Inhibitory Effects of 2-Aminoethoxydiphenyl Borate (2-APB) on Three KV1 Channel Currents
Abstract
1. Introduction
2. Results
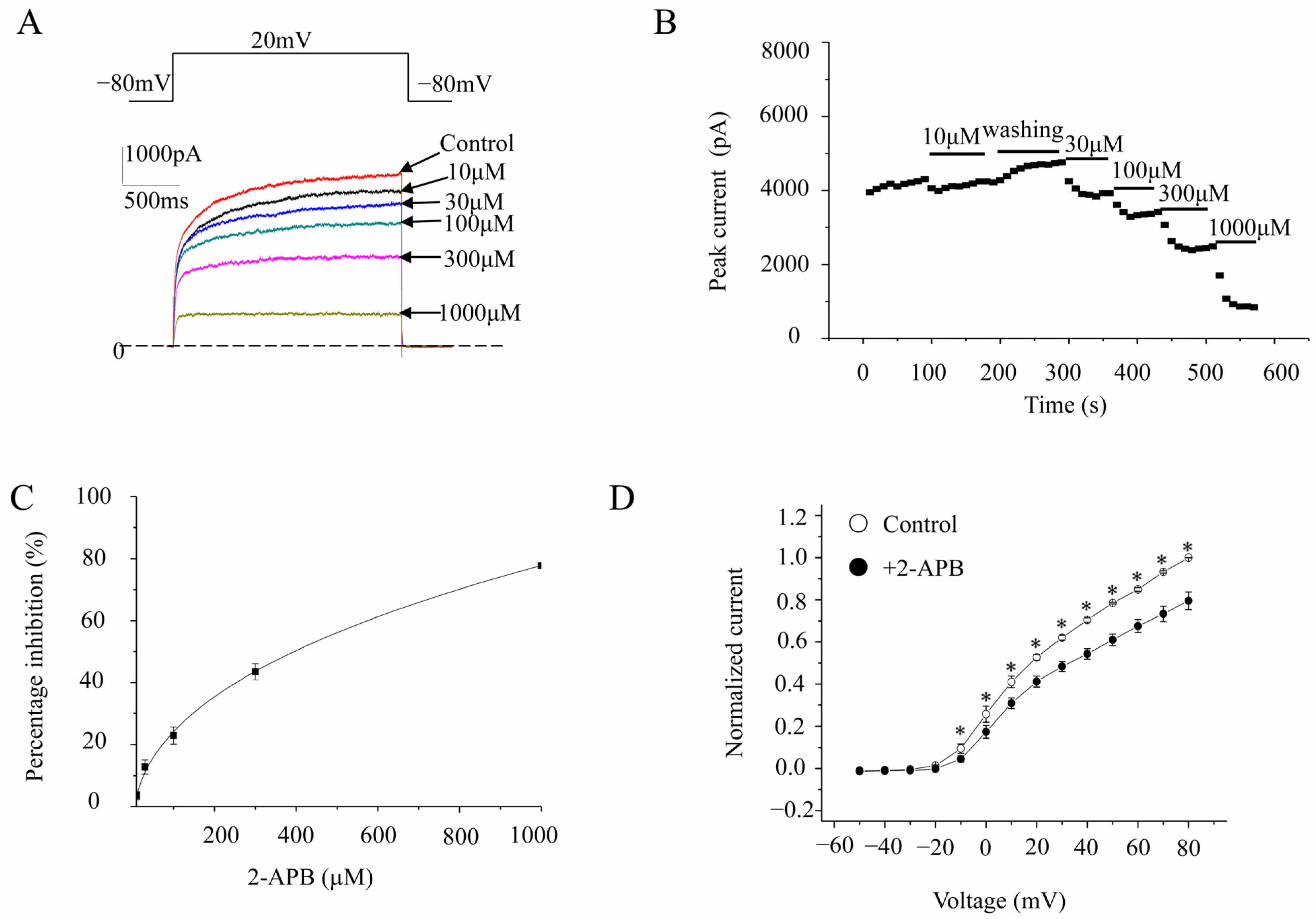
2.1. 2-APB Effects on Three hKV1 Channel Currents
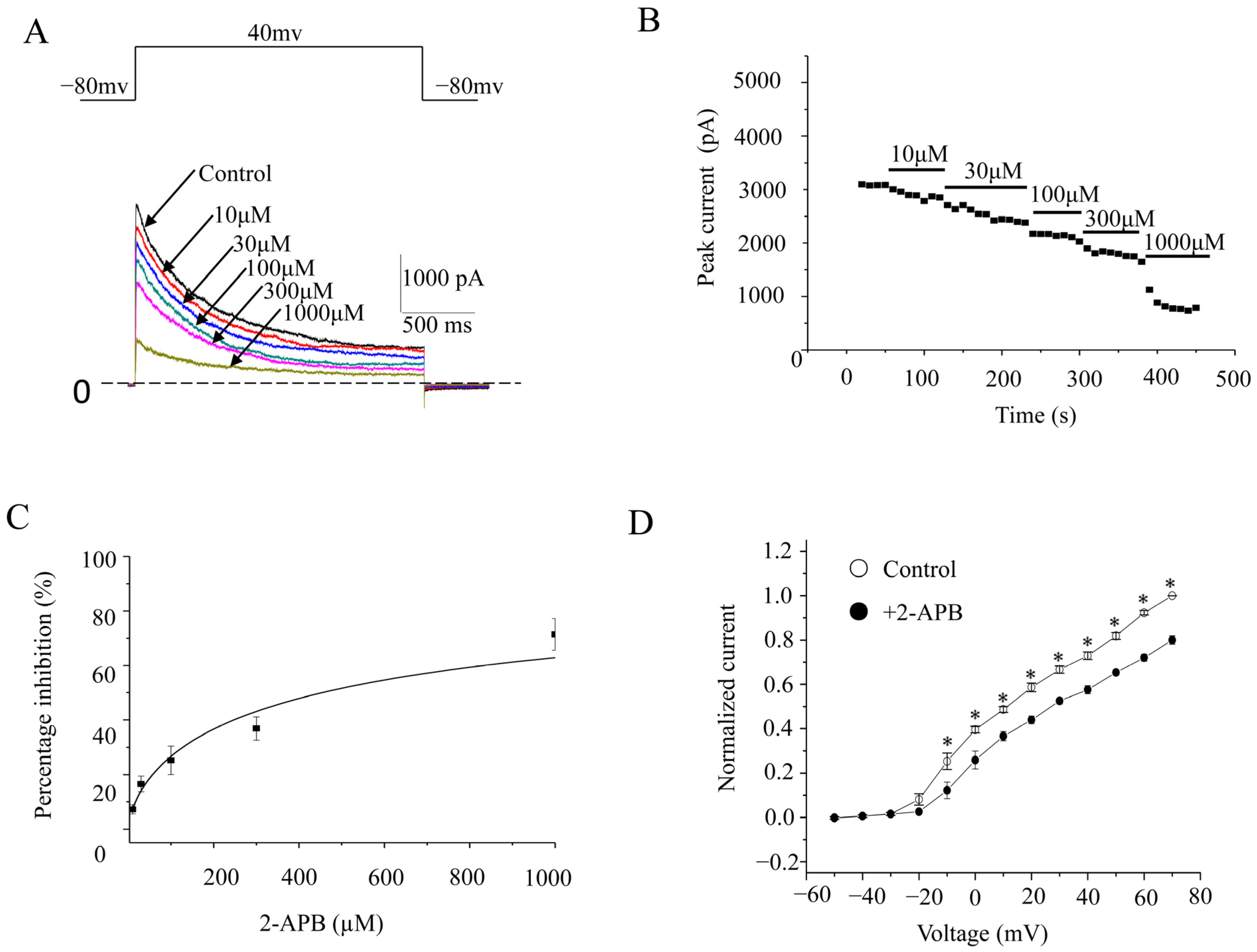
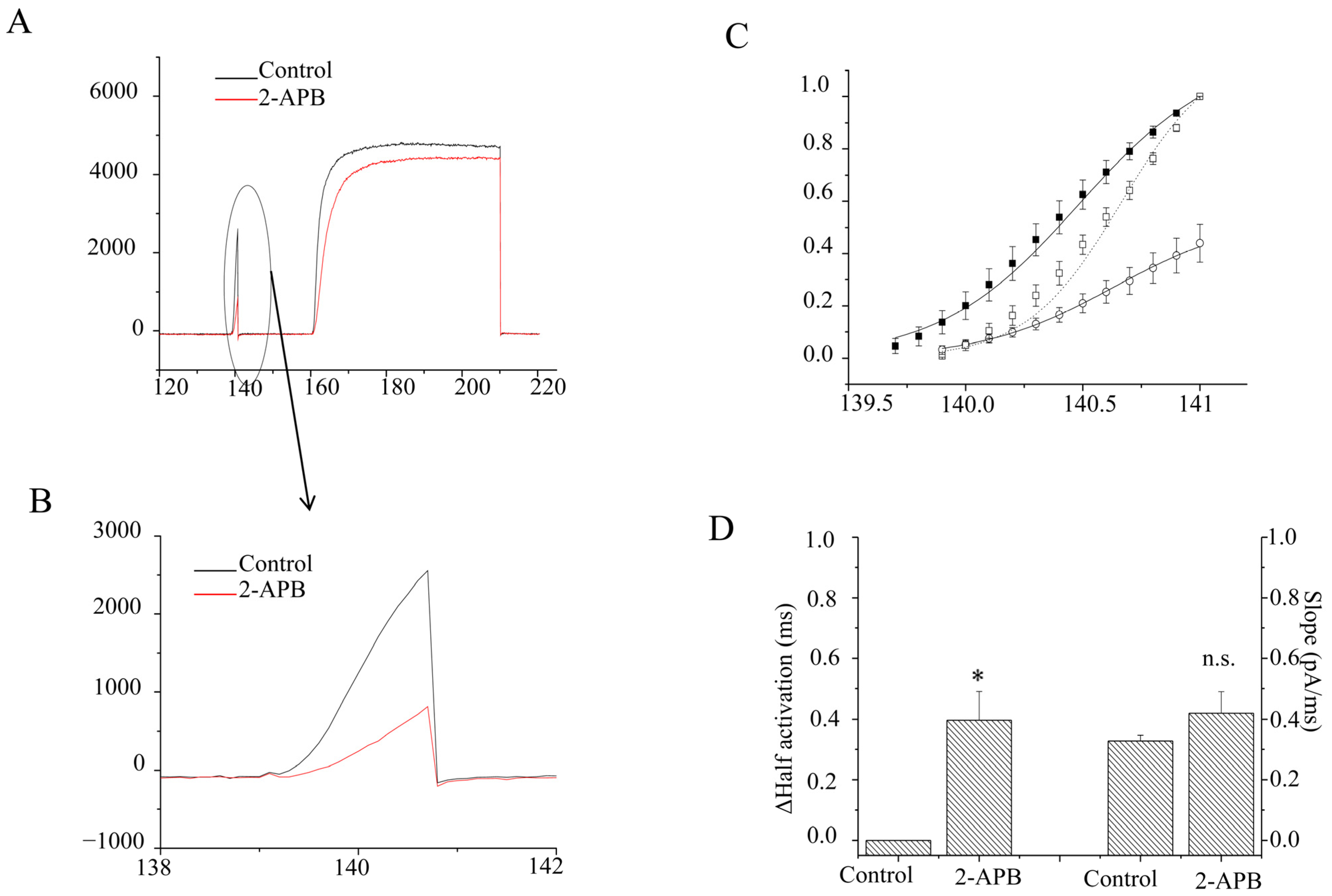
2.2. Characteristics of the Inhibition of 2-APB on Kv1.4
2.3. Effects of 2-APB on Mutation hKv1.4 Channels
2.3.1. Mutations at the Pore Region
2.3.2. N-Terminal Truncation
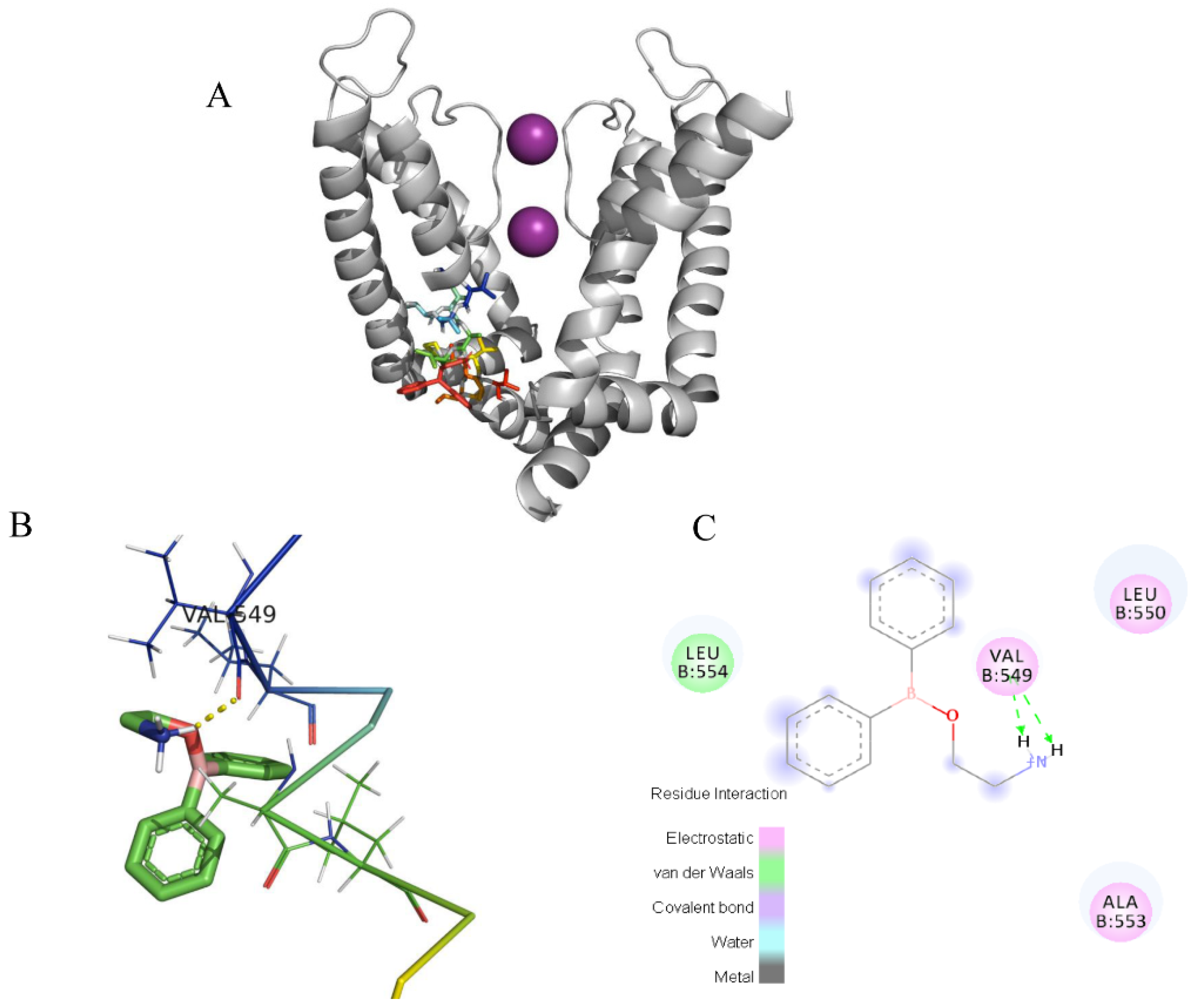
2.4. Docking of the Interactions between 2-APB and Three Channels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Site-Directed and N-Terminal Truncation Mutations of KV1.4
4.3. Data Recording
4.4. Statistical Analysis
4.5. Homology Modeling
4.6. Induced Fit Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Eroglu, E.; Unel, C.C.; Harmanci, N.; Erol, K.; Ari, N.S.; Ozatik, O. 2-Aminoethoxydiphenyl borate ameliorates functional and structural abnormalities in cisplatin-induced peripheral neuropathy. J. Trace Elem. Med. Biol. 2022, 70, 126909. [Google Scholar] [CrossRef] [PubMed]
