Detection of Various Traditional Chinese Medicinal Metabolites as Angiotensin-Converting Enzyme Inhibitors: Molecular Docking, Activity Testing, and Surface Plasmon Resonance Approaches
Abstract
1. Introduction
2. Results
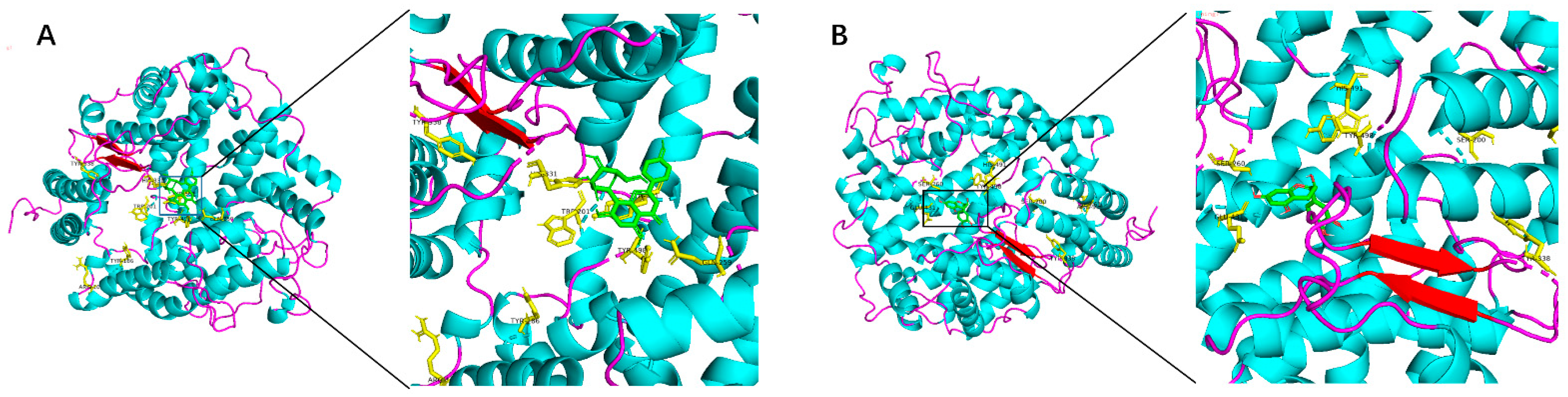
2.1. Molecular Docking Analysis
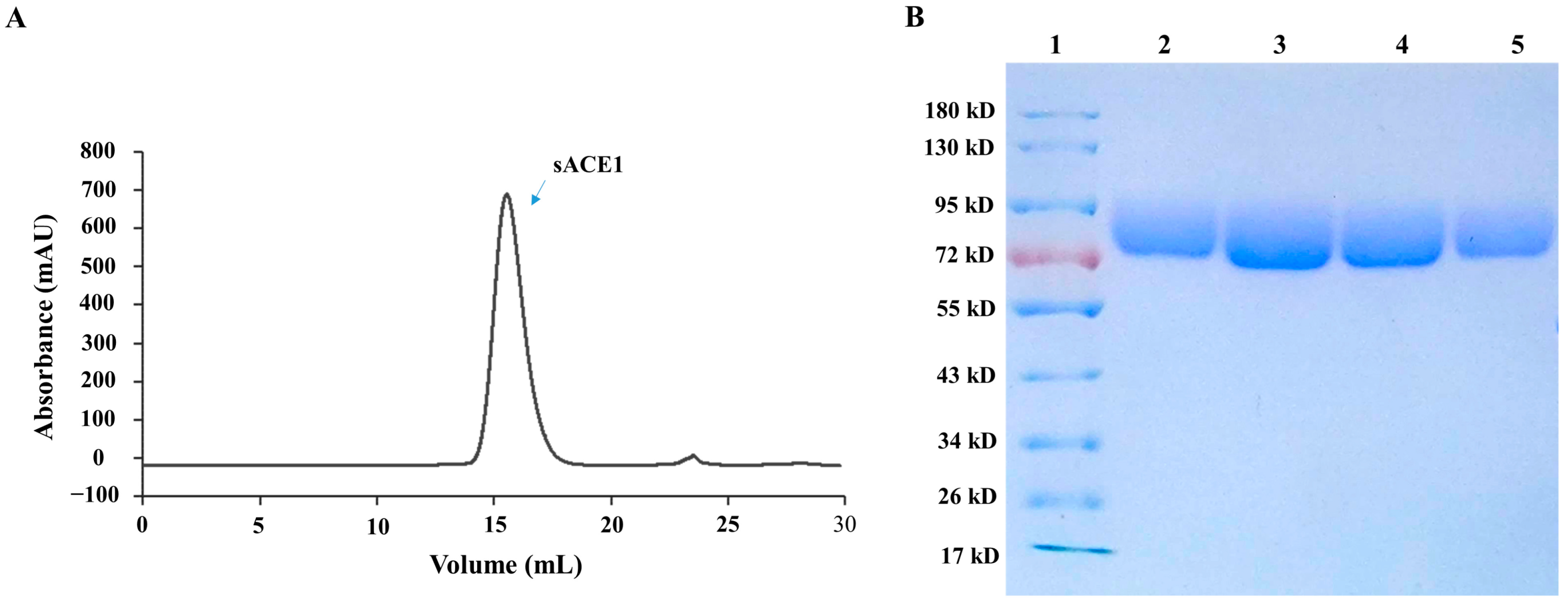
2.2. Preparation of the Recombinant sACE1 Protein
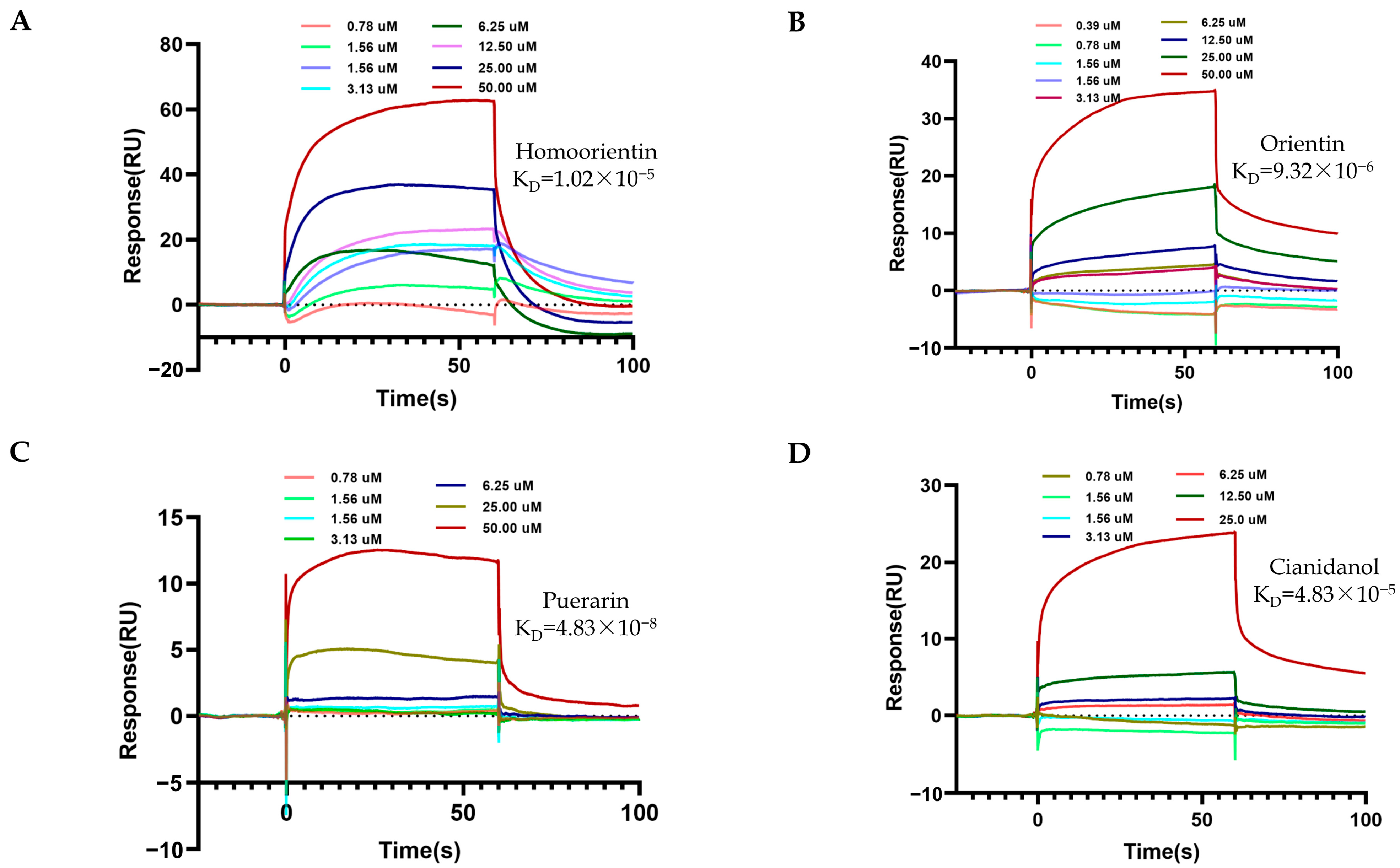
2.3. SPR Determination between TCM Metabolites and sACE1
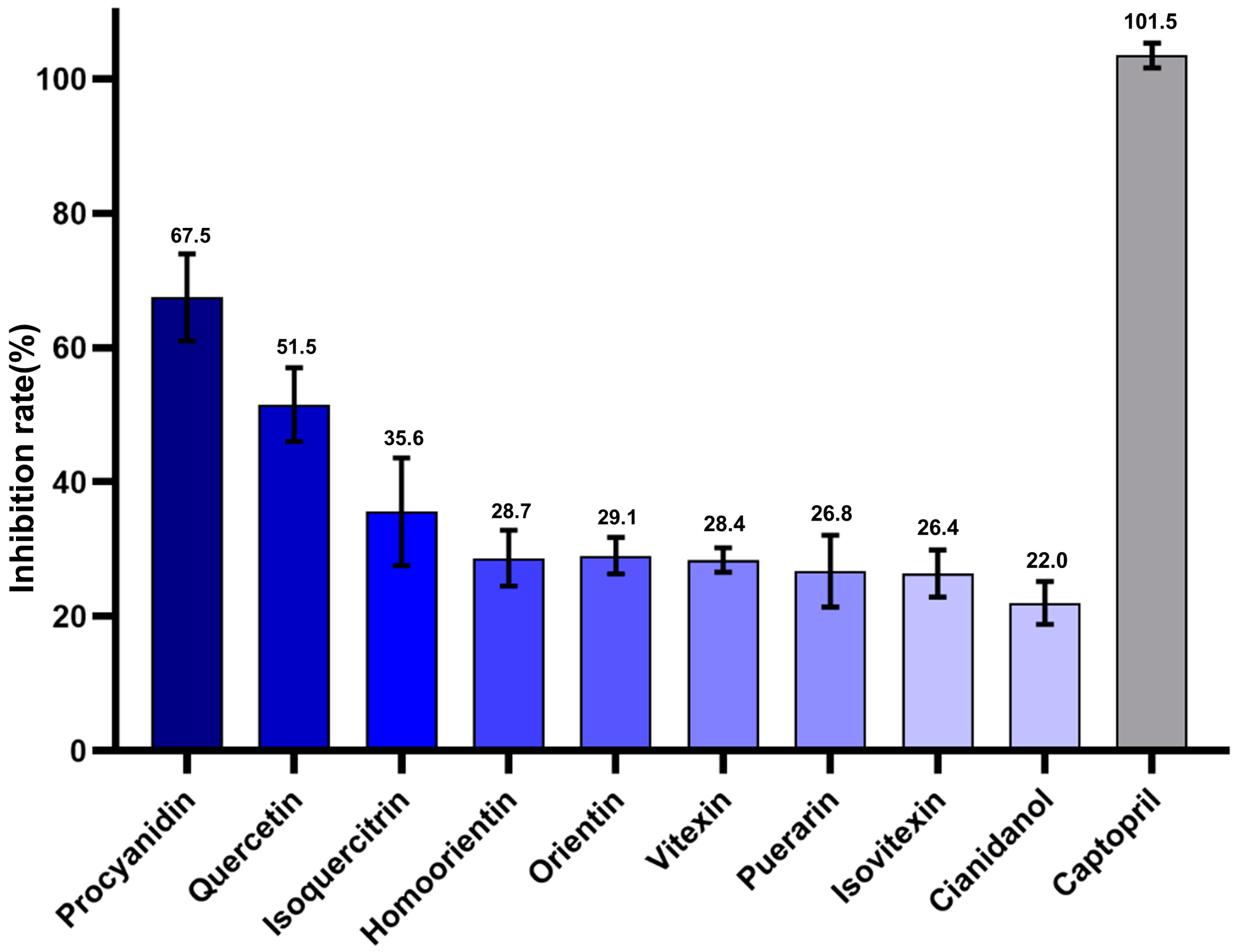
2.4. ACE1 Inhibitory Activity of Various Candidate TCM Metabolites
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Instruments and Materials
4.2. Molecular Docking Simulation
4.3. Construction of the Expression Plasmids
4.4. Expression and Purification of sACE1 Recombinant Protein
4.5. SPR Sensor Measurements
4.6. ACE Inhibition Measurement
4.7. Analysis of Inhibition Rate Data
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A



References
- Carey, R.M.; Moran, A.E.; Whelton, P.K. Treatment of Hypertension. JAMA 2022, 328, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.V.; Gil Lorenzo, A.F.; Bocanegra, V.; Vallés, P.G. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan through Hsp70. Cells 2021, 10, 3146. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A. Hypertension and the Brain: A Risk Factor for More Than Heart Disease. Cerebrovasc. Dis. 2016, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Bell, S.; Symes, R.; Vaze, A. Hypertensive eye disease: A review. Clin. Exp. Ophthalmol. 2017, 45, 45–53. [Google Scholar] [CrossRef]
- Desai, A.N. High Blood Pressure. JAMA 2020, 324, 1254–1255. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.J. Hypertension. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Kuboki, K.; Jiang, Z.Y.; Takahara, N.; Ha, S.W.; Igarashi, M.; Yamauchi, T.; Feener, E.P.; Herbert, T.P.; Rhodes, C.J.; King, G.L. Regulation of Endothelial Constitutive Nitric Oxide Synthase Gene Expression in Endothelial Cells and In Vivo. Circulation 2000, 101, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Linz, W.; Wohlfart, P.; Schölkens, B.A.; Malinski, T.; Wiemer, G. Interactions among ACE, kinins and NO. Cardiovasc. Res. 1999, 43, 549–561. [Google Scholar] [CrossRef]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The Renin-Angiotensin-Aldosterone System in Vascular Inflammation and Remodeling. Int. J. Inflamm. 2014, 2014, 689360. [Google Scholar] [CrossRef] [PubMed]
- Shanks, J.; Ramchandra, R. Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension. Int. J. Mol. Sci. 2021, 22, 12305. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.F. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003, 4, 225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baudin, B. New Aspects on Angiotensin-Converting Enzyme: From Gene to Disease. Clin. Chem. Lab. Med. 2002, 40, 256–265. [Google Scholar] [CrossRef]
- Sleight, P. The role of angiotensinconverting enzyme inhibitors in the treatment of hypertension. Curr. Cardiol. Rep. 2001, 3, 511–518. [Google Scholar] [CrossRef]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef]
- Manoharan, S.; Shuib, A.S.; Abdullah, N. Structural Characteristics and Antihypertensive Effects of Angiotensin-I-Converting Enzyme Inhibitory Peptides in the Renin-Angiotensin and Kallikrein Kinin Systems. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 383–406. [Google Scholar] [CrossRef]
- Bezalel, S.; Mahlab-Guri, K.; Asher, I.; Werner, B.; Sthoeger, Z.M. Angiotensin-converting Enzyme Inhibitor-induced Angioedema. Am. J. Med. 2015, 128, 120–125. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Seong, S.H.; Jung, H.A.; Choi, J.S. Angiotensin-I-Converting Enzyme Inhibitory Activity of Coumarins from Angelica decursiva. Molecules 2019, 24, 3937. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [PubMed]
- Olszanecki, R.; Bujak-Gizycka, B.; Madej, J.; Suski, M.; Wołkow, P.P.; Jawień, J.; Korbut, R. Kaempferol, but not resveratrol inhibits angiotensin converting enzyme. J. Physiol. Pharmacol. 2008, 59, 387–392. [Google Scholar]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.-S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef]
