Synthesis Routes on Electrochemical Behavior of Co-Free Layered LiNi0.5Mn0.5O2 Cathode for Li-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Materials and Preparation
2.2. Basic Characterization
2.3. Electrochemical Characterization
3. Results
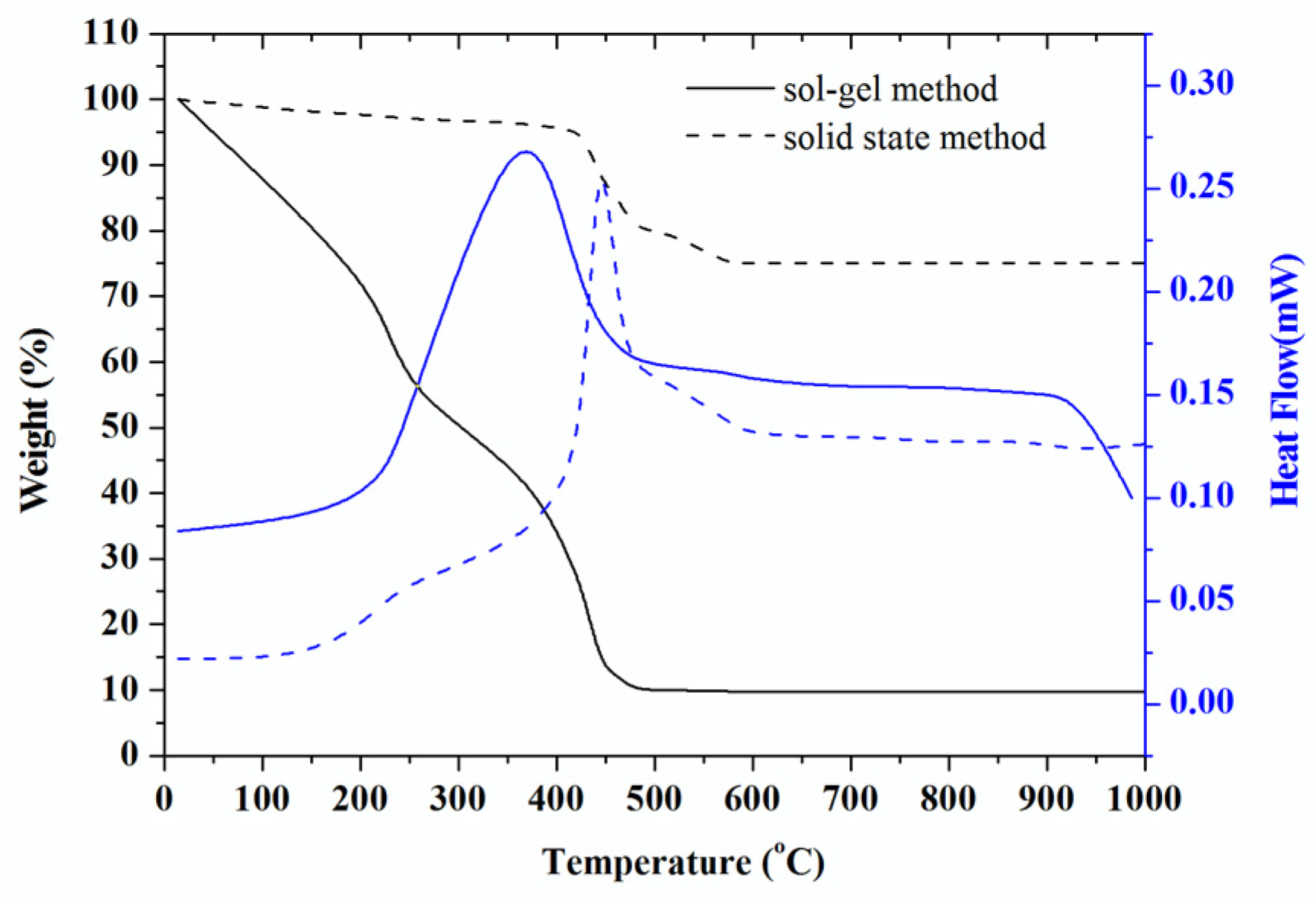
3.1. Weight Loss Decomposition
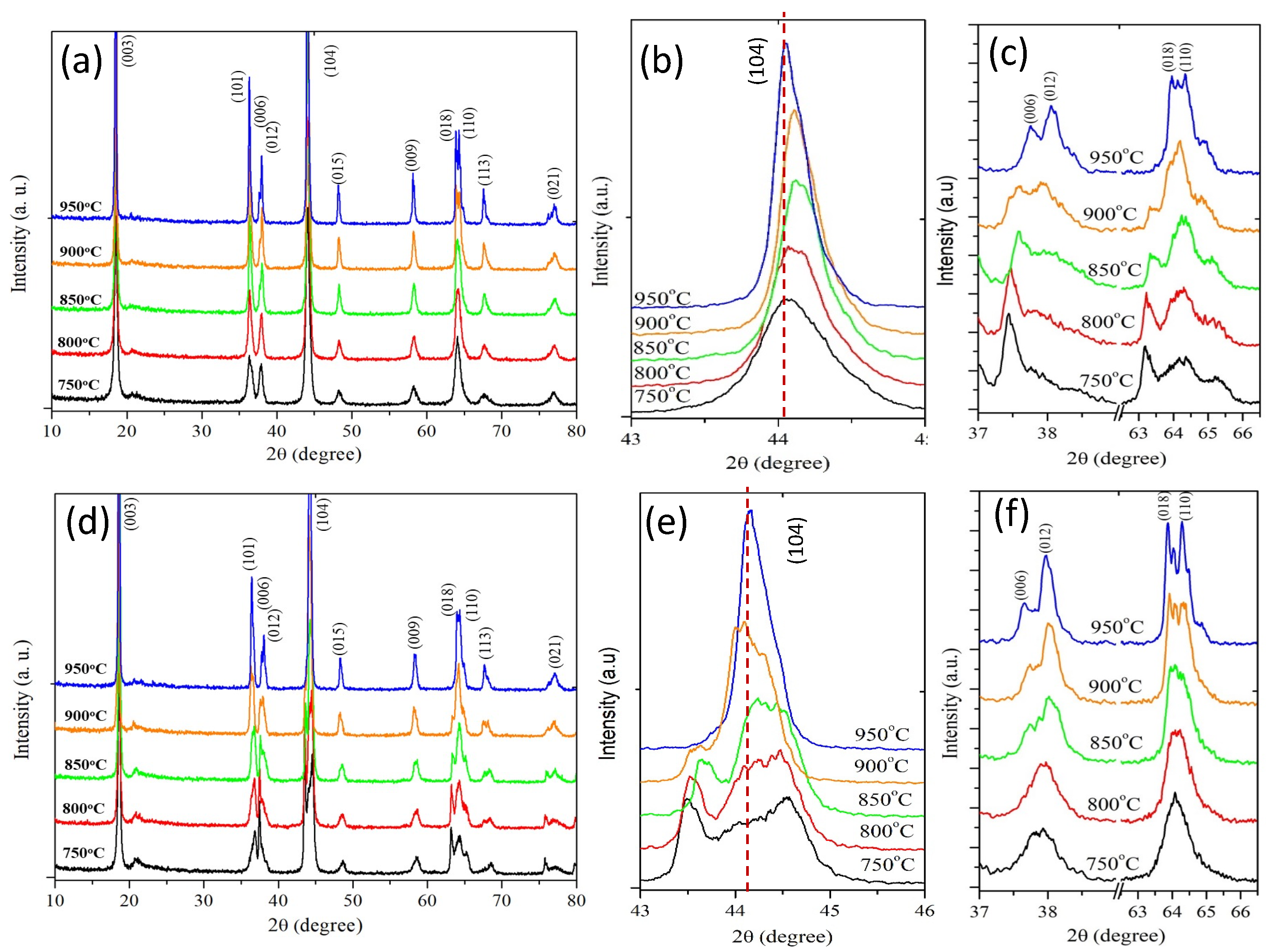
