Guttapercha Improves In Vitro Bioactivity and Dentin Remineralization Ability of a Bioglass Containing Polydimethylsiloxane-Based Root Canal Sealer
Abstract
1. Introduction
2. Results
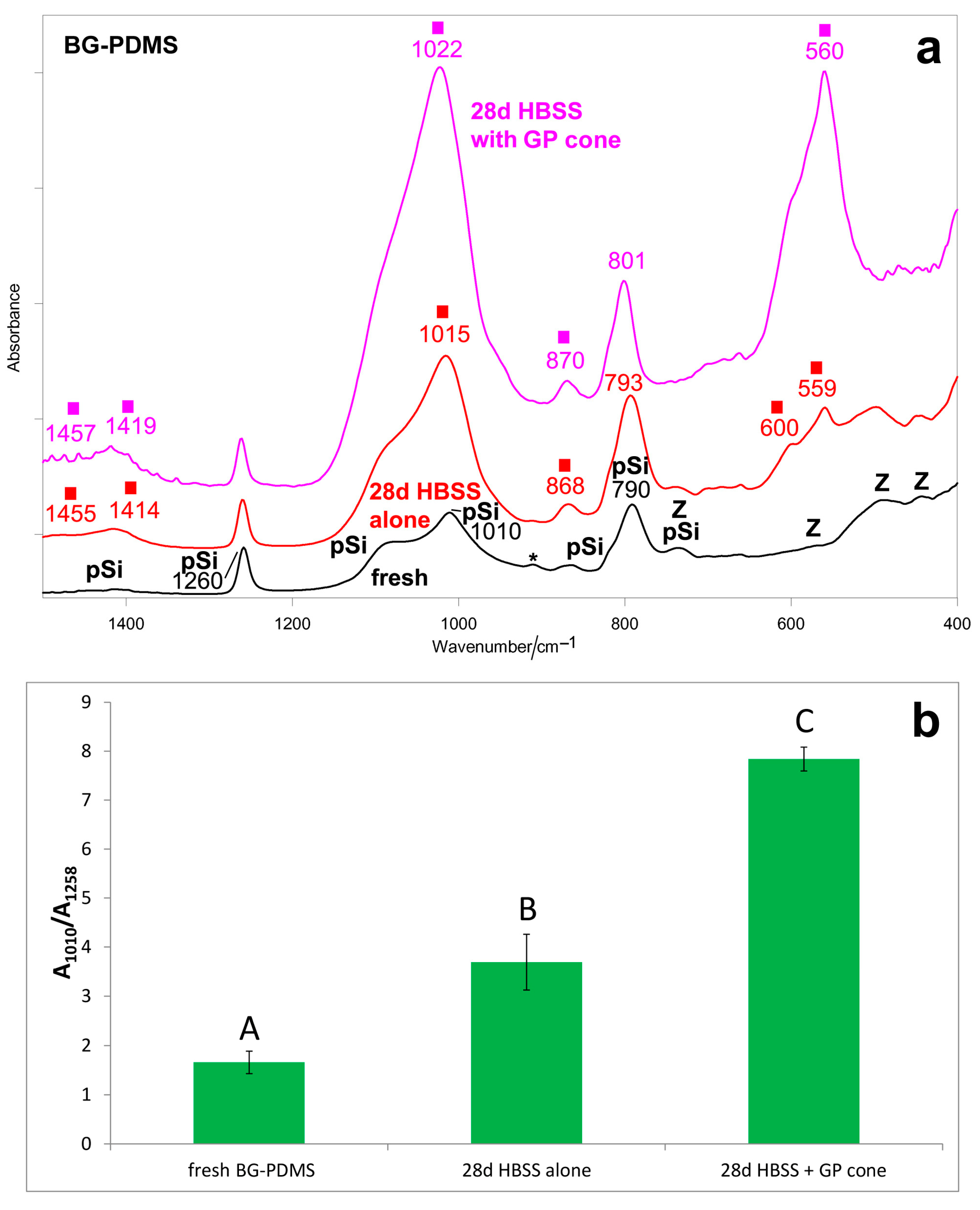
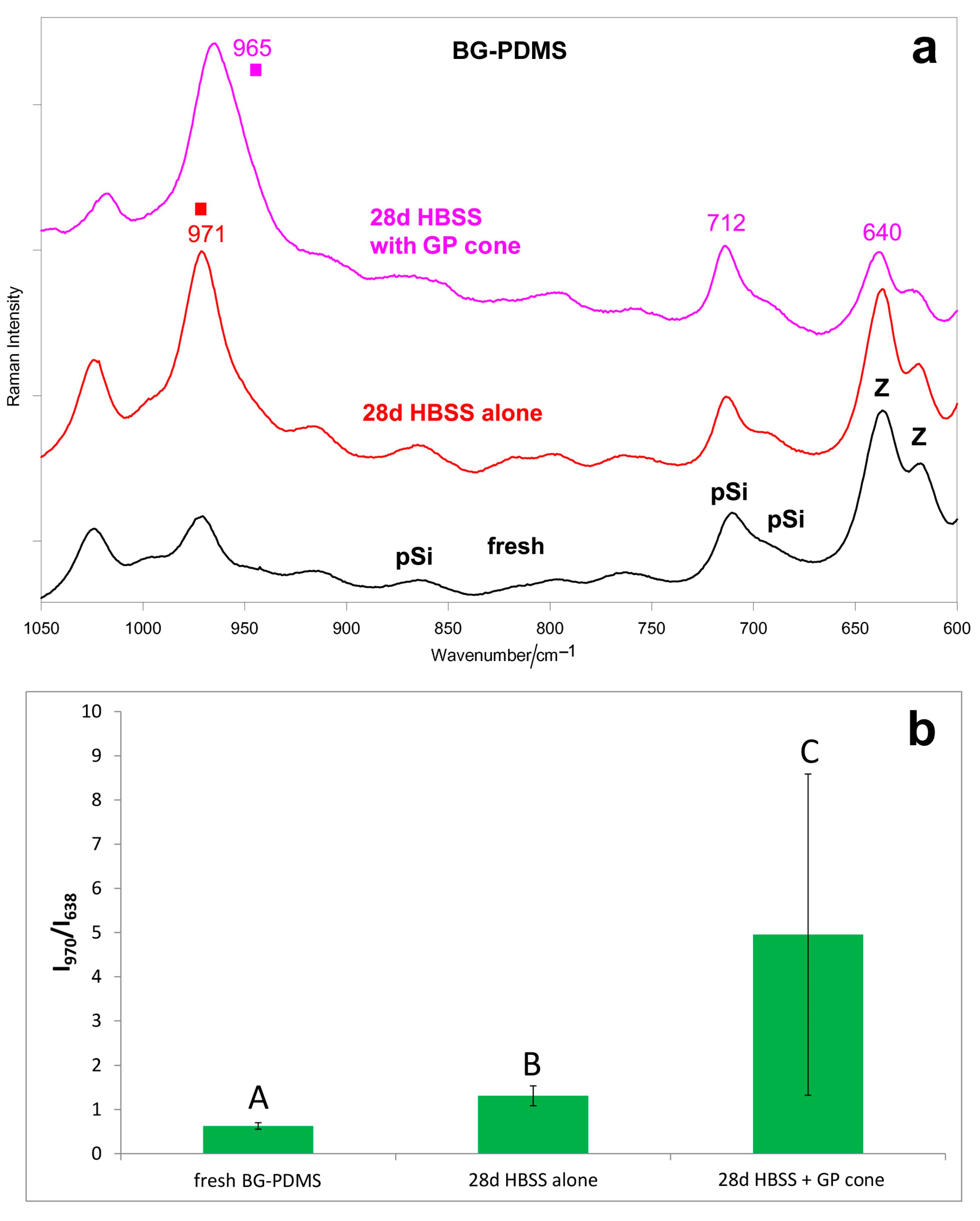
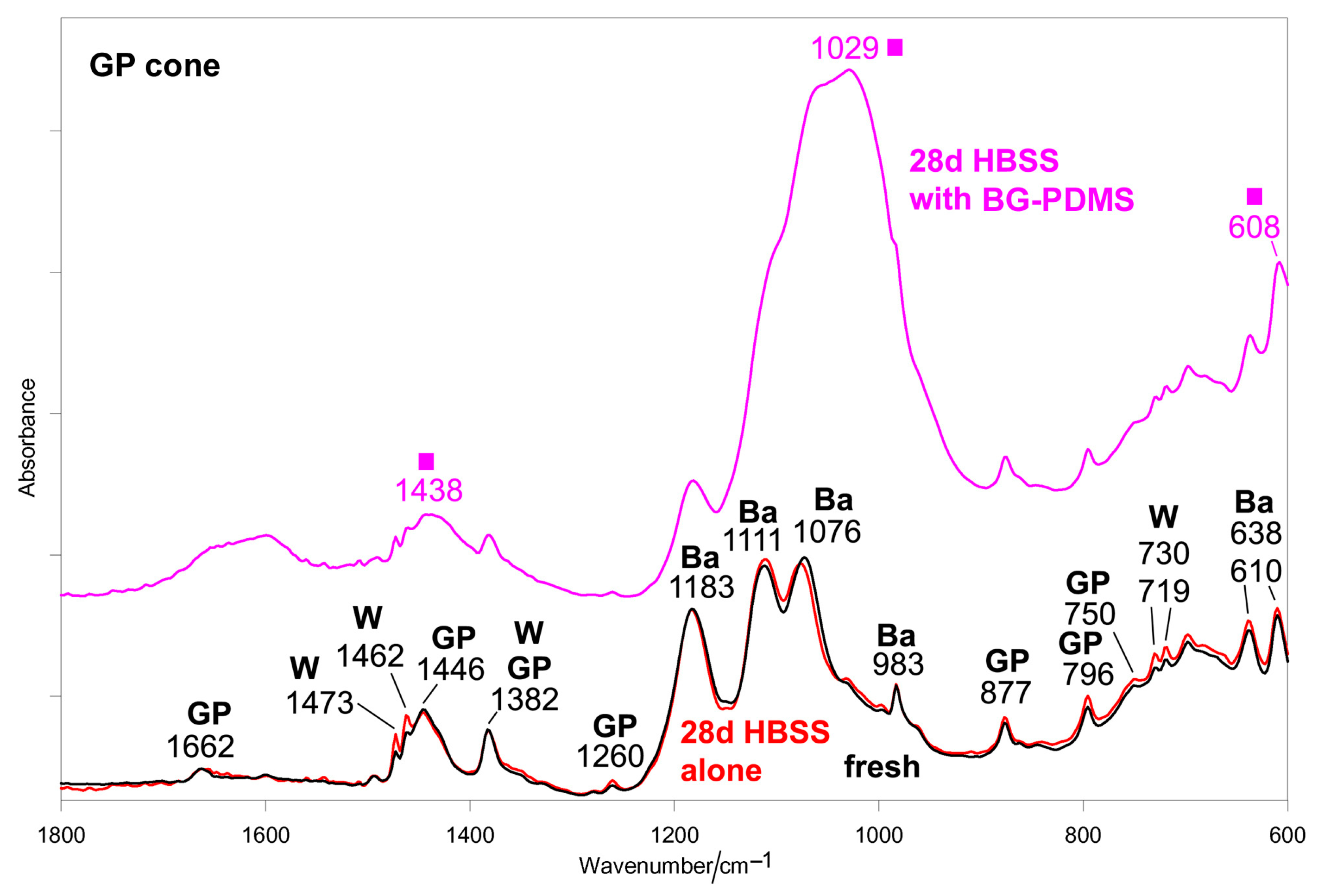
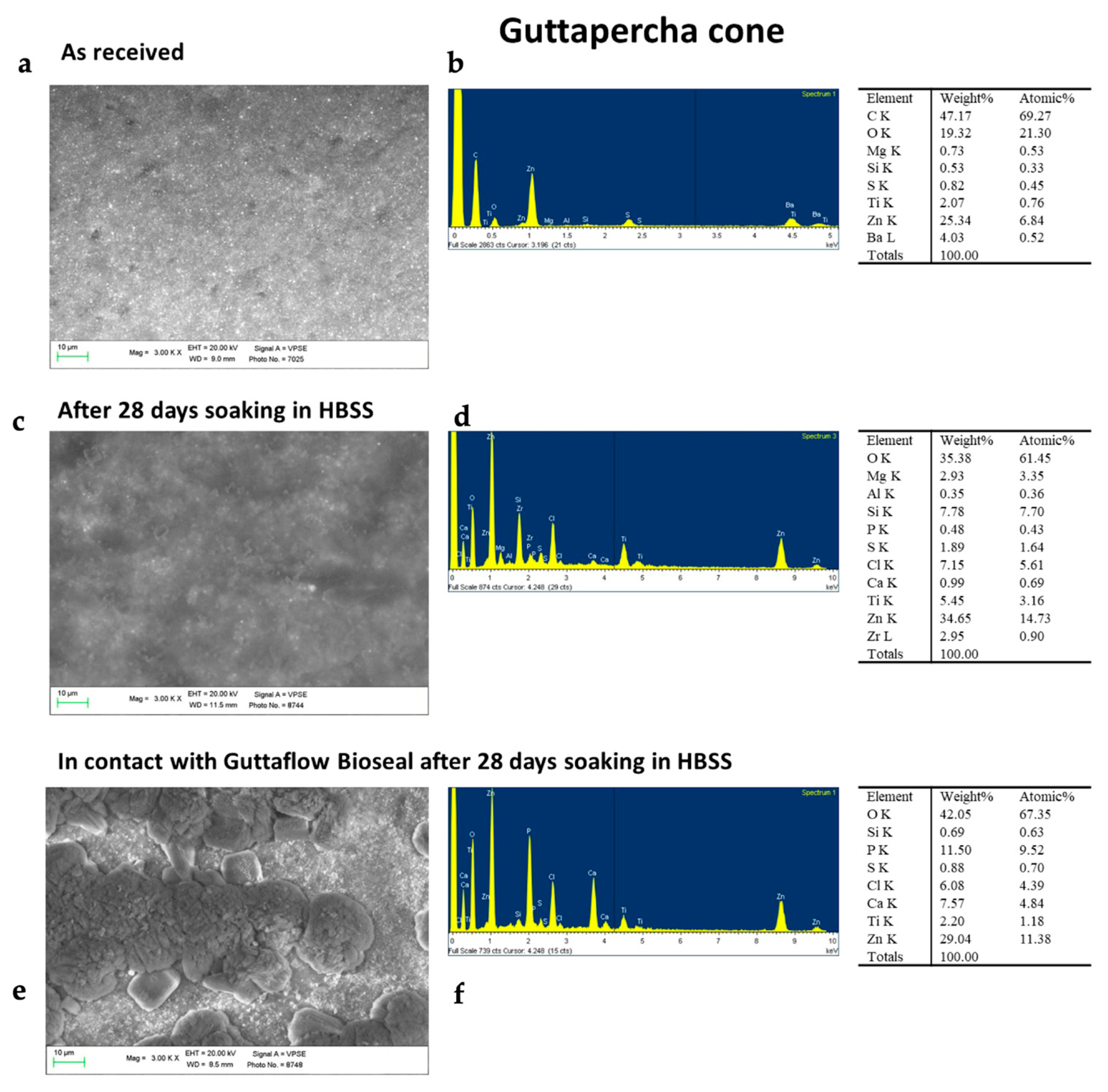
2.1. Bioactivity Tests
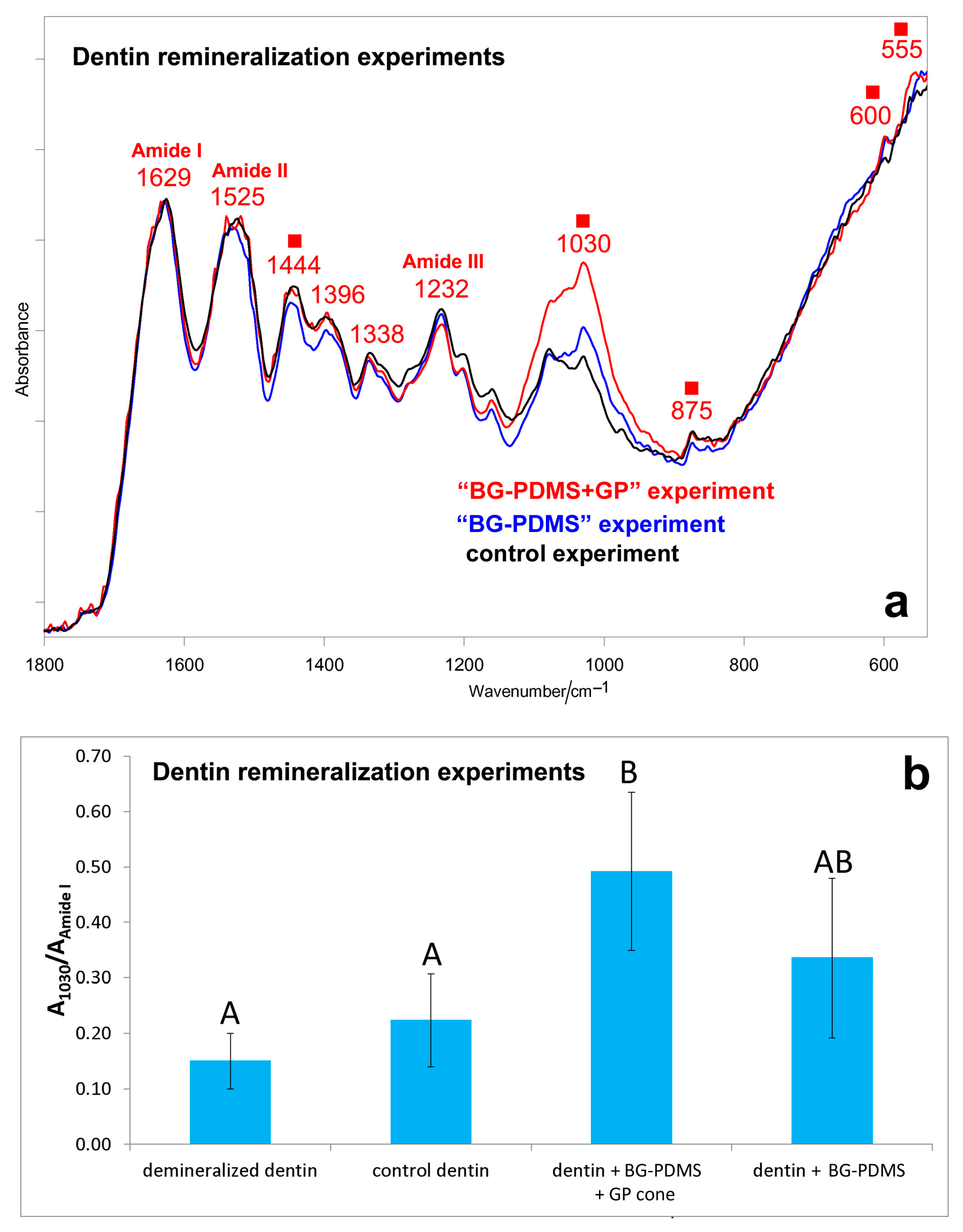
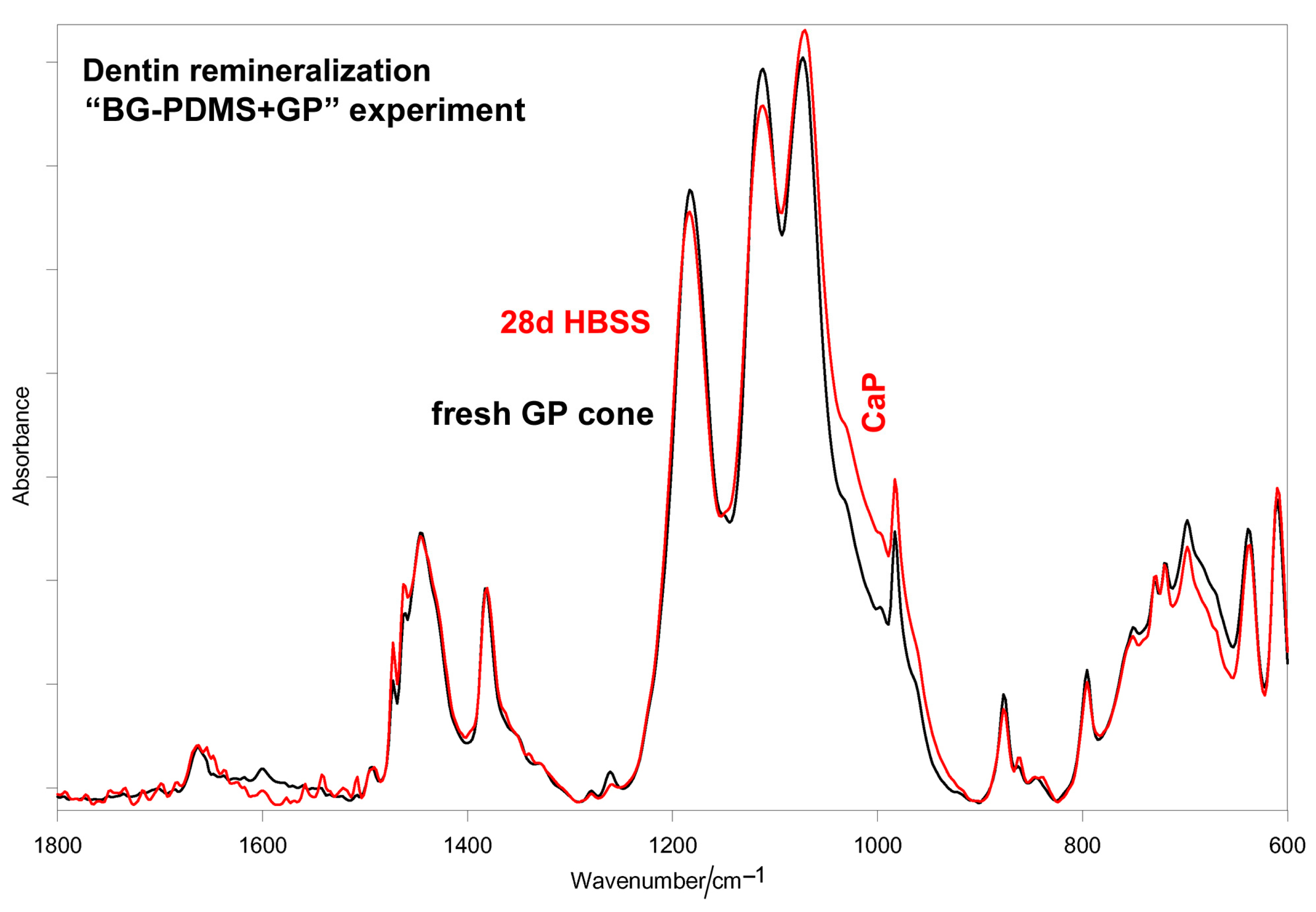
2.2. Dentin Remineralization Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bioactivity Tests
4.3. Dentin Remineralization Tests
4.4. Raman and IR Analyses
4.5. Statistical Analysis
4.6. ESEM-EDX Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marciano, J.; Michailesco, P.M.; Abadie, J.M. Stereochemical structure characterization of dental gutta-percha. J. Endod. 1993, 19, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Rootare, H.M.; Powers, J.M. Determination of phase transitions in gutta-percha by differential thermal analysis. J. Dent. Res. 1977, 56, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Maniglia-Ferreira, C.; Gurgel-Filho, E.D.; de Araújo Silva, J.B.; de Paula, R.M.; de Andrade Feitosa, J.P.; de Sousa-Filho, F.J. Chemical composition and thermal behavior of five brands of thermoplasticized gutta-percha. Eur. J. Dent. 2013, 7, 201–206. [Google Scholar] [CrossRef]
- Maniglia-Ferreira, C.; Silva, J.B.A., Jr.; de Paula, R.C.M.; Feitosa, J.P.A.; Cortez, D.G.N.; Zaia, A.A.; Souza-Filho, F.J. Brazilian gutta-percha points. Part I: Chemical composition and X-ray diffraction analysis. Braz. Oral Res. 2005, 19, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Gurgel-Filho, E.D.; Feitosa, J.P.A.; Teixeira, F.B.; de Paula, R.C.M.; Silva, J.B.A., Jr.; Souza-Filho, F.J. Chemical and X-ray analyses of five brands of dental gutta-percha cone. Int. Endod. J. 2003, 36, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.M.; Sandrik, J.L.; Heuer, M.A.; Rapp, G.W. Composition and mechanical properties of Gutta-percha endodontic points. J. Dent. Res. 1975, 54, 921–925. [Google Scholar] [CrossRef]