- Rosalez, M.N.; Estevez-Fregoso, E.; Alatorre, A.; Antonio, A.G.; Marvin, A.S.U. 2-aminoethyldiphenyl borinate: A multitarget compound with potential as a drug precursor. Curr. Mol. Pharmacol. 2020, 13, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, L.; Beltrán, M.; Aguado, A.; Gisselmann, G.; Hatt, H. 2-Aminoethoxydiphenyl borate activates the mechanically gated human KCNK channels KCNK2 (TREK-1), KCNK 4 (TRAAK), and KCNK 10 (TREK-2). Front. Pharmacol. 2013, 4, 63. [Google Scholar] [CrossRef]
- Maruyama, T.; Kanaji, T.; Nakade, S.; Kanno, T.; Mikoshiba, K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997, 122, 498–505. [Google Scholar] [CrossRef]
- Zhuo, R.G.; Liu, X.Y.; Zhang, S.Z.; Wei, X.L.; Zheng, J.Q.; Xu, J.P.; Ma, X.Y. Insights into the stimulatory mechanism of 2-aminoethoxydiphenyl borate on TREK-2 potassium channel. Neuroscience 2015, 300, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Littlechild, R.; Zaidman, N.; Khodaverdi, D.; Mason, M.J. Inhibition of KCa3.1 by depolarisation and 2-aminoethoxydiphenyl borate (2-APB) during Ca2+ release activated Ca2+ (CRAC) entry in human erythroleukemia (HEL) cells: Implications for the interpretation of 2-APB inhibition of CRAC entry. Cell Calcium 2015, 57, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deshpande, M.; Payne, R. 2-Aminoethoxydiphenyl borate inhibits phototransduction and blocks voltage-gated potassium channels in Limulus ventral photoreceptors. Cell Calcium 2002, 32, 209–216. [Google Scholar] [CrossRef]
- Ma, K.T.; Guan, B.C.; Yang, Y.Q.; Nuttall, A.L.; Jiang, Z.G. 2-Aminoethoxydiphenyl borate blocks electrical coupling and inhibits voltage-gated K+ channels in guinea pig arteriole cells. Am. J. Physiol. 2011, 300, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Nimigean, C.M. Voltage-gated potassium channels: A structural examination of selectivity and gating. Cold Spring Harb. Perspect. Biol. 2016, 5, a029231. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, Y. Progress in research of Kv1.1 and Kv1.3 channels as therapeutic targets. Curr. Top. Med. Chem. 2016, 16, 1877–1885. [Google Scholar] [CrossRef]
