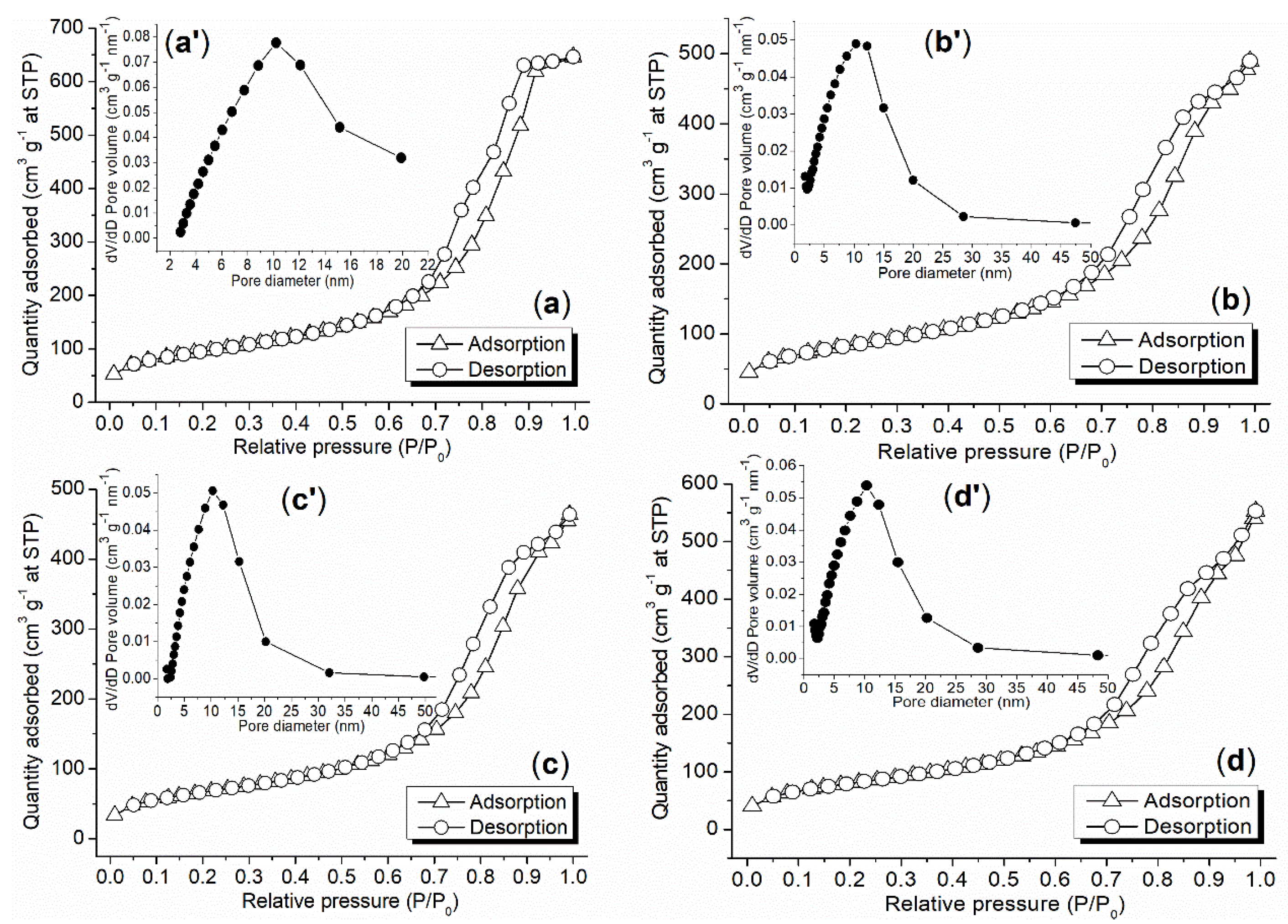
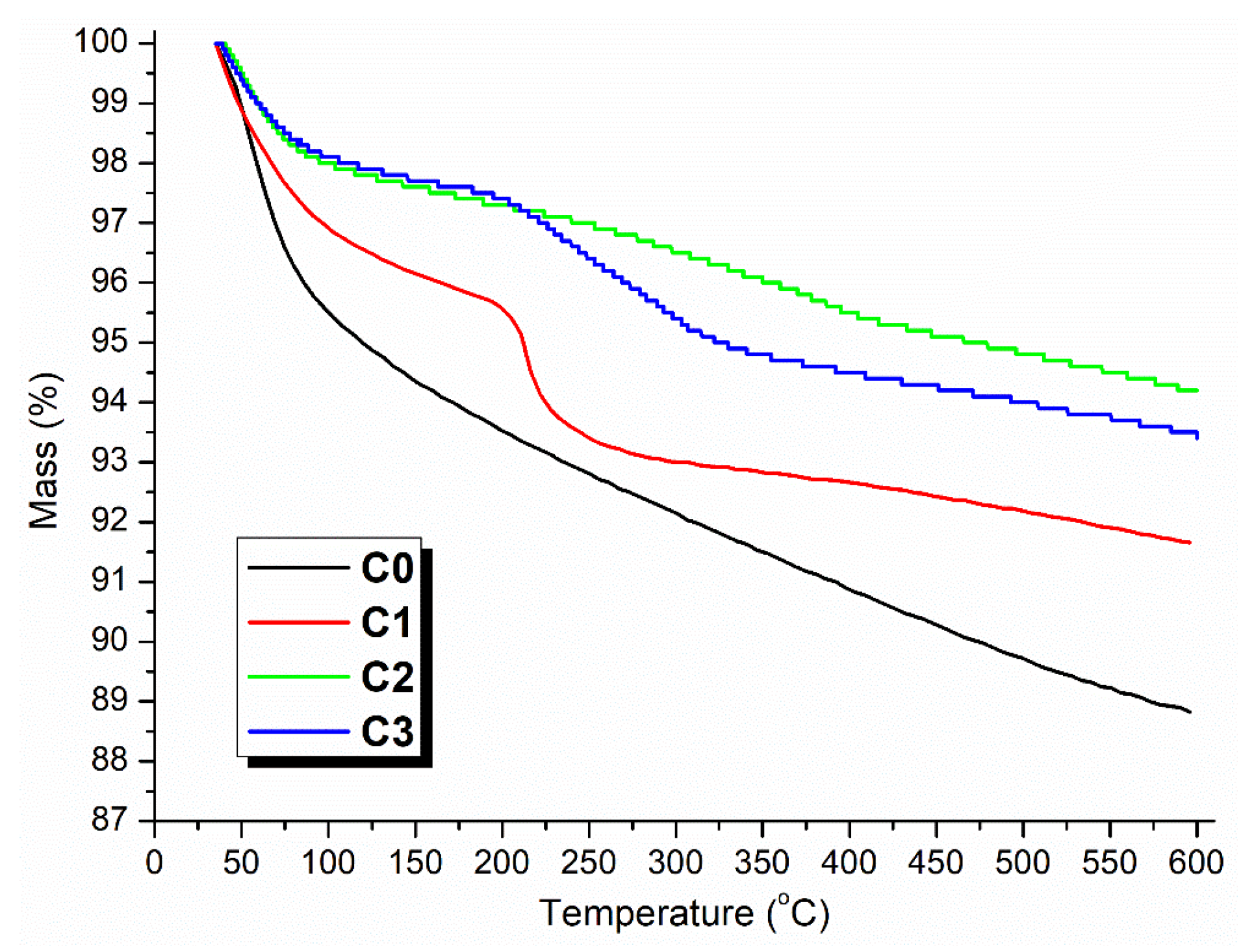
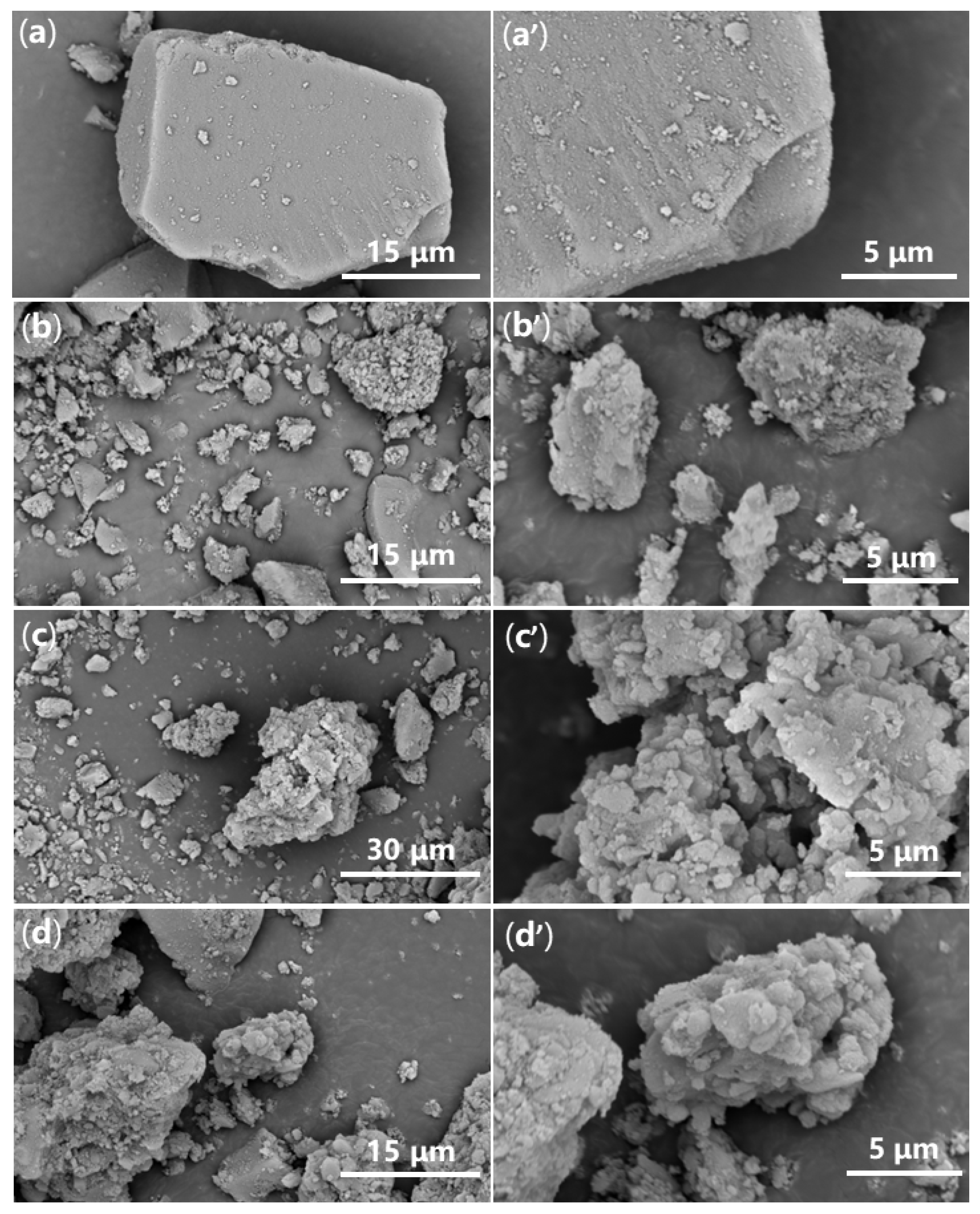
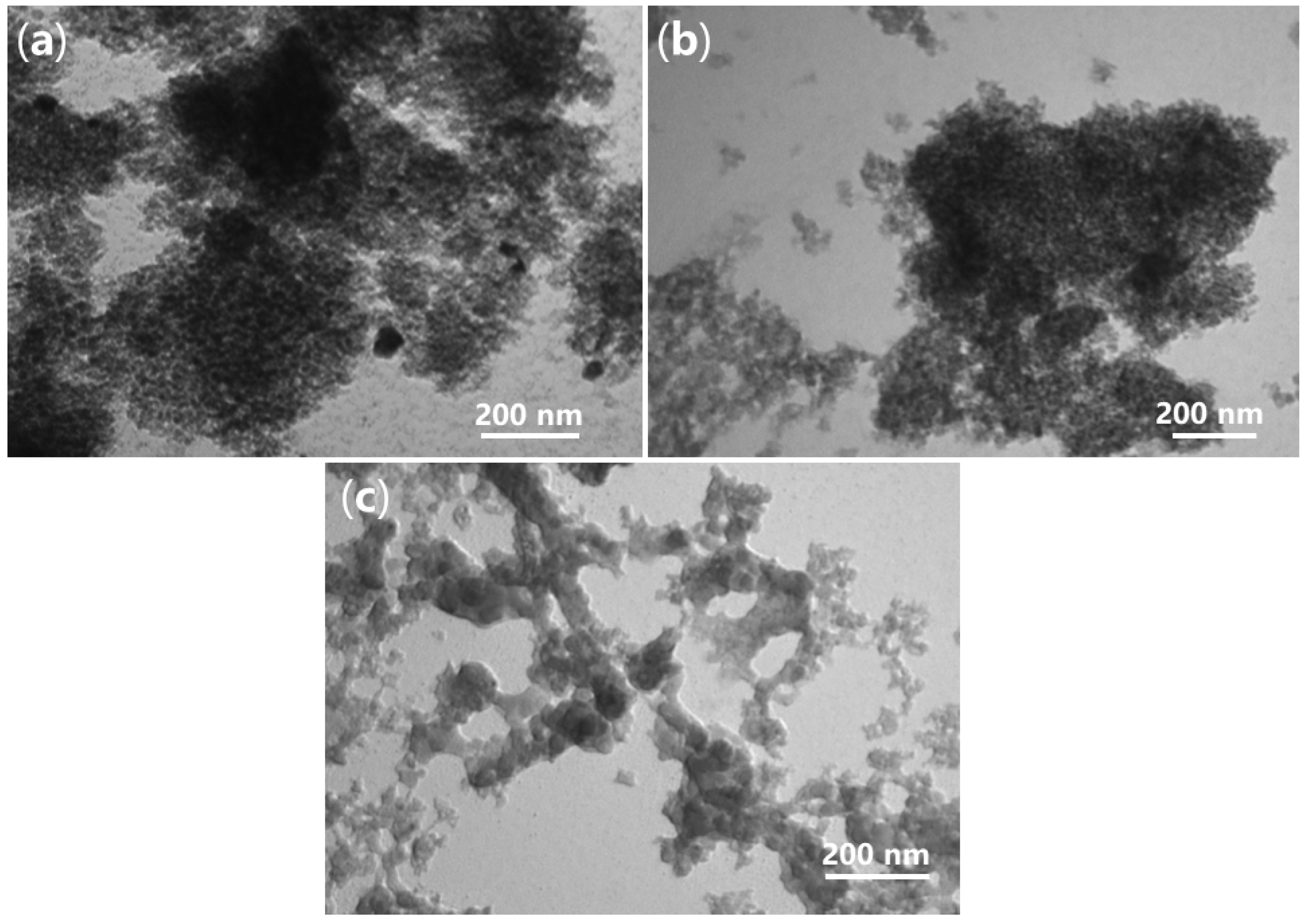


| Sample | C (1s) | O (1s) | Si (2p) | Pd (3d) | P (2p) |
|---|---|---|---|---|---|
| C0 | 284.80 (4.27) a | 532.80 (48.01) | 103.80 (47.72) | -b | - |
| C1 | 284.80 (27.50) | 532.80 (38.78) | 102.80 (33.18) | 336.80 (0.55) | - |
| C2 | 284.80 (19.19) | 532.80 (42.93) | 102.80 (37.48) | 336.80 (0.40) | - |
| C3 | 284.80 (31.08) | 532.80 (33.49) | 102.80 (29.35) | 334.80 (0.36) | 123.80 (5.72) |
| Sample | SBET | PV | PVmicro | Pore Size | ρ | dS | dSEM | dPdO2 | Acid Amount |
|---|---|---|---|---|---|---|---|---|---|
| (m2 g−1) a | (cm3 g−1) b | (cm3 g−1) c | (nm) d | (g cm−3) e | (nm) f | (μm) g | (nm) h | (mmol g−1) i | |
| C0 | 336 | 0.96 | 0.01 | 10.5 | 0.41 | 43 | 40 | not found | 0.40 |
| C1 | 295 | 0.75 | 0.004 | 9.9 | 0.54 | 37 | 5 | 25 | 0.72 |