- Moorer, W.R.; Genet, J.M. Anti-bacterial activity of Gutta-percha cones attributed to the zinc oxide component. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 508–517. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Osorio, E.; Prati, C.; Carvalho, R.M. Microhardness of acid-treated and resin infiltrated human dentine. J. Dent. 2005, 33, 349–354. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, I.; Vignoletti, F.; Vignoletti, G.; Martin, C.; Sanz, M. Long-term tooth survival and success following primary root canal treatment: A 5- to 37-year retrospective observation. Clin. Oral Investig. 2023, 27, 3233–3244. [Google Scholar] [CrossRef] [PubMed]
- Gulabivala, K.; Ng, Y.L. Factors that affect the outcomes of root canal treatment and retreatment—A reframing of the principles. Int. Endod. J. 2023, 56, 82–115. [Google Scholar] [CrossRef]
- Ricucci, D.; Loghin, S.; Gonçalves, L.S.; Rôças, I.N.; Siqueira, J.F., Jr. Histobacteriologic Conditions of the Apical Root Canal System and Periapical Tissues in Teeth Associated with Sinus Tracts. J. Endod. 2018, 44, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Laranjo, M.; Marto, C.M.; Casalta-Lopes, J.; Serambeque, B.; Gonçcalves, A.C.; Sarmento-Ribeiro, A.B.; Carrilho, E.; Botelho, M.F.; Paula, A.B.; et al. Guttaflow® Bioseal cytotoxicity assessment: In vitro study. Molecules 2020, 25, 4297. [Google Scholar] [CrossRef]
- Demétrio, K.B.; Giotti Cioato, J.M.; Moreschi, A.; Oliveira, G.A.; Lorenzi, W.; Hehn de Oliveira, F.; Vieira de Macedo Neto, A.; Stefani Sanchez, P.R.; Gastal Xavier, R.; Loureiro dos Santos, L.A. Polydimethylsiloxane/nano calcium phosphate composite tracheal stents: Mechanical and physiological properties. J. Biomed. Mater. Res. Part B 2019, 107, 545–553. [Google Scholar] [CrossRef]
- Redondo, F.L.; Giaroli, M.C.; Ciolino, A.E.; Ninago, M.D. Hydroxyapatite growth on poly(dimethylsiloxane-block-ε-caprolactone)/tricalcium phosphate coatings obtained by electrophoretic deposition. Front. Mater. 2022, 8, 803054. [Google Scholar] [CrossRef]
- Elsayed, M.A. Aging of TotalFill BC Sealer and MTA Fillapex in simulated body fluid. Eur. Endod. J. 2021, 6, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Jiao, K.; Wang, T.; Zhang, W.; Camilleri, J.; Bergeron, B.E.; Feng, H.I.; Mao, J.; Chen, J.; Pashley, D.H.; et al. A review of the bioactivity of hydraulic calcium silicate cements. J. Dent. 2014, 42, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Cao, C.Y.; Mei, M.L.; Li, Q.L.; Lo, E.C.M.; Chu, C.H. Methods for Biomimetic Remineralization of Human Dentine: A Systematic Review. Int. J. Mol. Sci. 2015, 16, 4615–4627. [Google Scholar] [CrossRef]