- Patel, S.P.; Campbell, D.L. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: Underlying molecular, cellular and biophysical mechanisms. J. physiol. 2005, 569, 7–39. [Google Scholar] [CrossRef] [PubMed]
- Marzian, S.; Stansfeld, P.J.; Rapedius, M.; Rinne, S.; Nematian-Ardestani, E.; Abbruzzese, J.L.; Steinmeyer, K.; Sansom, M.S.; Sanguinetti, M.C.; Baukrowitz, T.; et al. Side pockets provide the basis for a new mechanism of Kv channel-specific inhibition. Nat. Chem. Biol. 2013, 9, 507–513. [Google Scholar] [CrossRef]
- Long, S.B.; Campbell, E.B.; MacKinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 2005, 309, 897–903. [Google Scholar] [CrossRef]
- Chen, R.; Robinson, A.; Gordon, D.; Chung, S.H. Modeling the binding of three toxins to the voltage-gated potassium channel (Kv1.3). Biophys. J. 2011, 101, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Khabiri, M.; Nikouee, A.; Cwiklik, L.; Grissmer, S.; Ettrich, R. Charybdotoxin unbinding from the mKv1.3 potassium channel: A combined computational and experimental study. J. Phys. Chem. B 2011, 115, 11490–11500. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.H.; Choi, S.H.; Yoon, I.S.; Pyo, M.K.; Shin, T.J.; Choi, W.S.; Lim, Y.; Rhim, H.; Won, K.H.; et al. Ginsenoside Rg3 inhibits human Kv1.4 channel currents by interacting with the Lys531 residue. Mol. Pharmacol. 2008, 73, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Danthi, S.J.; Enyeart, J.J. Curcumin potently blocks Kv1.4 potassium channels. Biochem. Biophys. Res. Commun. 2006, 344, 1161–1165. [Google Scholar] [CrossRef]
- Yeom, H.D.; Lee, J.H. Regulation of human Kv1.4 channel activity by the antidepressant metergoline. Biol. Pharm. Bull. 2016, 39, 1069–1072. [Google Scholar] [CrossRef]
- Binzen, U.; Greffrath, W.; Hennessy, S.; Bausen, M.; Saaler-Reinhardt, S.; Treede, R.D. Co-expression of the voltage-gated potassium channel Kv1.4 with transient receptor potential channels (TRPV1 and TRPV2) and the cannabinoid receptor CB1 in rat dorsal root ganglion neurons. Neuroscience 2006, 142, 527–539. [Google Scholar] [CrossRef]
