Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds
Abstract
1. Introduction
2. Results
2.1. Dry Mass and Percentage Yield of the Extracts
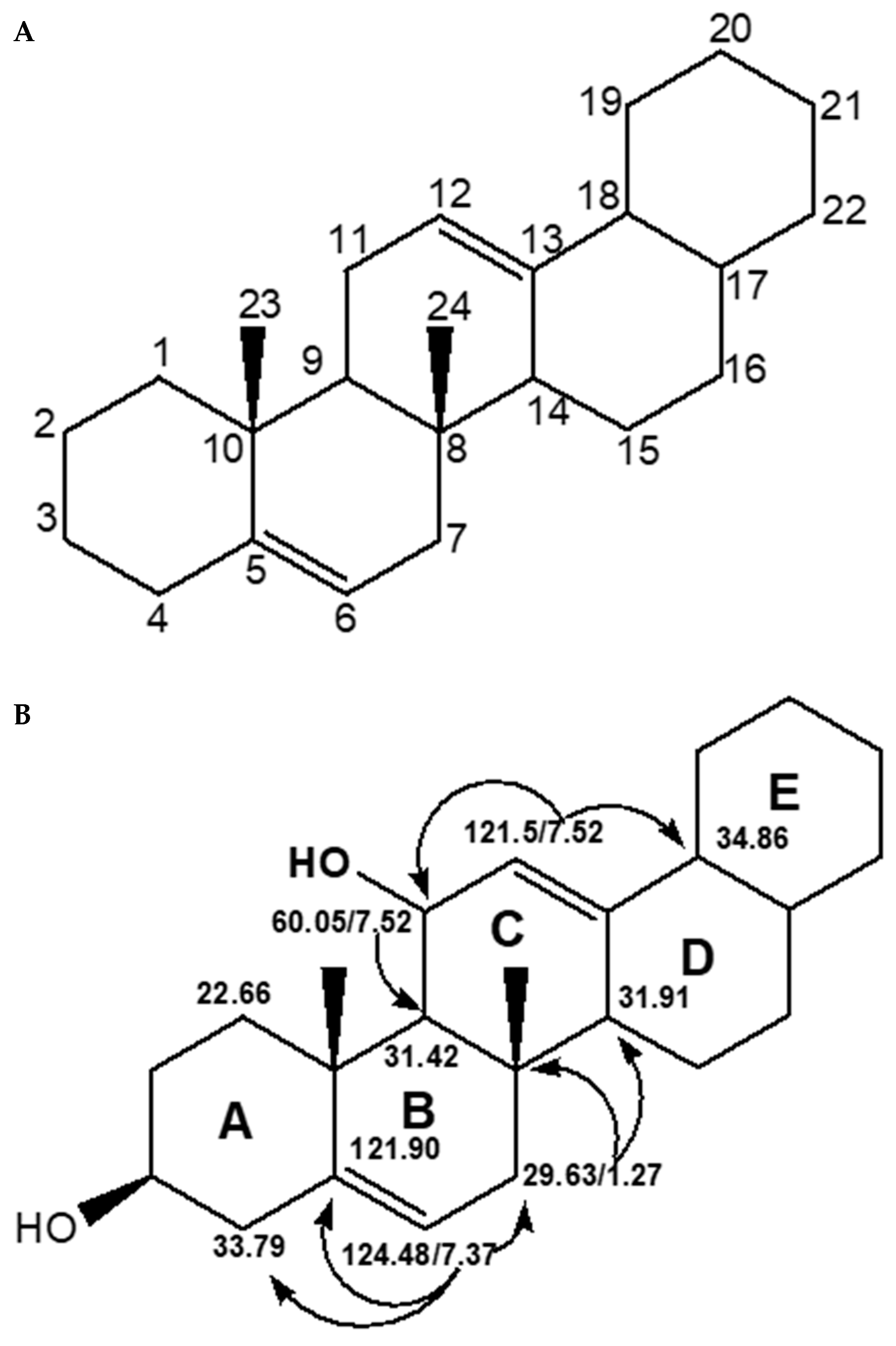
2.2. Structural Characterization and Elucidation of Compound E1
2.2.1. HPLC-PDA and UPLC-MS of Compound E1
2.2.2. One- and Two-Dimension NMR Analysis of E1
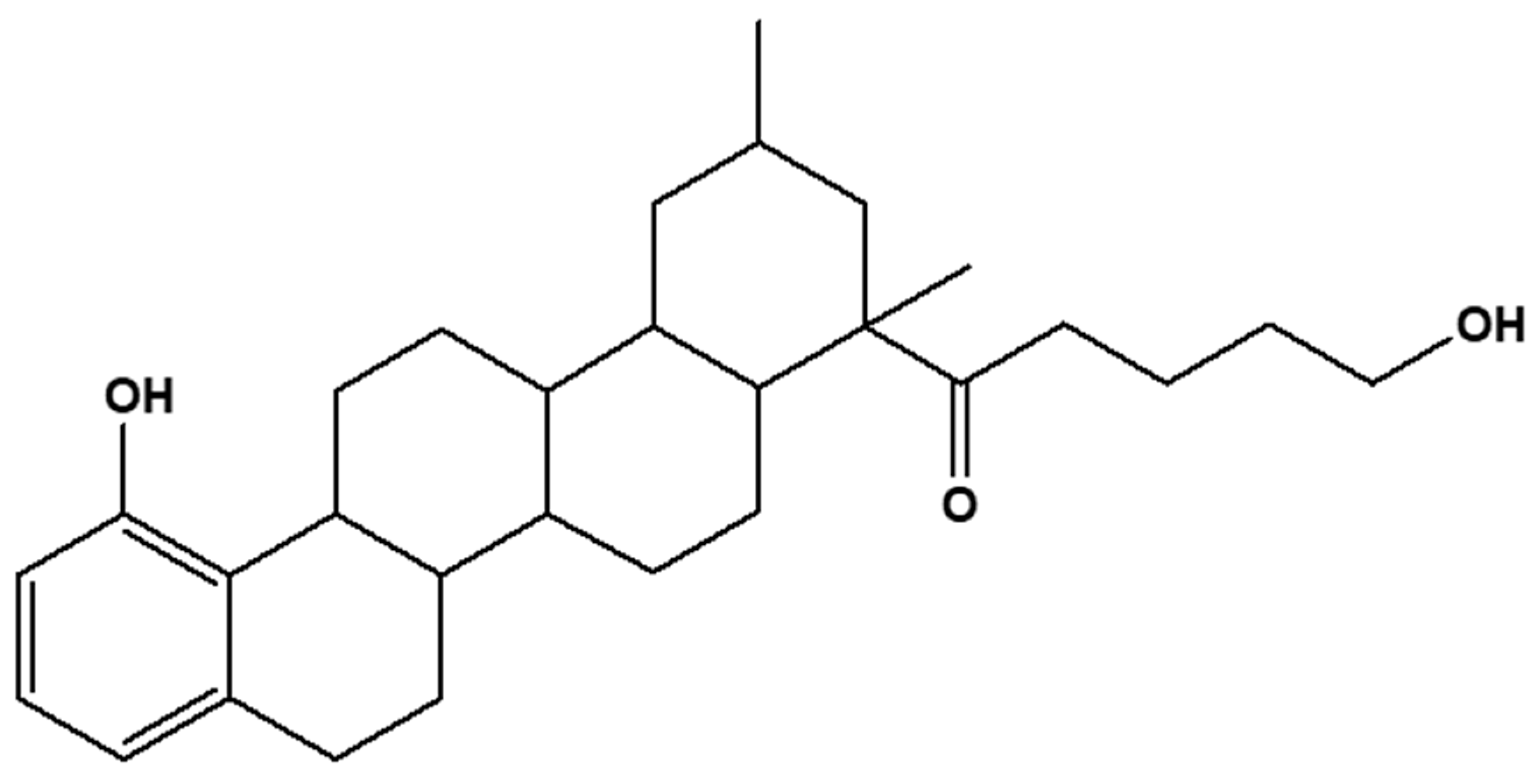
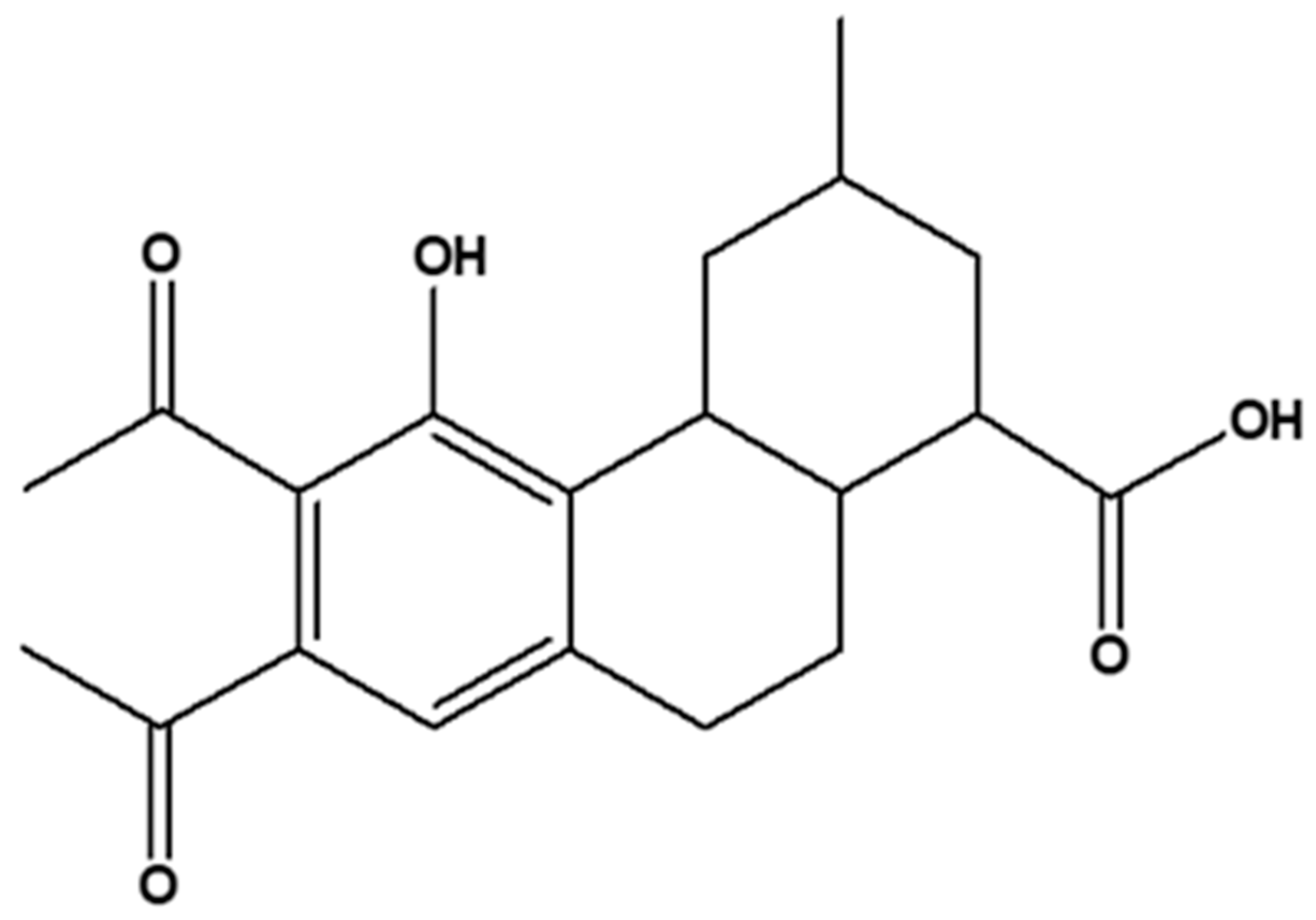
2.3. Structural Characterization and Elucidation of Compound E3
2.3.1. HPLC-PDA and UPLC-MS of Compound E3
2.3.2. One- and Two-Dimensional NMR Analysis of E3
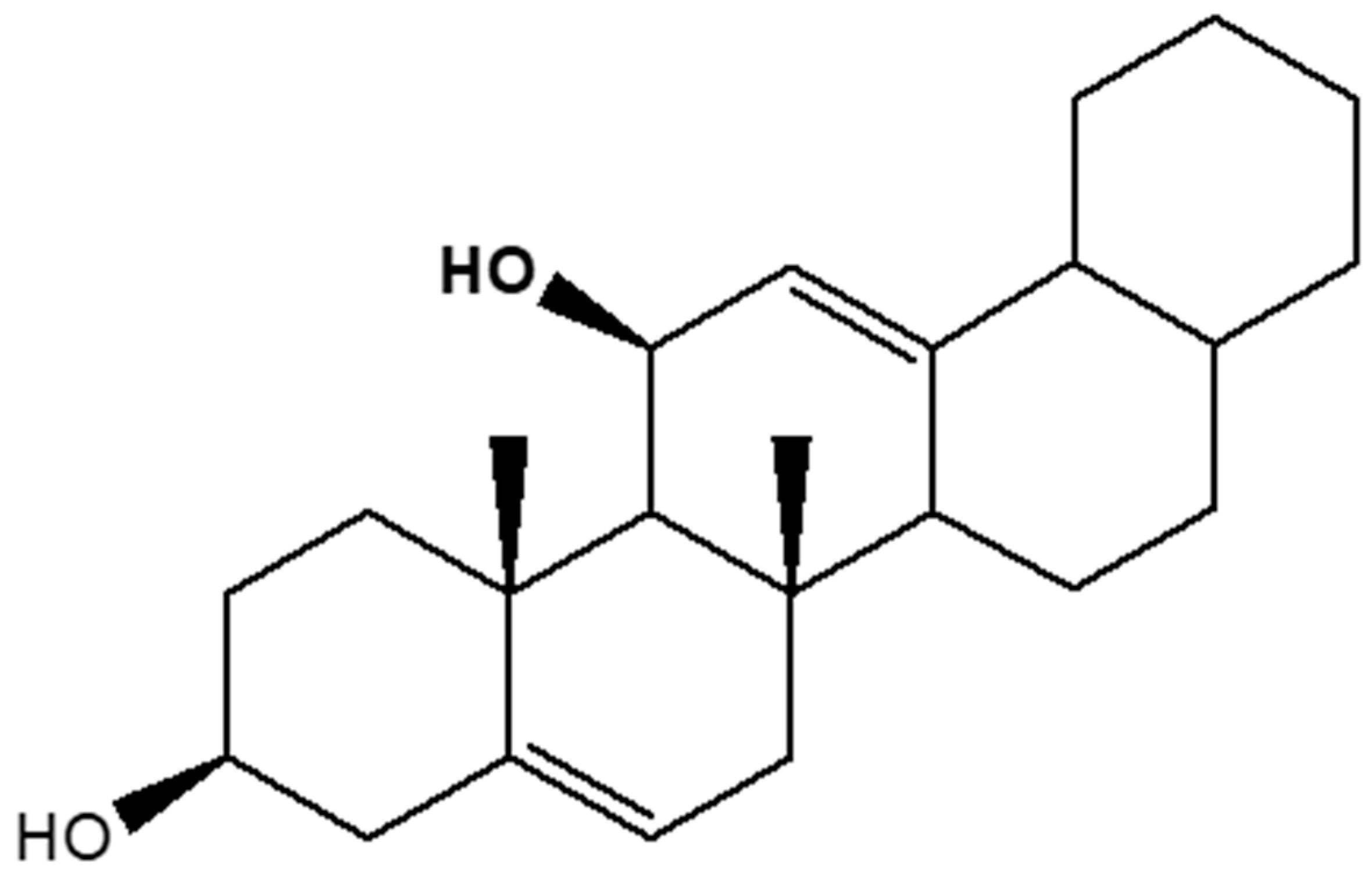
2.4. Charaterization and Structural Elucidation of Isolated Compound Ra
2.4.1. Structural Characterization and Elucidation of Compound Ra
2.4.2. One- and Two-Dimensional NMR Analysis of Ra
2.5. Qualitative Antimicrobial Assay
2.6. Qualitative Antioxidant Evaluation
2.7. Quantitative Antioxidant
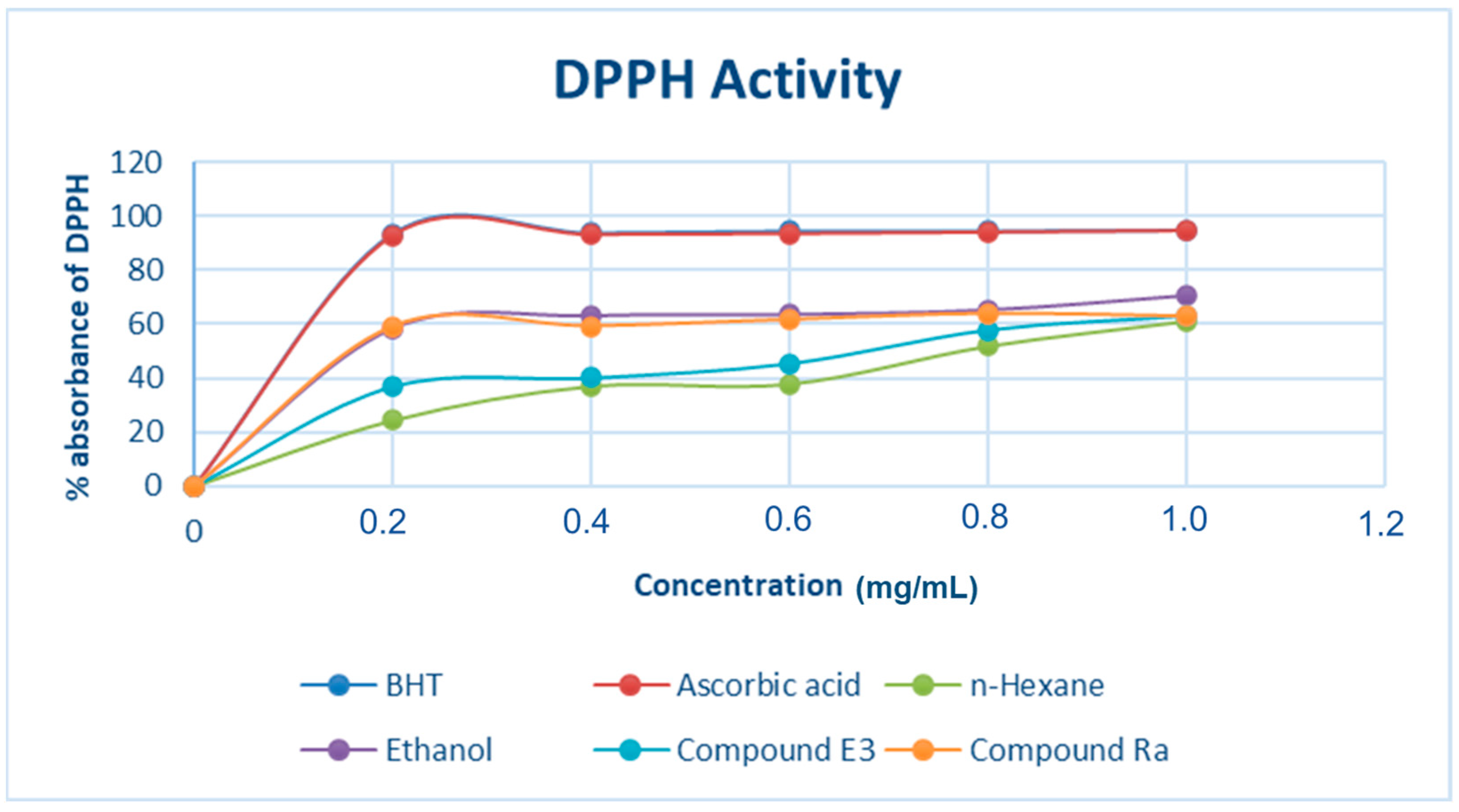
2.7.1. DPPH Free Radical Scavenging Assay
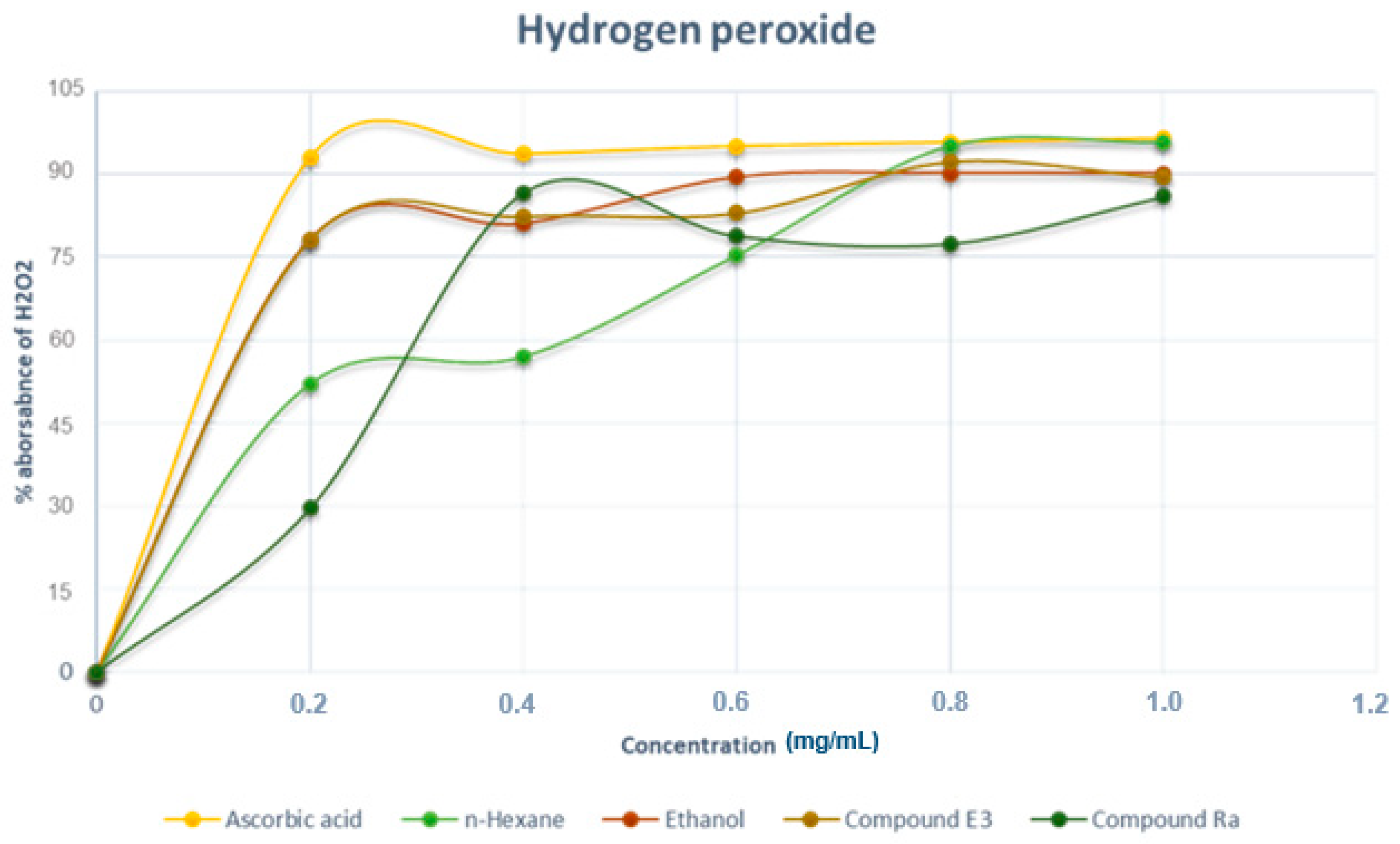
2.7.2. Hydrogen Peroxide Free Radical Scavenging Assay
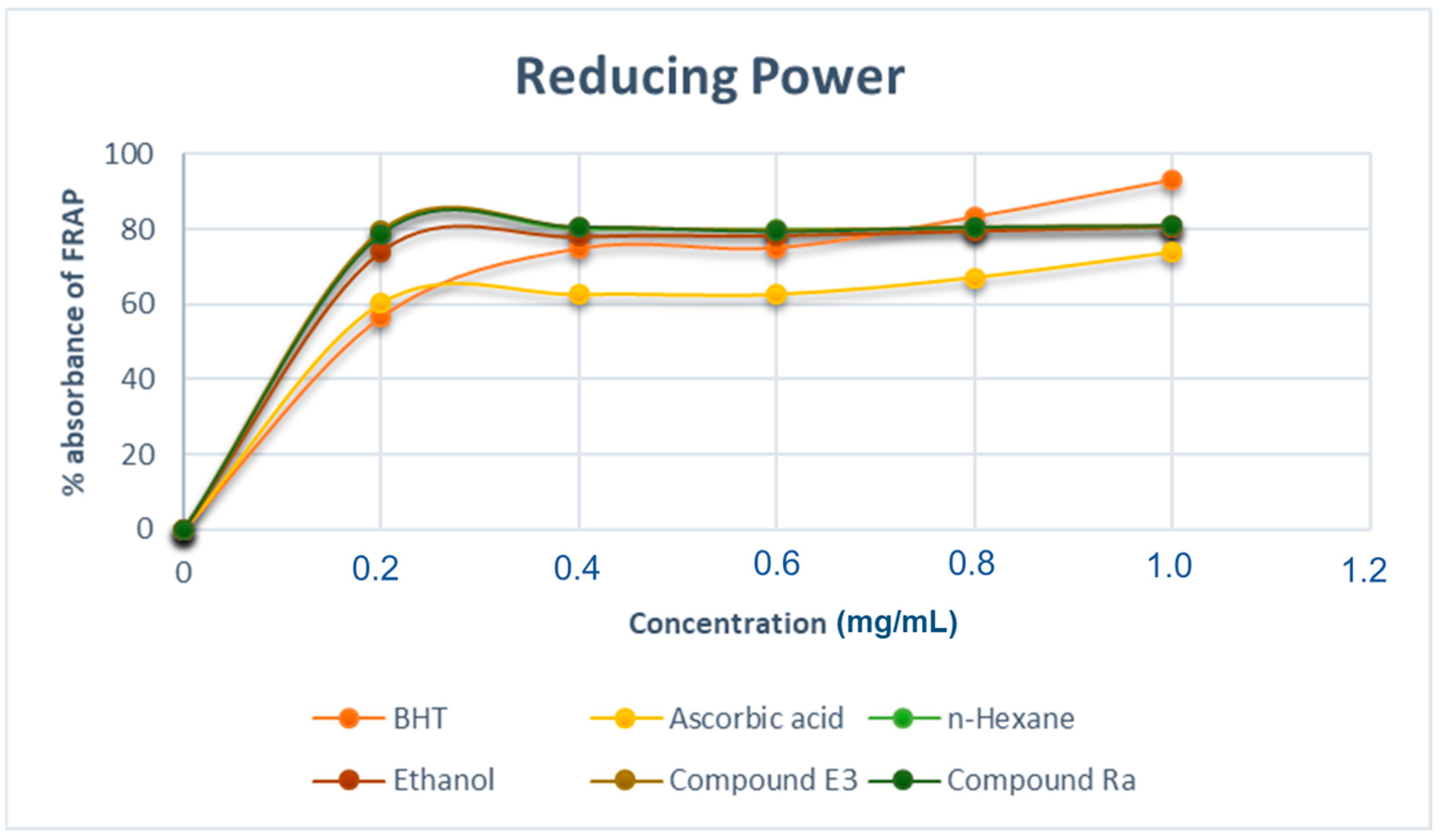
2.7.3. Ferric Reducing Power Assay
2.8. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Isolation of Compounds from the Hexane Extract
4.2.1. Bulking of Fractions from Hexane Extract Column
4.2.2. Re-Chromatography of Major Fraction A
4.2.3. Re-Chromatography of Major Fraction A2
4.3. Isolation of Compounds from the Ethanol Extract
4.3.1. Bulking of Fractions from Ethanol Extract Column
4.3.2. Bacterial Culture and Maintenance
4.3.3. Preparation of Inoculum
4.4. TLC Bioautography