- Rabadjieva, D.; Gergulova, R.; Titorenkova, R.; Tepavitcharova, S.; Dyulgerova, E.; Balarew, C.; Petrov, O. Biomimetic transformations of amorphous calcium phosphate: Kinetic and thermodynamic studies. J. Mater. Sci. Mater. Med. 2010, 21, 2501–2509. [Google Scholar] [CrossRef]
- Jiao, K.; Niu, L.N.; Ma, C.F.; Huang, X.Q.; Pei, D.D.; Luo, T.; Huang, Q.; Chen, J.H.; Tay, F.R. Complementarity and Uncertainty in Intrafibrillar Mineralization of Collagen. Adv. Funct. Mater. 2016, 26, 6858–6875. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Prati, C. Properties of a novel polysiloxane-guttapercha calcium silicate-bioglass-containing root canal sealer. Dent. Mater. 2016, 32, e113–e126. [Google Scholar] [CrossRef]
- Hoikkala, N.P.J.; Wang, X.; Hupa, L.; Smatt, J.H.; Peltonen, J.; Vallittu, P.K. Dissolution and mineralization characterization of bioactive glass-ceramic endodontic Guttaflow Biosealer. Dent. Mater. J. 2018, 37, 988–994. [Google Scholar] [CrossRef]
- Hoikkala, N.P.J.; Siekkinen, M.; Hupa, L.; Vallittu, P.K. Behaviour of different bioactive glasses incorporated in polydimethylsiloxane endodontic sealer. Dent. Mater. 2021, 37, 321–327. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Aparicio, C.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Polylactic acid-based porous scaffolds doped with calcium silicates and dicalcium phosphate dihydrate designated for biomedical application. Mater. Sci. Eng. C 2018, 82, 163–181. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Hench, L.L.; Oonishi, H. Quantitative rates of in vivo bone generation for Bioglass® and hydroxyapatite particles as bone graft substitute. J. Mater. Sci. Mater. Med. 1997, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, M.; Tomás-Catalá, C.J.; Oñate-Sánchez, R.E.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Cytotoxicity of GuttaFlow Bioseal, GuttaFlow2, MTA Fillapex, and AH Plus on Human Periodontal Ligament Stem Cells. J. Endod. 2017, 43, 816–822. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Collado-González, M.; Tomás-Catalá, C.J.; García-Bernal, D.; López, S.; Oñate-Sánchez, R.E.; Moraleda, J.M.; Murcia, L. GuttaFlow Bioseal promotes spontaneous differentiation of human periodontal ligament stem cells into cementoblast-like cells. Dent. Mater. 2019, 35, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.; Di Foggia, M.; Zamparini, F.; Prati, C.; Gandolfi, M.G. The Influence of the Matrix on the Apatite-Forming Ability of Calcium Containing Polydimethylsiloxane-Based Cements for Endodontics. Molecules 2022, 27, 5750. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Yu, Y.; Wei, M.T.; Chang, C.C.; Ricotta, V.; Feng, K.C.; Wang, L.; Bherwani, A.K.; Yang, H.D.O.; Simon, M.; et al. Regulating substrate mechanics to achieve odontogenic differentiation for dental pulp stem cells on TiO2 filled and unfilled polyisoprene. Acta Biomater. 2019, 89, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, Y.; Joubert, C.; Bruder, G.; Liu, Y.; Chang, C.C.; Simon, M.; Walker, G.S.; Rafailovich, M. Differentiation of dental pulp stem cells on gutta-percha scaffolds. Polymers 2016, 8, 193. [Google Scholar] [CrossRef]
- do Nascimento, R.M.; de Paula, A.J.; Oliveira, N.C.; Alves, A.C.; de Oliveira Aquino, Y.M.L.; Souza Filho, A.G.; Soares Rodrigues, J.E.F.; Hernandes, A.C. Towards the production of natural rubber-calcium phosphate hybrid for applications as bioactive coatings. Mater. Sci. Eng. C 2019, 94, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.A.; de Almeida Filho, E.; Romeiro Miranda, M.C.; dos Santos, M.L.; Herculano, R.D.; Guastaldi, A.C. Natural rubber latex coated with calcium phosphate for biomedical application. J. Biomater. Sci. Polym. Ed. 2015, 26, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Utara, S.; Klinkaewnarong, J. Sonochemical synthesis of nano-hydroxyapatite using natural rubber latex as a templating agent. Ceram. Int. 2015, 41, 14860–14867. [Google Scholar] [CrossRef]
- Lopes, C.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Pretorius, N.E.; Power, A.; Tennant, M.; Forrest, A.; Cozzolino, D. The use of vibrational spectroscopy in the geographic characterization of human teeth: A systematic review. Appl. Spectrosc. Rev. 2020, 55, 105–127. [Google Scholar] [CrossRef]
- Carden, A.; Morris, M.D. Application of vibrational spectroscopy to the study of mineralized tissues (Review). J. Biomed. Opt. 2000, 5, 259–268. [Google Scholar] [CrossRef]
- Orsini, G.; Orilisi, G.; Notarstefano, V.; Monterubbianesi, R.; Vitiello, F.; Tosco, V.; Belloni, A.; Putignano, A.; Giorgini, E. Vibrational Imaging Techniques for the Characterisation of Hard Dental Tissues: From Bench-Top to Chair-Side. Appl. Sci. 2021, 11, 11953. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface Modification of Sylgard-184 Poly(dimethylsiloxane) Networks by Ultraviolet and Ultraviolet/Ozone Treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, mid-infrared, near-infrared and ultraviolet-visible spectroscopy of PDMS silicone rubber for characterization of polymer optical waveguide materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