- Rasmusson, R.L.; Morales, M.J.; Castellino, R.C.; Zhang, Y.; Campbell, D.L.; Strauss, H.C. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J. Physiol. 1995, 489, 709–721. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, W.; Pan, L.; Zhang, A.; Chen, Q.; Xu, K.; Lu, H.; Chen, Y. Peimine, a main active ingredient of Fritillaria, exhibits anti-inflammatory and pain suppression properties at the cellular level. Fitoterapia 2016, 111, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Pan, L.; Zhao, W.; Xu, L.; Xu, J.; Lu, H.; Chen, Y. Neochamaejasmin A inhibits KV1.4 channel activity via direct binding to the pore. Brain Res. 2018, 1683, 17–26. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef] [PubMed]

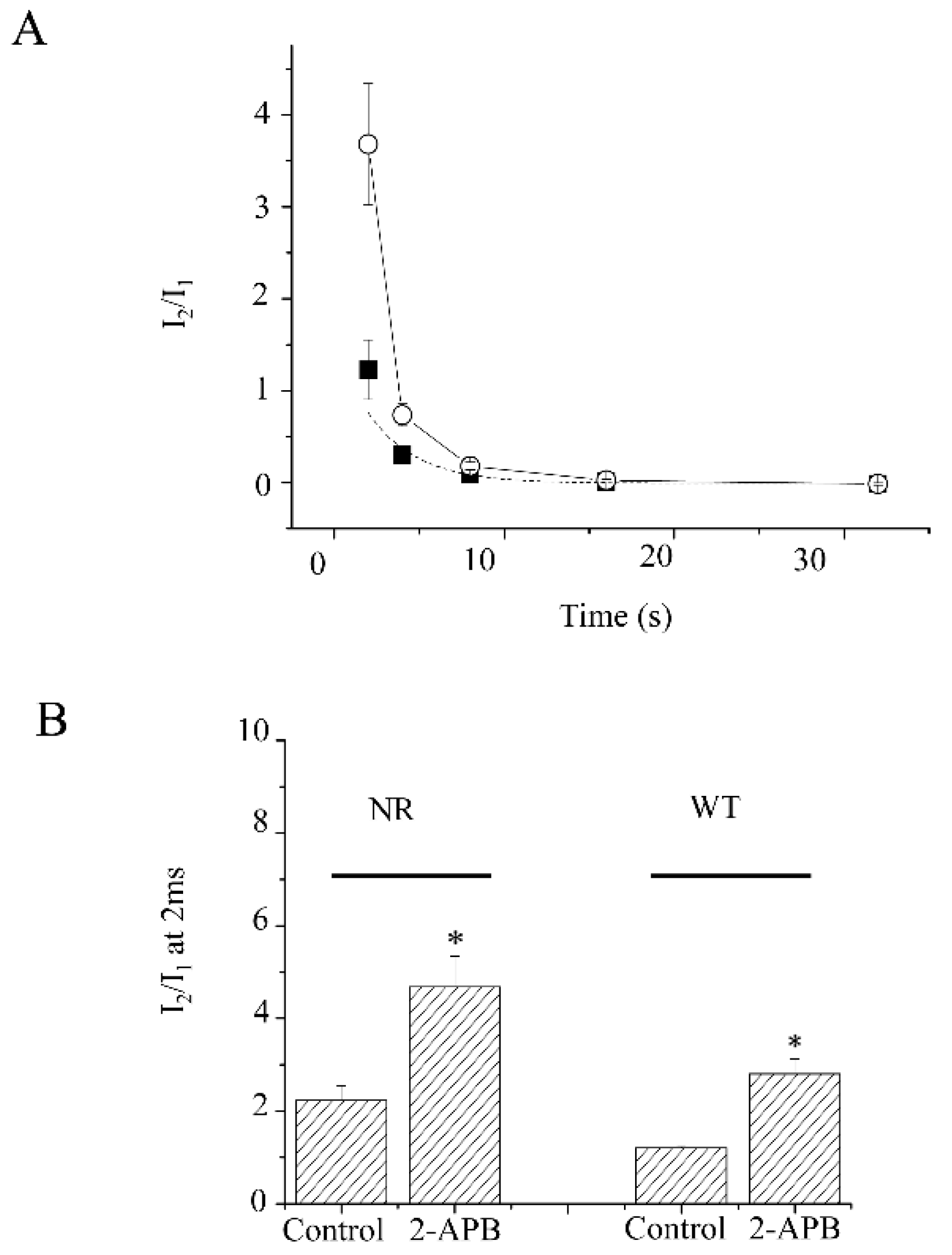
 points to the current amplitudes facilitated at a 2 ms prepulse length. (D). The onset of the slow inactivation of KV1.4 channels in the absence or presence of 2-APB. The prepulse length varied from 32 ms to 1024 ms.