4.4.1. In Vitro Qualitative and Quantitative Antioxidant Assay
4.4.2. DPPH Antioxidant Activity Assay
4.4.3. Hydrogen Peroxide Activity Assay
4.4.4. Reducing Power Activity Assay
4.4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation, and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Jima, T.T.; Megersa, M. Ethnobotanical study of medicinal plants used to treat human diseases in Berbere District, Bale Zone of Oromia Regional State, Southeast Ethiopia. Evid. Based Complement. Altern. Med. 2018, 2018, 8602945. [Google Scholar]
- Shanmugavel, G.; Prabakaran, K.; George, B. Evaluation of phytochemical constituents of Moringa oleifera (Lam) leaves collected from Puducherry region, South India. Int. J. Zool. Appl. Biosci. 2018, 3, 1–8. [Google Scholar]
- Mahmood, K.T.; Mugal, T.; Ul Haq, I. Moringa oleifera: A natural gift-a review. J. Pharm. Sci. Res. 2010, 2, 775–781. [Google Scholar]
- Sher, H.; Aldosari, A.; Ali, A.; De Boer, H.J. Economic benefits of high value medicinal plants to Pakistani communities: An analysis of current practice and potential. J. Ethnobiol. Ethnomed. 2014, 10, 71. [Google Scholar] [CrossRef]
- Nawaz, Y.; Asim, M. Child Malnutrition in Pakistan: Evidence from Literature. Children 2018, 5, 60. [Google Scholar]
- Uilah, N.; Zahoor, M.; Khan, F.A.; Khan, S. A review on general introduction to medicinal plants, it’s phytochemicals and roles of heavy metal and inorganic constituents. Life Sci. J. 2014, 11, 520–527. [Google Scholar]
- Helal, I.M.; El-Bessoumy, A.; Al-Bataineh, E.; Martin, R.P.; Rajagopalan, J.P.; Chandramoorthy, H.C.; Ahmed, S.B.H. Antimicrobial efficiency of essential oils from traditional medicinal plants of Asir Region, Saudi Arabia, over drug resistant isolates. Biomed Res. Int. 2019, 2019, 8928306. [Google Scholar] [CrossRef]