| C2 | 238 | 0.72 | 0.002 | 11 | 0.76 | 33 | 2 | 38 | 0.54 |
| C3 | 288 | 0.86 | 0 | 11 | 0.70 | 29 | 10 | 66 | 0.58 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry a | Catalyst b | Loading (mol%) c | Na2CO3 (mmol) | Solvent(/Co-Solvent) | T (°C) | Conversion (%) c | Yield (%) d |
| 1 | C0 | as Si a | 4 | THE | 80 | 0 | 0 |
| 2 | as Si a | 4 | THE/BMImBF4 | 80 | 0 | 0 | |
| 3 | Pd(OAc)2 | 4 | 4 | THE | 80 | 32 | 32 |
| 4 | C1 | 4 | 4 | THE | 80 | 91 | 91 |
| 5 | 2 | 4 | THE | 80 | 93 | 93 | |
| 6 | 4 | 2 | THE | 80 | 86 | 86 | |
| 7 | 4 | 4 | THE/BMImBF4 | 80 | 6 | 6 | |
| 8 | 4 | 4 | THE/BMImPF6 | 80 | 41 | 41 | |
| 9 | 4 | 4 | THE | 60 | 72 | 72 | |
| 10 | 4 | 4 | THE | 40 | 62 | 62 | |
| 11 | PdCl2(dppf) | 4 | 4 | THE | 80 | 77 | 77 |
| 12 | C2 | 4 | 4 | THE | 80 | 85 | 85 |
| 13 | 4 | 4 | THE | 60 | 86 | 86 | |
| 14 | 4 | 4 | THE/BMImBF4 | 80 | 40 | 40 | |
| 15 | 4 | 4 | THE/BMImPF6 | 80 | 35 | 35 | |