- Ye, H.; Gu, Z.; Gracias, D.H. Kinetics of Ultraviolet and Plasma Surface Modification of Poly(dimethylsiloxane) Probed by Sum Frequency Vibrational Spectroscopy. Langmuir 2006, 22, 1863–1868. [Google Scholar] [CrossRef]
- Phillippi, C.M.; Mazdiyasni, K.S. Infrared and Raman spectra of zirconia polymorphs. J. Am. Ceram. Soc. 1971, 54, 254–258. [Google Scholar] [CrossRef]
- Apfelbaum, F.; Diab, H.; Mayer, I.; Featherstone, J.D.B. An FTIR Study of Carbonate in Synthetic Apatites. J. Inorg. Biochem. 1992, 45, 211–282. [Google Scholar] [CrossRef]
- Ramos Coelho, S.A.; Almeida, J.C.; Unalan, I.; Detsch, R.; Salvado, I.M.M.; Boccaccini, A.R.; Vaz Fernandes, M.H. Cellular Response to Sol−Gel Hybrid Materials Releasing Boron and Calcium Ions. ACS Biomater. Sci. Eng. 2021, 7, 491–506. [Google Scholar] [CrossRef]
- Xu, G.; Aksay, I.A.; Grooves, J.T. Continuous crystalline carbonate apatite thin films. A biomimetic approach. J. Am. Chem. Soc. 2001, 123, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, K.A.M.; Suarez, P.A.Z.; Rubim, J.C. Photo-degradation of synthetic and natural polyisoprenes at specific UV radiations. Polym. Degrad. Stab. 2005, 90, 34–43. [Google Scholar] [CrossRef]
- Abdulov, K.S. Calculation of polarized IR absorption spectra for trans-1,4-polyisoprenes of various conformations. J. Appl. Spectrosc. 2008, 75, 494–499. [Google Scholar] [CrossRef]
- Ratri, P.J.; Tashiro, K. Application of the simultaneous measurement system of WAXD, SAXS and transmission FTIR spectra to the study of structural changes in the cold- and melt-crystallization processes of trans-1,4-polyisoprene. Polym. J. 2013, 45, 1019–1026. [Google Scholar] [CrossRef]
- Saunders, R.A.; Smith, D.C. Infra-Red Spectra and Structure of Hevea and Gutta Elastomers. J. Appl. Phys. 1949, 20, 953–965. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Putzig, C.L.; Leugers, M.A. The Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Baglioni, M.; Poggi, G.; Ciolli, G.; Fratini, E.; Giorgi, R.; Baglioni, P. A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: SAXS Characterization and Application. Materials 2018, 11, 1144. [Google Scholar] [CrossRef]