- El-Dahiyat, F.; Rashrash, M.; Abuhamdah, S.; Farha, R.A.; Babar, Z. Herbal medicines: A cross-sectional study to evaluate the prevalence and predictors of use among Jordanian adults. J. Pharm. Policy Pract. 2020, 13, 2. [Google Scholar] [CrossRef]
- Antasionasti, I.; Riyanto, S.; Rohman, A. Antioxidant activities and phenolics contents of Avocado (Persea americana Mill.) peel in vitro. Res. J. Med. Plants 2017, 11, 55–61. [Google Scholar]
- Mapfumari, S.; Nogbou, N.D.; Musyoki, A.; Gololo, S.; Mothibe, M.; Bassey, K. Phytochemical Screening, antioxidant and antibacterial properties of extracts of Viscum continuum E. Mey. Ex Sprague, a South African Mistletoe. Plants 2022, 11, 2094. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.A.; Rahman, H.; Niaz, Z.; Qasim, M.; Khan, J.; Tayyaba; Rehman, B. Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. Eur. J. Microbiol. Immunol. 2013, 3, 272–274. [Google Scholar] [CrossRef]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I.; et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Das, S.; Singh, S.; Gajamer, V.R.; Pradhan, N.; Lepcha, Y.D.; Tiwari, H.K. Prevalence of antibiotic resistance in commensal Escherichia coli among the children in rural hill communities of Northeast India. PLoS ONE 2018, 13, e0199179. [Google Scholar] [CrossRef] [PubMed]
- Romandini, A.; Pani, A.; Schenardi, P.A.; Pattarino, G.A.C.; De Giacomo, C.; Scaglione, F. Antibiotic resistance in pediatric infections: Global emerging threats, predicting the Near Future. Antibiotics 2021, 10, 393. [Google Scholar] [CrossRef]