- Junior, A.G.; Gasparotto, F.M.; Lourenço, E.L.B.; Crestani, S.; Stefanello, M.E.A.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef]
- Kwon, E.-K.; Lee, D.-Y.; Lee, H.; Kim, D.-O.; Baek, N.-I.; Kim, Y.-E.; Kim, H.-Y. Flavonoids from the Buds of Rosa damascene Inhibit the Activity of 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase and Angiotensin I-Converting Enzyme. J. Agric. Food Chem. 2010, 58, 882–886. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Actisgoretta, L.; Villordo, J.J.; Fraga, C.G. Procyanidin structure defines the extent and specificity of angiotensin I converting enzyme inhibition. Biochimie 2006, 88, 359–365. [Google Scholar] [CrossRef]
- Kang, D.G.; Oh, H.; Chung, H.T.; Lee, H.S. Inhibition of angiotensin converting enzyme by lithospermic acid B isolated from radixSalviae miltiorrhiza Bunge. Phytother. Res. 2003, 17, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Bolaji-Alabi, F.B.; Ajibade, T.O.; Adejumobi, O.A.; Ajani, O.S.; Jarikre, T.A.; Omobowale, T.O.; Ola-Davies, O.E.; Soetan, K.O.; Aro, A.O.; et al. Novel antihypertensive action of rutin is mediated via inhibition of angiotensin converting enzyme/mineralocorticoid receptor/angiotensin 2 type 1 receptor (ATR1) signaling pathways in uninephrectomized hypertensive rats. J. Food Biochem. 2020, 44, e13534. [Google Scholar] [CrossRef] [PubMed]
- Al Shukor, N.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-Converting Enzyme Inhibitory Effects by Plant Phenolic Compounds: A Study of Structure Activity Relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Toson, N.S.; Pimentel, M.C.; Bordignon, S.A.; Mendez, A.S.; Henriques, A.T. Polyphenols composition from leaves of Cuphea spp. and inhibitor potential, in vitro, of angiotensin I-converting enzyme (ACE). J. Ethnopharmacol. 2020, 255, 112781. [Google Scholar] [CrossRef]