- Parker, F.S. Applications of Infrared, Raman, and Resonance Raman Spectroscopy in Biochemistry; Plenum Press: New York, NY, USA, 1983. [Google Scholar]
- Shen, L.; Bu, H.; Yang, H.; Liu, W.; Li, G. Investigation on the behavior of collagen self-assembly in vitro via adding sodium silicate. Int. J. Biol. Macromol. 2018, 115, 635–642. [Google Scholar] [CrossRef]
- Kaneko, T.; Ito, S.; Minakawa, T.; Hirai, N.; Ohki, Y. Degradation mechanisms of silicone rubber under different aging conditions. Polym. Degrad. Stab. 2019, 168, 108936. [Google Scholar] [CrossRef]
- Ghanbari-Siahkali, A.; Mitra, S.; Kingshott, P.; Almdal, K.; Bloch, C.; Rehmeier, H.K. Investigation of the hydrothermal stability of crosslinked liquid silicone rubber (LSR). Polym. Degrad. Stab. 2005, 90, 471–480. [Google Scholar] [CrossRef]
- Maniglia-Ferreira, C.; Bönecker, G.; Silva, J.B.A., Jr.; de Paula, R.C.M.; Feitosa, J.P.A.; Souza-Filho, F.J. Degradation of trans-polyisoprene after root filling with thermoplasticized techniques. Int. Endod. J. 2008, 41, 296–302. [Google Scholar] [CrossRef][Green Version]
- Silva, J.B.A., Jr.; de Paula, R.C.M.; Feitosa, J.P.A.; Gurgel-Filho, E.D.; Maniglia-Ferreira, C.; de Souza Filho, F.J. In Vivo Aging of Gutta-percha Dental Cone. J. Appl. Polym. Sci. 2006, 100, 4082–4088. [Google Scholar] [CrossRef]
- Dem, K.; Wu, Y.; Kaminga, A.C.; Dai, Z.; Cao, X.; Zhu, B. The push out bond strength of polydimethylsiloxane endodontic sealers to dentin. BMC Oral Health 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hench, L.L. Bioactive Materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Notingher, I.; Jones, J.R.; Verrier, S.; Bisson, I.; Embanga, P.; Edwards, P.; Polak, J.M.; Hench, L.L. Application of FTIR and Raman spectroscopy to characterization of bioactive materials and living cells. J. Spectrosc. 2003, 17, 275–288. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Shipper, G.; Ørstavik, D.; Teixeira, F.B.; Trope, M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon). J. Endod. 2004, 30, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Kutty, M.G.; Abu Kasim, N.H.; Ab Aziz, Z.A.C. Physicochemical Properties of Calcium Phosphate Based Coating on Gutta-percha Root Canal Filling. Int. J. Polym. Sci. 2015, 2015, 414521. [Google Scholar] [CrossRef]