- Yadav, A.; Kumari, R.; Yadav, A.; Mishra, J.P.; Srivatva, S.; Prabha, S. Antioxidants, and its functions in human body—A Review. Res. Environ. Life 2016, 9, 1328–1331. [Google Scholar]
- Karaman, R.; Jubeh, B.; Breijyeh, Z. Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules 2020, 25, 2888. [Google Scholar]
- Seanego, C.T.; Ndip, R.N. Identification, and antibacterial evaluation of bioactive compounds from Garcinia kola (Heckel) Seeds. Molecules 2012, 7, 6569–6584. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Budkaew, J.; Chumworathayi, B.; Pientong, C.; Ekalaksananan, T. Prevalence and factors associated with gonorrhea infection with respect to anatomic distributions among men who have sex with men. PLoS ONE 2019, 14, e0211682. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.; Jyot, J.; Ravi, M.; Ramphal, R. High pyocyanin production and non-motility of Pseudomonas aeruginosa isolates are correlated with septic shock or death in bacteraemia patients. PLoS ONE 2021, 16, e0253259. [Google Scholar] [CrossRef] [PubMed]
- Iwu-Jaja, C.J.; Jaca, A.; Jaja, I.F.; Jordan, P.; Bhengu, P.; Iwu, C.D.; Okeibunor, J.; Karamagi, H.; Tumusiime, P.; Fuller, W.; et al. Preventing and managing antimicrobial resistance in the African region: A scoping review protocol. PLoS ONE 2021, 16, e0254737. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, M.; Scibisz, I.; Przybył, J.L.; Laudy, A.E.; Majewska, E.; Tarnowska, K.; Małajowicz, J.; Ziarno, M. Antioxidant and Antibacterial Activity of Extracts from Selected Plant Material. Appl. Sci. 2022, 12, 9871. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Wang, Z.; Li, P.; Zhao, C.; Liu, J. Comparative Analysis of Chemical Constituents of Moringa oleifera Leaves from China and India by Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-Of-Flight Mass Spectrometry. Molecules 2019, 24, 942. [Google Scholar] [CrossRef]
- Ojiako, E.N. Phytochemical analysis and antimicrobial screening of Moringa oleifera leaves extract. Int. J. Eng. Sci. 2014, 3, 32–35. [Google Scholar]
- Sousa, G.F.; Duarte, L.P.; Alcantara, A.F.C.; Silva, G.D.F.; Vieira-Filho, S.A.; Silva, R.R.; Oliveira, D.M.; Takahash, J.A. New Triterpenes from Maytenus robusta: Structural Elucidation Based on NMR Experimental Data and Theoretical Calculation. Molecules 2012, 17, 13439–13456. [Google Scholar] [CrossRef]
- Salimi, Y.; Bialangi, N.; Widysusanti Abdulkadir, W.; Situmeang, B. Triterpenoid Compound From n-Hexane Extract of Kelor Leaves (Moringa oleifera Lamk.) And Antibacterial Activity Test Against Staphylococus aureus and Escherichia coli. Indones. J. Chem. Res. 2019, 7, 32–40. [Google Scholar] [CrossRef]
- Okorondu, S.I.; Akujobi, C.O.; Okorondu, J.N.; Anyado-Nwadike, S.O. Antimicrobial activity of the leaf extracts of Moringa oleifera and Jatropha curcas on pathogenic bacteria. Int. J. Biol. Chem. Sci. 2013, 7, 195–202. [Google Scholar] [CrossRef]
- Semenya, C.A. Phytochemical Fingerprinting of South African Medicinal Moringa oleifera Lam. Leaves and Quality Control Ofmoringa Leaf-Based Products. Master’s Thesis, Sefako Makgatho Health Sciences University, Pretoria, South Africa, 2020. [Google Scholar]