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, High-Throughput Assay for Screening Angiotensin I-Converting Enzyme Inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef]
- Ahmad, I.; Yanuar, A.; Mulia, K.; Mun’Im, A. Review of angiotensin-converting enzyme inhibitory assay: Rapid method in drug discovery of herbal plants. Pharmacogn. Rev. 2017, 11, 1–7. [Google Scholar] [CrossRef]
- de Almeida, F.G.; Vanzolini, K.L.; Cass, Q.B. Angiotensin converting enzyme immobilized on magnetic beads as a tool for ligand fishing. J. Pharm. Biomed. Anal. 2017, 132, 159–164. [Google Scholar] [CrossRef]
- Piliarik, M.; Vaisocherová, H.; Homola, J. Surface Plasmon Resonance Biosensing. Methods Mol. Biol. 2009, 503, 65–88. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef]
- Stigter, E.; de Jong, G.; van Bennekom, W. Coupling surface-plasmon resonance and mass spectrometry to quantify and to identify ligands. TrAC Trends Anal. Chem. 2013, 45, 107–120. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, Y.-W.; Kim, T.-H. Recent Advances in Surface Plasmon Resonance Sensors for Sensitive Optical Detection of Pathogens. Biosensors 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Barderas, R.; Pingarrón, J.M.; Campuzano, S.; Merino, S. Surface plasmon resonance immunosensor for ErbB2 breast cancer biomarker determination in human serum and raw cancer cell lysates. Anal. Chim. Acta 2016, 905, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Safina, G. Application of surface plasmon resonance for the detection of carbohydrates, glycoconjugates, and measurement of the carbohydrate-specific interactions: A comparison with conventional analytical techniques. A critical review. Anal. Chim. Acta 2012, 712, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Devireddy, R.; Gartia, M.R. Surface Plasmon Resonance (SPR) Sensor for Cancer Biomarker Detection. Biosensors 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Minunni, M.; Bilia, A.R. SPR in Drug Discovery: Searching Bioactive Compounds in Plant Extracts. Methods Mol. Biol. 2009, 572, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.K.R. Systematic screening of viral entry inhibitors using surface plasmon resonance. Rev. Med. Virol. 2017, 27, 131–145. [Google Scholar] [CrossRef]