- Taddei, P.; Prati, C.; Gandolfi, M.G. A poly(2-hydroxyethyl methacrylate)-based resin improves the dentin remineralizing ability of calcium silicates. Mater. Sci. Eng. C 2017, 77, 755–764. [Google Scholar] [CrossRef]
- Serper, A.; Çalt, S. The Demineralizing Effects of EDTA at Different Concentrations and pH. J. Endod. 2002, 28, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Di Foggia, M.; Prati, C.; Gandolfi, M.G.; Taddei, P. An in vitro study on dentin demineralization and remineralization: Collagen rearrangements and influence on the enucleated phase. J. Inorg. Biochem. 2019, 193, 84–93. [Google Scholar] [CrossRef]
- He, G.; Dahl, T.; Veis, A.; George, A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003, 2, 552–558. [Google Scholar] [CrossRef]
- Takeuchi, A.; Ohtsuki, C.; Kamitakahara, M.; Ogata, S.I.; Tanihara, M.; Miyazaki, T. Apatite formation on synthetic polypeptide with β sheet structure in a solution mimicking body environment. Key Engin. Mater. 2006, 309–311, 489–492. [Google Scholar] [CrossRef]
- Efflandt, S.E.; Magne, P.; Douglas, W.H.; Francis, L.F. Interaction between bioactive glasses and human dentin. J. Mater. Sci. Mater. Med. 2002, 13, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Z.L.; Liao, S.S.; Cui, F.Z. Nucleation Sites of Calcium Phosphate Crystals during Collagen Mineralization. J. Am. Ceram. Soc. 2003, 86, 1052–1054. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ginebra, M.P.; Prati, C. Fluoride-containing nanoporous calcium-silicate MTA cements for endodontics and oral surgery: Early fluorapatite formation in a phosphate-containing solution. Int. Endod. J. 2011, 44, 938–949. [Google Scholar] [CrossRef]
- Bradt, J.H.; Mertig, M.; Teresiak, A.; Pompe, W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem. Mater. 1999, 11, 2694–2701. [Google Scholar] [CrossRef]
- Zhu, W.; Robey, P.G.; Boskey, A.L. The regulatory role of matrix proteins in mineralization of bone. In Osteoporosis; Markus, R., Feldman, D., Nelson, D.A., Rosen, C.J., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2008; Volume 1. [Google Scholar]