- Nuthan, B.R.; Rakshith, D.; Marulasiddaswamy, K.M.; Rao, H.C.Y.; Ramesha, K.P.; Mohana, N.C.; Siddappa, S.; Darshan, D.; Kumara, K.K.S.; Satish, S. Application of optimized and validated agar overlay TLC–Bioautography assay for detecting the antimicrobial metabolites of pharmaceutical interest. J. Chromatogr. Sci. 2020, 58, 737–746. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity using DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camaraleaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Gurumurthy, P.; Borra, S.K.; Cherian, K.M. Antioxidant, and free radical scavenging activity of triphala determined by using different in vitro models. J. Med. Plant Res. 2013, 39, 2898–2905. [Google Scholar]
- Bhoomi, B.J.; Piyush, C.; Jagdish, P.; Kinnari, N.M. In vitro evaluation of antioxidant and antiproliferative activity in crude extracts of argyreia nervosa roots. World J. Pharm. Pharm. Sci. 2014, 3, 1235–1250. [Google Scholar]
- Omede, A. Total polyphenolic content, and antioxidant properties of Moringa oleifera leaf extracts. Anim. Res. Int. 2016, 13, 2454–2462. [Google Scholar]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Moyo, B.; Oyedemi, S.; Masika, P.J.; Muchenje, V. Polyphenolic content, and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 2012, 91, 441–447. [Google Scholar] [CrossRef]
- Olivier, M.T.; Muganza, F.M.; Shai, L.J.; Gololo, S.S.; Nemutavhanani, L.D. Phytochemical screening, antioxidant, and antibacterial activities of ethanol extracts of Asparagus suaveolens aerial parts. S. Afr. J. Bot. 2017, 108, 41–46. [Google Scholar] [CrossRef]
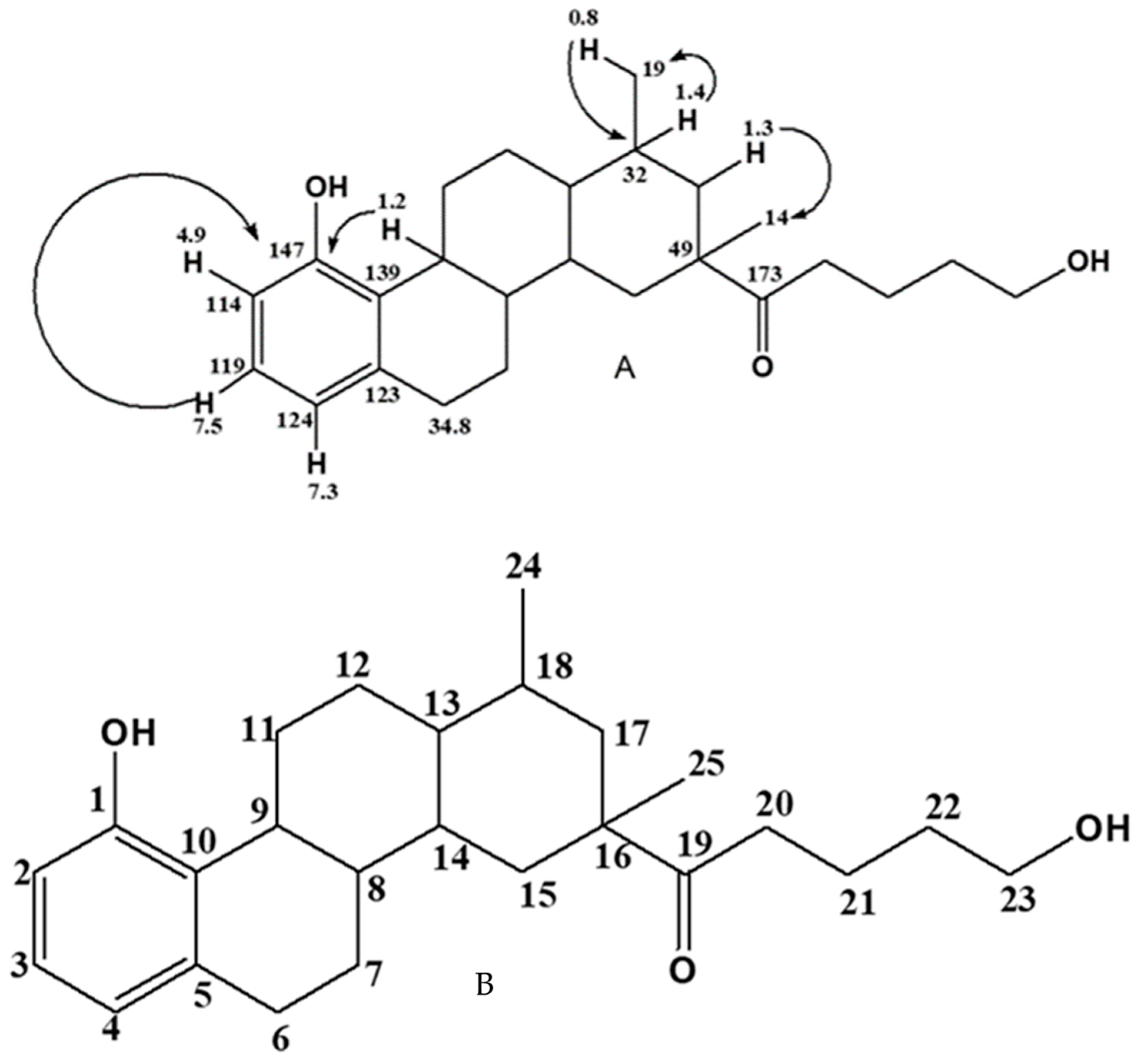
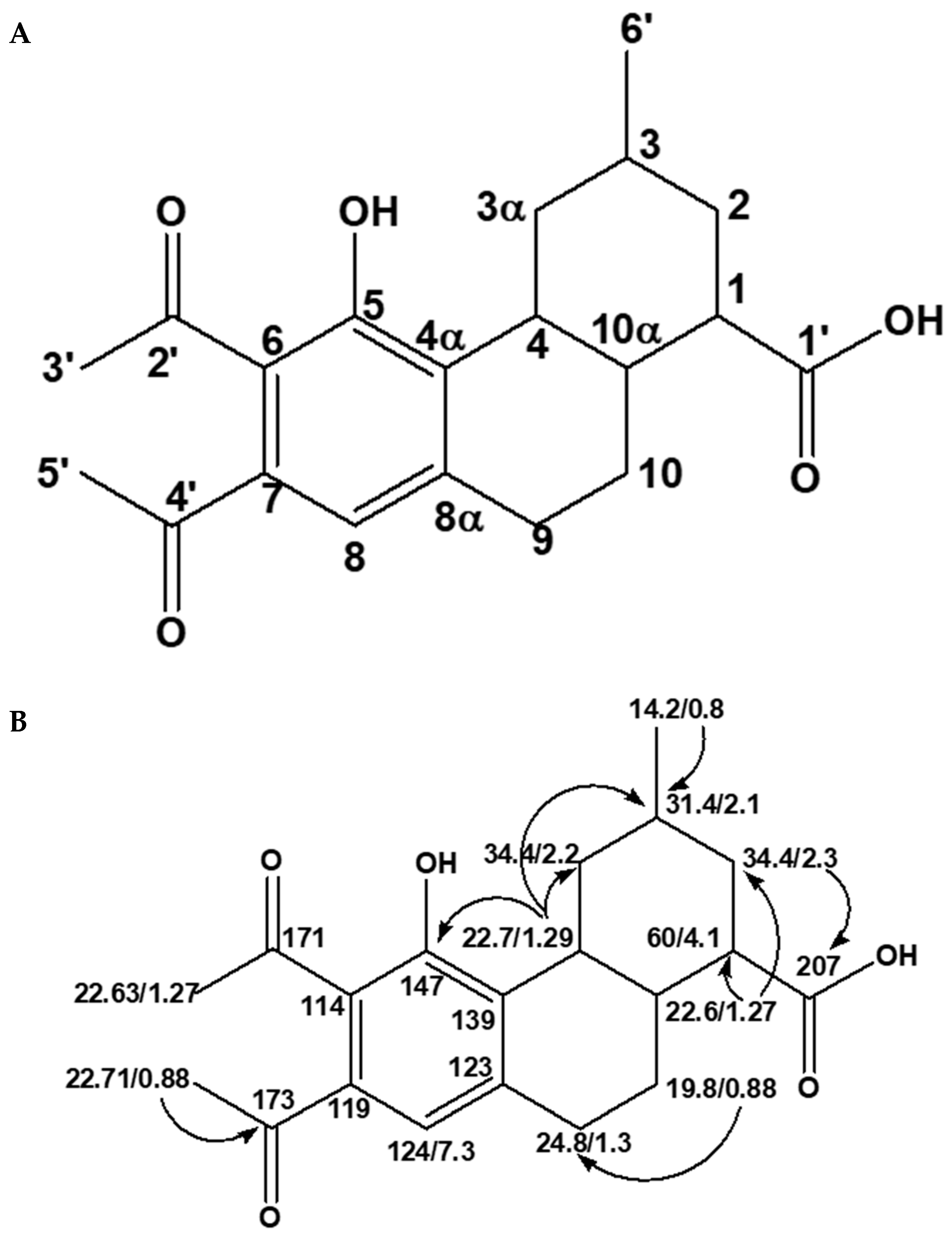

| Position | C-δ (ppm) | H-δ (ppm), J (Hz) |