- Yakes, B.; Buijs, J.; Elliott, C.; Campbell, K. Surface plasmon resonance biosensing: Approaches for screening and characterising antibodies for food diagnostics. Talanta 2016, 156–157, 55–63. [Google Scholar] [CrossRef]
- Navratilova, I.; Besnard, J.; Hopkins, A.L. Screening for GPCR Ligands Using Surface Plasmon Resonance. ACS Med. Chem. Lett. 2011, 2, 549–554. [Google Scholar] [CrossRef]
- Patil, S.; Sistla, S.; Jadhav, J. Screening of Inhibitors for Mushroom Tyrosinase Using Surface Plasmon Resonance. J. Agric. Food Chem. 2014, 62, 11594–11601. [Google Scholar] [CrossRef]
- Olaru, A.; Bala, C.; Jaffrezic-Renault, N.; Aboul-Enein, H.Y. Surface Plasmon Resonance (SPR) Biosensors in Pharmaceutical Analysis. Crit. Rev. Anal. Chem. 2015, 45, 97–105. [Google Scholar] [CrossRef]
- Riedel, T.; Majek, P.; Rodriguez-Emmenegger, C.; Brynda, E. Surface plasmon resonance: Advances of label-free approaches in the analysis of biological samples. Bioanalysis 2014, 6, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, L.; Sewell, B.T.; Sturrock, E.D. The influence of angiotensin converting enzyme mutations on the kinetics and dynamics of N-domain selective inhibition. FEBS J. 2016, 283, 3941–3961. [Google Scholar] [CrossRef] [PubMed]
- Polakovičová, M.; Jampílek, J. Advances in Structural Biology of ACE and Development of Domain Selective ACE-inhibitors. Med. Chem. 2019, 15, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-S.; Doerksen, R.J.; Chen, S.-H.; Sung, W.-C.; Juan, C.-C.; Rawendra, R.D.; Chen, C.-R.; Li, J.-W.; Aisha; Huang, T.-C.; et al. Screening and profiling stilbene-type natural products with angiotensin-converting enzyme inhibitory activity from Ampelopsis brevipedunculata var. hancei (Planch.) Rehder. J. Pharm. Biomed. Anal. 2015, 108, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