points to the current amplitudes facilitated at a 2 ms prepulse length. (D). The onset of the slow inactivation of KV1.4 channels in the absence or presence of 2-APB. The prepulse length varied from 32 ms to 1024 ms.
 points to the current amplitudes facilitated at a 2 ms prepulse length. (D). The onset of the slow inactivation of KV1.4 channels in the absence or presence of 2-APB. The prepulse length varied from 32 ms to 1024 ms.
points to the current amplitudes facilitated at a 2 ms prepulse length. (D). The onset of the slow inactivation of KV1.4 channels in the absence or presence of 2-APB. The prepulse length varied from 32 ms to 1024 ms.





| Mutations | Inhibition (%) | S.E.M | Number of Cells |
|---|---|---|---|
| WT | 56.8 | 2.7 | 6 |
| KV1.4_G548A | N.D. | N.D. | N.D. |
| KV1.4_V549A | 44.1 * | 0.2 | 4 |
| KV1.4_L550A | 50.0 | 4.4 | 4 |
| KV1.4_T551A | 37.8 * | 6.0 | 4 |
| KV1.4_I552A | N.D. | N.D. | N.D. |
| KV1.4_A553V | 18.4 * | 7.0 | 6 |
| KV1.4_L554A | 32.9 * | 9.7 | 4 |
| KV1.4_P555A | N.D. | N.D. | N.D. |
| KV1.4_V556A | 60.8 | 0.5 | 3 |
| KV1.4_P557A | N.D. | N.D. | N.D. |
| KV1.4_V558A | 57.3 | 4.1 | 5 |
| KV1.4_I559A | N.D. | N.D. | N.D. |
| KV1.4_V560A | N.D. | N.D. | N.D. |
| KV1.4_S561A | N.D. | N.D. | N.D. |
| KV1.4_ truncation | 23.1 * | 2.5 | 5 |
| Ligand | Protein | Binding Amino Acid Residues | Binding Energy (kcal/mol) | Inhibition Constant, Ki (μM) | VDW_HB Desolv_Energy (kcal/mol) | Ligand Efficiency |
|---|---|---|---|---|---|---|
| 2-APB | Kv1.2 (2A79) | T401 | −6.04 | 218.87 | −6.00 | 0.36 |
| Kv1.3 (model) | A473 | −5.56 | 350.52 | −6.56 | 0.30 | |
| Kv1.4 (model) | V549 | −7.26 | 118.52 | −7.56 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Pan, L.; Stalin, A.; Xu, J.; Wu, L.; Ke, X.; Chen, Y. Inhibitory Effects of 2-Aminoethoxydiphenyl Borate (2-APB) on Three KV1 Channel Currents. Molecules 2023, 28, 871. https://doi.org/10.3390/molecules28020871
Zhao W, Pan L, Stalin A, Xu J, Wu L, Ke X, Chen Y. Inhibitory Effects of 2-Aminoethoxydiphenyl Borate (2-APB) on Three KV1 Channel Currents. Molecules. 2023; 28(2):871. https://doi.org/10.3390/molecules28020871
Chicago/Turabian StyleZhao, Wei, Lanying Pan, Antony Stalin, Jianwei Xu, Liren Wu, Xianfu Ke, and Yuan Chen. 2023. "Inhibitory Effects of 2-Aminoethoxydiphenyl Borate (2-APB) on Three KV1 Channel Currents" Molecules 28, no. 2: 871. https://doi.org/10.3390/molecules28020871
APA StyleZhao, W., Pan, L., Stalin, A., Xu, J., Wu, L., Ke, X., & Chen, Y. (2023). Inhibitory Effects of 2-Aminoethoxydiphenyl Borate (2-APB) on Three KV1 Channel Currents. Molecules, 28(2), 871. https://doi.org/10.3390/molecules28020871