|---|---|---|
| 1 | 147 | Cq |
| 2 | 114 | 7.14 (H, d, J = 8.4) |
| 3 | 119 | 7.54 (H, dd, 11.1, 3.1) |
| 4 | 124 | 7.37 (H, d, J = 14.0) |
| 5 | 123 | Cq |
| 6 | 34 | 2.29 (2H, m) |
| 7 | 24 | 1.69 (2H, dt, J = 11.4, 7.4) |
| 8 | 39 | 1.30 (H, m) |
| 9 | 28 | 2.02 (H, m) |
| 10 | 139 | Cq |
| 11 | 27 | 2.09 (2H, dd, J = 11.3, 4.2) |
| 12 | 31 | 2.36 (2H, m) |
| 13 | 50 | 3.51 (H, |
| 14 | 29.6 | 1.30 (H, m) |
| 15 | 30 | 2.10 (2H, t, J = 5.7) |
| 16 | 49 | Cq |
| 17 | 37 | 1.32 (2H, m) |
| 18 | 32 | 1.44 (H, dd, J = 12.1, 2.9) |
| 19 | 173 | Cq |
| 20 | 33 | 2.36 (2H, dd, J = 11.3, 3.2) |
| 21 | 22 | 0.89 (2H, m) |
| 22 | 29.5 | 1.30 (2H, m) |
| 23 | 60 | 4.17 (2H, dd, J = 11.5, 3.0) |
| 24 | 19 | 0.89 (3H, s) |
| 25 | 14 | 0.90 (3H, s) |
| Position | C-δ (ppm) | H-δ (ppm), J (Hz) |
|---|---|---|
| 1 | 60.16 | 4.13 (q, H, J = 7.2) |
| 2 | 39.89 | 2.02 (dd, 2H, J = 11.8, 7.0), |
| 3 | 28.96 | 1.63 (m, H) |
| 3α | 34.42 | 2.33 (dd, 2H, J = 11.7, 7.2), |
| 4 | 31.44 | 2.19 (m, H) |
| 4α | 139.03 | Cq |
| 5 | 147.09 | Cq |
| 6 | 114.08 | Cq |
| 7 | 119.09 | Cq |
| 8 | 124.47 | 7.38 (s, H) |
| 8α | 123.99 | Cq |
| 9 | 30.94 | 1.5 (dd, 2H, J = 13.3, 3.6) |
| 10 | 24.81 | 1.30 (m, 2H) |
| 10α | 29.68 | 2.02 (m, H, J = 10.8, 7.6) |
| 1′ | 207.02 | Cq |
| 2′ | 171.00 | Cq |
| 3′ | 22.63 | 1.27 (s, 3H) |
| 4′ | 173.98 | Cq |
| 5′ | 22.71 | 0.88 (s, 3H), |
| 6′ | 14.21 | 0.87 (s, 3H) |
| Position | C-δ (ppm) | H-δ (ppm), J (Hz) |
|---|---|---|
| 1 | 22.66 | 1.36 (2H, dd, J = 10, 4) |
| 2 | 29.33 | 1.14 (2H, td, J = 12, 4) |
| 3 | 68.32 | 3.87 (H, m) |
| 4 | 34.49 | 1.23 (2H, d, J = 8) |
| 5 | 119.90 | Cq |
| 6 | 124.48 | 7.37 (H, m) |