- Rhee, S.H.; Tanaka, J. Effect of citric acid on the nucleation of hydroxyapatite in a simulated body fluid. Biomaterials 1999, 20, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Butler, I.S.; Gilson, D.F.R. FT-Raman and high-pressure infrared spectroscopic studies of dicalcium phosphate dihydrate (CaHPO4●2H2O) and anhydrous dicalcium phosphate (CaHPO4). Spectrochim. Acta A 1999, 55, 2801–2809. [Google Scholar] [CrossRef]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier transform Raman spectroscopy of synthetic and biological calcium phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Termine, J.D.; Eanes, E.D.; Greenfield, D.J.; Nylen, M.U.; Harper, R.A. Hydrazine-deproteinated bone mineral. Physical and chemical properties. Calcif. Tissue Int. 1973, 12, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Sauer, G.R.; Wuthier, R.E. Fourier transform infrared characterization of mineral phases formed during induction of mineralization by collagenase-released matrix vesicles in vitro. J. Biol. Chem. 1988, 263, 13718–13724. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Taddei, P.; Modena, E.; Siboni, F.; Prati, C. Biointeractivity-related versus chemi/physisorption-related apatite precursor-forming ability of current root end filling materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly porous polycaprolactone scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for bone regeneration. Mater. Sci. Eng. C 2019, 102, 341–361. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taddei, P.; Di Foggia, M.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Guttapercha Improves In Vitro Bioactivity and Dentin Remineralization Ability of a Bioglass Containing Polydimethylsiloxane-Based Root Canal Sealer. Molecules 2023, 28, 7088. https://doi.org/10.3390/molecules28207088
Taddei P, Di Foggia M, Zamparini F, Prati C, Gandolfi MG. Guttapercha Improves In Vitro Bioactivity and Dentin Remineralization Ability of a Bioglass Containing Polydimethylsiloxane-Based Root Canal Sealer. Molecules. 2023; 28(20):7088. https://doi.org/10.3390/molecules28207088
Chicago/Turabian StyleTaddei, Paola, Michele Di Foggia, Fausto Zamparini, Carlo Prati, and Maria Giovanna Gandolfi. 2023. "Guttapercha Improves In Vitro Bioactivity and Dentin Remineralization Ability of a Bioglass Containing Polydimethylsiloxane-Based Root Canal Sealer" Molecules 28, no. 20: 7088. https://doi.org/10.3390/molecules28207088
APA StyleTaddei, P., Di Foggia, M., Zamparini, F., Prati, C., & Gandolfi, M. G. (2023). Guttapercha Improves In Vitro Bioactivity and Dentin Remineralization Ability of a Bioglass Containing Polydimethylsiloxane-Based Root Canal Sealer. Molecules, 28(20), 7088. https://doi.org/10.3390/molecules28207088