| 16 | PdCl2(dtbpf) | 4 | 4 | THE | 80 | 76 | 76 |
| 17 | C3 | 4 | 4 | THE | 80 | 88 | 88 |
| 18 | 4 | 4 | THE/BMImBF4 | 80 | 45 | 45 | |
| 19 | 4 | 4 | THE/BMImPF6 | 80 | 58 | 58 | |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry a | Catalyst b | Loading (mol%) c | Na2CO3 (mmol) | Solvent(/Co-Solvent) | T (°C) | Conversion (%) c | Yield (%) d |
| 1 | C0 | as Si a | 4 | THE | 80 | 0 | 0 |
| 2 | as Si a | 4 | THE/BMImBF4 | 80 | 0 | 0 | |
| 3 | Pd(OAc)2 | 4 | 4 | THE | 80 | 26 | 26 |
| 4 | C1 | 4 | 4 | THE | 80 | 93 | 93 |
| 5 | 2 | 4 | THE | 80 | 92 | 92 | |
| 6 | 4 | 2 | THE | 80 | 87 | 87 | |
| 7 | 4 | 4 | THE/BMImBF4 | 80 | 13 | 13 | |
| 8 | 4 | 4 | THE/BMImPF6 | 80 | 40 | 40 | |
| 9 | 4 | 4 | THE | 60 | 75 | 75 | |
| 10 | 4 | 4 | THE | 40 | 70 | 70 | |
| 11 | PdCl2(dppf) | 4 | 4 | THE | 80 | 86 | 86 |
| 12 | C2 | 4 | 4 | THE | 80 | 88 | 88 |
| 13 | 4 | 4 | THE | 60 | 89 | 89 | |