| 7 | 29.63 | 1.30 (2H, m) |
| 8 | 29.67 | Cq |
| 9 | 31.42 | 2.02 (H, m) |
| 10 | 30.21 | Cq |
| 11 | 68.03 | 3.59 (H, td, J = 12, 4) |
| 12 | 114.03 | 7.51 (H, m) |
| 13 | 123.96 | Cq |
| 14 | 31.91 | 2.06 (H, t, J= 8) |
| 15 | 29.49 | 1.31 (2H, td, J = 8, 4) |
| 16 | 28.94 | 1.15 (2H, dt, J = 16, 4) |
| 17 | 34.52 | 1.30 (H, m) |
| 18 | 34.86 | 2.00 (H, m) |
| 19 | 29.14 | 1.99 (2H, m) |
| 20 | 28.55 | 1.29 (2H, m) |
| 21 | 27.07 | 1.13 (2H, tt, J = 16, 4) |
| 22 | 30.02 | 1.26 (2H, td, J = 8, 4) |
| 23 | 18.81 | 1.24 (3H, s) |
| 24 | 14.04 | 0.93 (3H, s) |
| Analyte | DPPH IC50 (mg/mL) | H2O2 IC50 (mg/mL) | FRAP IC50 (mg/mL) |
|---|---|---|---|
| BHT | 0.08 | Nd | 0.323 |
| Ascorbic acid | 0.88 | 0.443 | 0.421 |
| n-Hexane | 0.761 | 0.639 | 0.211 |
| Ethanol | 0.435 | 0.541 | 0.249 |
| Compound E3 | 0.671 | 0.559 | 0.208 |
| Compound Ra | 0.475 | 0.689 | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segwatibe, M.K.; Cosa, S.; Bassey, K. Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds. Molecules 2023, 28, 899. https://doi.org/10.3390/molecules28020899
Segwatibe MK, Cosa S, Bassey K. Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds. Molecules. 2023; 28(2):899. https://doi.org/10.3390/molecules28020899
Chicago/Turabian StyleSegwatibe, Mmabatho Kgongoane, Sekelwa Cosa, and Kokoette Bassey. 2023. "Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds" Molecules 28, no. 2: 899. https://doi.org/10.3390/molecules28020899
APA StyleSegwatibe, M. K., Cosa, S., & Bassey, K. (2023). Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds. Molecules, 28(2), 899. https://doi.org/10.3390/molecules28020899