- Trettel, G.; Bertoncini, C.R.A.; Lima-Landman, M.T. The mechanisms of calcium mobilization by procyanidins, flavonols and flavonoids from Cecropia glaziovii Sneth in pulmonary endothelial cell cultures endorse its popular use as vasodilator phytomedicine. Biomed. Pharmacother. 2021, 144, 112231. [Google Scholar] [CrossRef]
- Parellada, J.; Suárez, G.; Guinea, M. Inhibition of Zinc Metallopeptidases by Flavonoids and Related Phenolic Compounds: Structure-Activity Relationships. J. Enzym. Inhib. 1998, 13, 347–359. [Google Scholar] [CrossRef]
- Xiong, Y.; Shepherd, S.; Tibbs, J.; Bacon, A.; Liu, W.; Akin, L.D.; Ayupova, T.; Bhaskar, S.; Cunningham, B.T. Photonic Crystal Enhanced Fluorescence: A Review on Design Strategies and Applications. Micromachines 2023, 14, 668. [Google Scholar] [CrossRef]
- Choudhury, S.D.; Badugu, R.; Lakowicz, J.R. Directing Fluorescence with Plasmonic and Photonic Structures. Accounts Chem. Res. 2015, 48, 2171–2180. [Google Scholar] [CrossRef]
- Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Radiative decay engineering 4. Experimental studies of surface plasmon-coupled directional emission. Anal. Biochem. 2004, 324, 170–182. [Google Scholar] [CrossRef]
- Cheerala, V.S.K.; Ganesh, K.M.; Bhaskar, S.; Ramamurthy, S.S.; Neelakantan, S.C. Smartphone-Based Attomolar Cyanide Ion Sensing Using Au-Graphene Oxide Cryosoret Nanoassembly and Benzoxazolium-Based Fluorophore in a Surface Plasmon-Coupled Enhanced Fluorescence Interface. Langmuir 2023, 39, 7939–7957. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, Z.; Wang, J.; Wu, D. Green tea EGCG inhibits naïve CD4+ T cell division and progression in mice: An integration of network pharmacology, molecular docking and experimental validation. Curr. Res. Food Sci. 2023, 7, 100537. [Google Scholar] [CrossRef]
- Sharma, G.P.; Fish, B.L.; Frei, A.C.; Narayanan, J.; Gasperetti, T.; Scholler, D.; Pierce, L.; Szalewski, N.; Blue, N.; Medhora, M.; et al. Pharmacologic ACE-Inhibition Mitigates Radiation-Induced Pneumonitis by Suppressing ACE-Expressing Lung Myeloid Cells. Int. J. Radiat. Oncol. 2022, 113, 177–191. [Google Scholar] [CrossRef] [PubMed]