| 14 | 4 | 4 | THE/BMImBF4 | 80 | 45 | 45 | |
| 15 | 4 | 4 | THE/BMImPF6 | 80 | 38 | 38 | |
| 16 | PdCl2(dtbpf) | 4 | 4 | THE | 80 | 85 | 85 |
| 17 | C3 | 4 | 4 | THE | 80 | 92 | 92 |
| 18 | 4 | 4 | THE/BMImBF4 | 80 | 50 | 50 | |
| 19 | 4 | 4 | THE/BMImPF6 | 80 | 62 | 62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Wu, Y.; Zheng, A.; Wang, X.; Li, X.; Wang, W.; Gao, M.; Bibi, Z.; Chaudhary, S.; Sun, Y. Mechanochemical Synthesis of PdO2 Nanoparticles Immobilized over Silica Gel for Catalytic Suzuki–Miyaura Cross-Coupling Reactions Leading to the C-3 Modification of 1H-Indazole with Phenylboronic Acids. Molecules 2023, 28, 7190. https://doi.org/10.3390/molecules28207190
Pan Q, Wu Y, Zheng A, Wang X, Li X, Wang W, Gao M, Bibi Z, Chaudhary S, Sun Y. Mechanochemical Synthesis of PdO2 Nanoparticles Immobilized over Silica Gel for Catalytic Suzuki–Miyaura Cross-Coupling Reactions Leading to the C-3 Modification of 1H-Indazole with Phenylboronic Acids. Molecules. 2023; 28(20):7190. https://doi.org/10.3390/molecules28207190
Chicago/Turabian StylePan, Qin, Yong Wu, Aqun Zheng, Xiangdong Wang, Xiaoyong Li, Wanqin Wang, Min Gao, Zainab Bibi, Sidra Chaudhary, and Yang Sun. 2023. "Mechanochemical Synthesis of PdO2 Nanoparticles Immobilized over Silica Gel for Catalytic Suzuki–Miyaura Cross-Coupling Reactions Leading to the C-3 Modification of 1H-Indazole with Phenylboronic Acids" Molecules 28, no. 20: 7190. https://doi.org/10.3390/molecules28207190
APA StylePan, Q., Wu, Y., Zheng, A., Wang, X., Li, X., Wang, W., Gao, M., Bibi, Z., Chaudhary, S., & Sun, Y. (2023). Mechanochemical Synthesis of PdO2 Nanoparticles Immobilized over Silica Gel for Catalytic Suzuki–Miyaura Cross-Coupling Reactions Leading to the C-3 Modification of 1H-Indazole with Phenylboronic Acids. Molecules, 28(20), 7190. https://doi.org/10.3390/molecules28207190