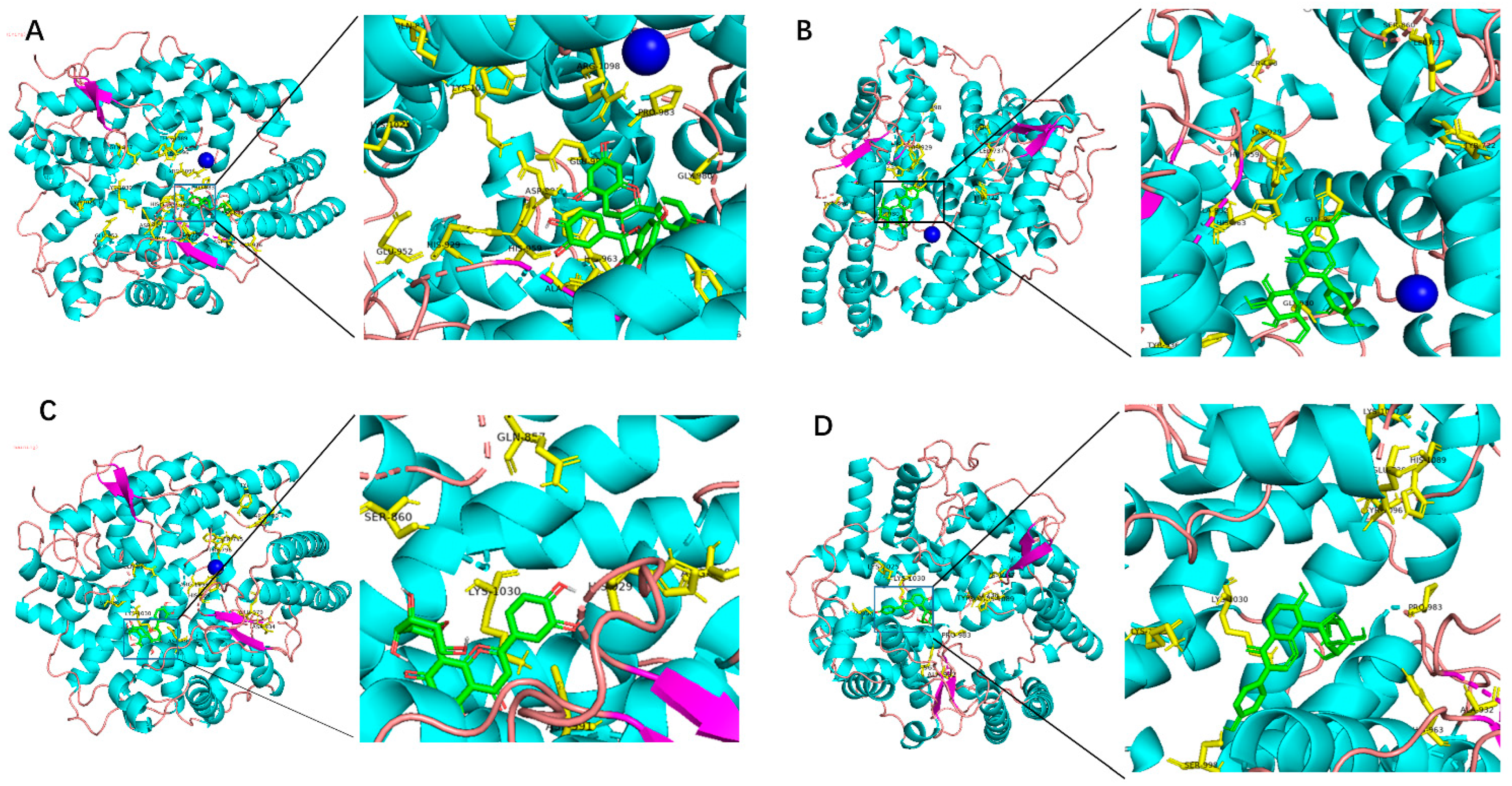




| ACE Inhibitors | Docked Energy (kcal/mol) | Specific Amino Acids for Hydrophobic Interactions |
|---|---|---|
| Procyanidin | −9.6 | GLN-857,HIS-1089,TYR-1096,ARG-1098,GLU-987,PRO-983,GLY-980,HIS-963,ASN-642,TYR-936,ASP-934,ALA-932,HIS-963,ASP-991,GLU-952,HIS-959 |
| Quercetin | −8.0 | ASN-787,SER-795,ALA-932,ARG-1098,ASP-697,GLY-980,LYS-1025,GLU-960,ALA-937,CYS-946,LYS-1030 |
| Isoquercitrin | −8.1 | GLU-986,GLY-980,TYR-936,HIS-963,ASP-934,ALA-932,HIS-959,HIS-929,LEU-737,SER-998,SER-860 |
| Resveratrol | −7.3 | HIS-1089,SER-795,HIS-963,ASP-934 |
| Homoorientin | −8.2 | HIS-959,ASP-991,LYS-1025,LYS-1030,SER-998,GLU-987,HIS-963,ASP-934,LYS-1087,ASP-1029 |
| Orientin | −8.4 | SER-860,GLN-857,LYS-1030,ASP-991,ARG-1098,TRP-796,SER-795,HIS-929,GLY-980,GLU-979,ASP-934,ARG-700 |
| Isovitexin | −7.9 | GLU-979,HIS-929,HIS-959,SER-931,ALA-932,LYS-1030 |
| Puerarin | −8.4 | LYS-1025,SER-998,LYS-1030,HIS-963,ALA-932,PRO-983,GLU-738,TRY-1096,HIS-1089,LYS-1087 |
| Vitexin | −8.1 | GLN-857,HIS-1081,HIS-1030,HIS-957,ASP-991,GLY-980 |
| Cianidanol | −7.2 | LYS-1030,SER-998,HIS-959,HIS-929,SER-931,SER-1093,SER-795,ALA-932,GLU-699,TRY-936,TYR-970 |
| ACE Inhibitors | Docked Energy (kcal/mol) | Specific Amino Acids for Hydrophobic Interactions |
|---|---|---|
| Procyanidin | −9.7 | GLN-259,LYS-489,HIS-491,TYR-498,LYS-432,THR-358,TYR-186,ASP-393,HIS-361,GLU-389,ALA-334,ARG-500,TYR-501,TYR-498 |
| Quercetin | −7.7 | TYR-501,TYR-498,HIS-491,GLN-259,GLY-382,ARG-381,ARG-90,TRY-369, ALA-334,HIS-361,SER-357 |
| Isoquercitrin | −9.7 | GLN-259,LYS-432,HIS-491,ARG-500,GLU-389,ASP-393,HIS-331,HIS-361,SER-333,GLU-362,TYR-369,ARG-381,ARG-500,HIS-491,TRP-198,THR-97,TYR-186,TYR-111 |
| Resveratrol | −7.0 | ARG-381,GLN-259,LYS-489,ARG-500,GLN-81,GLN-444 |
| Homoorientin | −8.0 | HIS-365,ALA-334,ALA-332,SER-357,GLY-382,ARG-381,ARG-500, TYR-501,TYR-424,HIS-491,GLN-259 |
| Orientin | −8.5 | ARG-381,PRO-385,ALA-334,ASP-393,TRP-201,ASN-203,SER-100,THR-97, TRP-201,GLN-259,LYS-432,ARG-96,GLY-93,ARG-89 |
| Isovitexin | −8.5 | ALA-334,TYR-369,PRO-385,ARG-500,TYR-501,SER-35,GLN-62,TYR-24,ARG-90,ALA-101,ARG-96,TYR-197,SER-200,ASN-203,GLN-81 |
| Puerarin | −7.9 | HIS-365,ALA-334,HIS-331,TYR-424,ARG-90,THR-97,ALN-81,GLN-259,SER-200 |
| Vitexin | −7.5 | GLN-259,TYR-498,TRP-201,TYR-501,HIS-331,TYR-186,TYR-338,ARG-108 |
| Cianidanol | −7.6 | SER-260,GLU-431,TYR-498,HIS-491,SER-200,TYR-338,SER-61,ARG-96 |
| Compounds | KD (M) |
|---|---|
| Captopril | 1.01 × 10−8 |
| Procyanidin | 4.34 × 10−6 |
| Quercetin | 4.38 × 10−5 |
| Isoquercitrin | 5.76 × 10−6 |
| Homoorientin | 1.02 × 10−5 |
| Orientin | 9.32 × 10−6 |
| Puerarin | 1.38 × 10−5 |
| Cianidanol | 4.83 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Jiao, Y.; Luo, M.; Wang, J.; Li, J.; Ma, Y.; Liu, C. Detection of Various Traditional Chinese Medicinal Metabolites as Angiotensin-Converting Enzyme Inhibitors: Molecular Docking, Activity Testing, and Surface Plasmon Resonance Approaches. Molecules 2023, 28, 7131. https://doi.org/10.3390/molecules28207131
Wu Q, Jiao Y, Luo M, Wang J, Li J, Ma Y, Liu C. Detection of Various Traditional Chinese Medicinal Metabolites as Angiotensin-Converting Enzyme Inhibitors: Molecular Docking, Activity Testing, and Surface Plasmon Resonance Approaches. Molecules. 2023; 28(20):7131. https://doi.org/10.3390/molecules28207131
Chicago/Turabian StyleWu, Qixin, Yue Jiao, Mingzhu Luo, Jingyi Wang, Jingzhe Li, Yanyan Ma, and Changzhen Liu. 2023. "Detection of Various Traditional Chinese Medicinal Metabolites as Angiotensin-Converting Enzyme Inhibitors: Molecular Docking, Activity Testing, and Surface Plasmon Resonance Approaches" Molecules 28, no. 20: 7131. https://doi.org/10.3390/molecules28207131
APA StyleWu, Q., Jiao, Y., Luo, M., Wang, J., Li, J., Ma, Y., & Liu, C. (2023). Detection of Various Traditional Chinese Medicinal Metabolites as Angiotensin-Converting Enzyme Inhibitors: Molecular Docking, Activity Testing, and Surface Plasmon Resonance Approaches. Molecules, 28(20), 7131. https://doi.org/10.3390/molecules28207131