3.2. X–ray Diffraction Analysis
3.3. Morphology
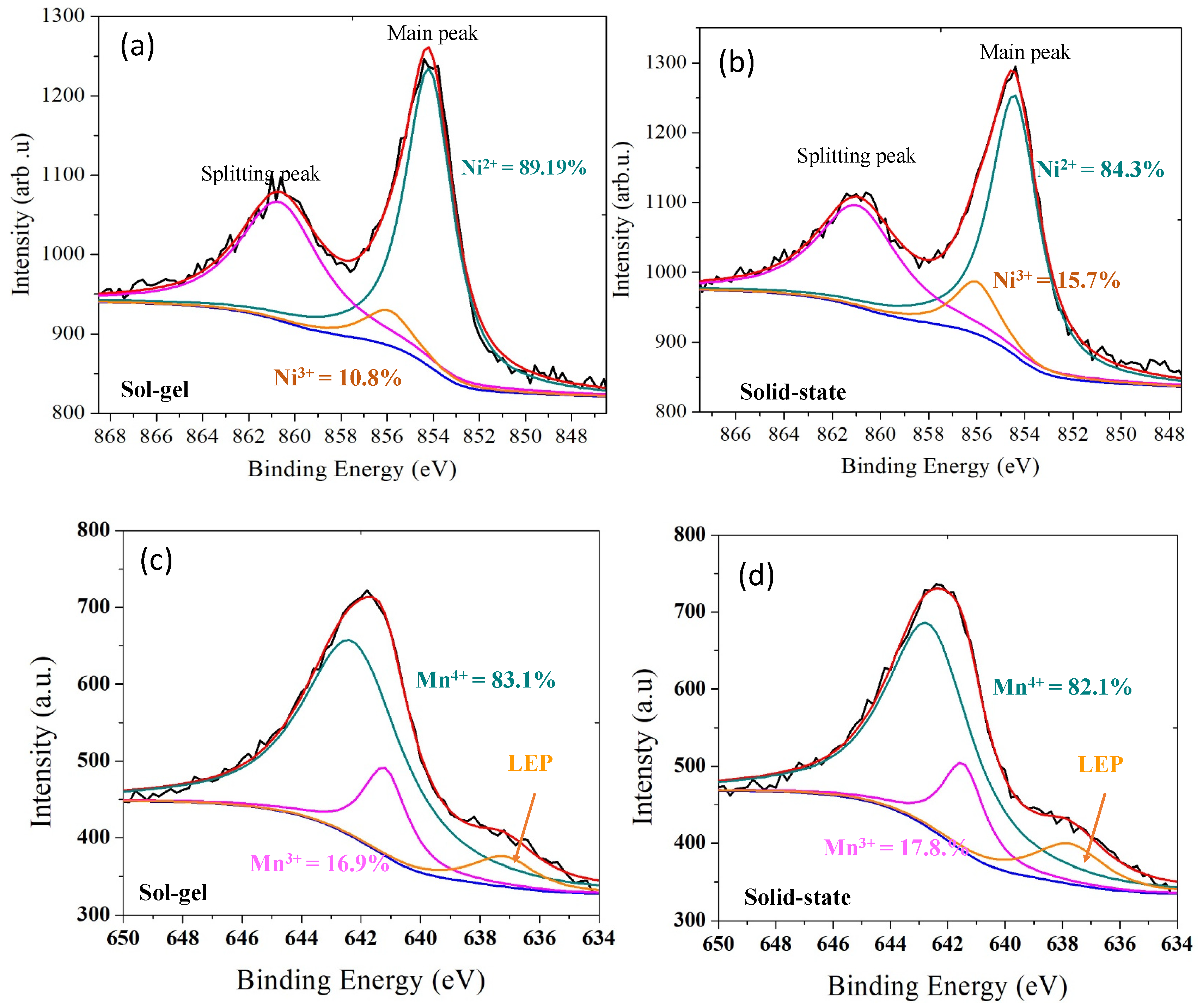
3.4. XPS
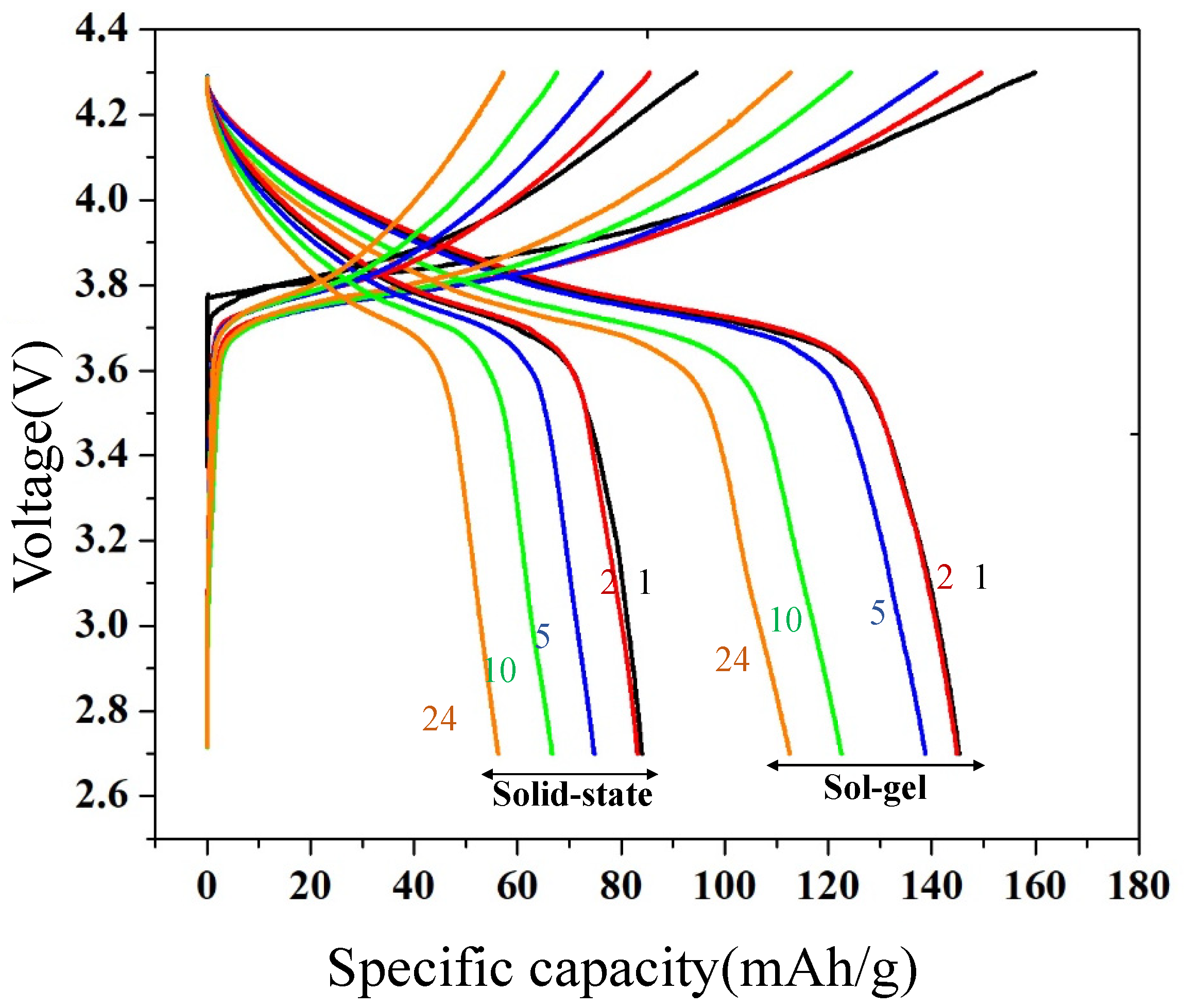
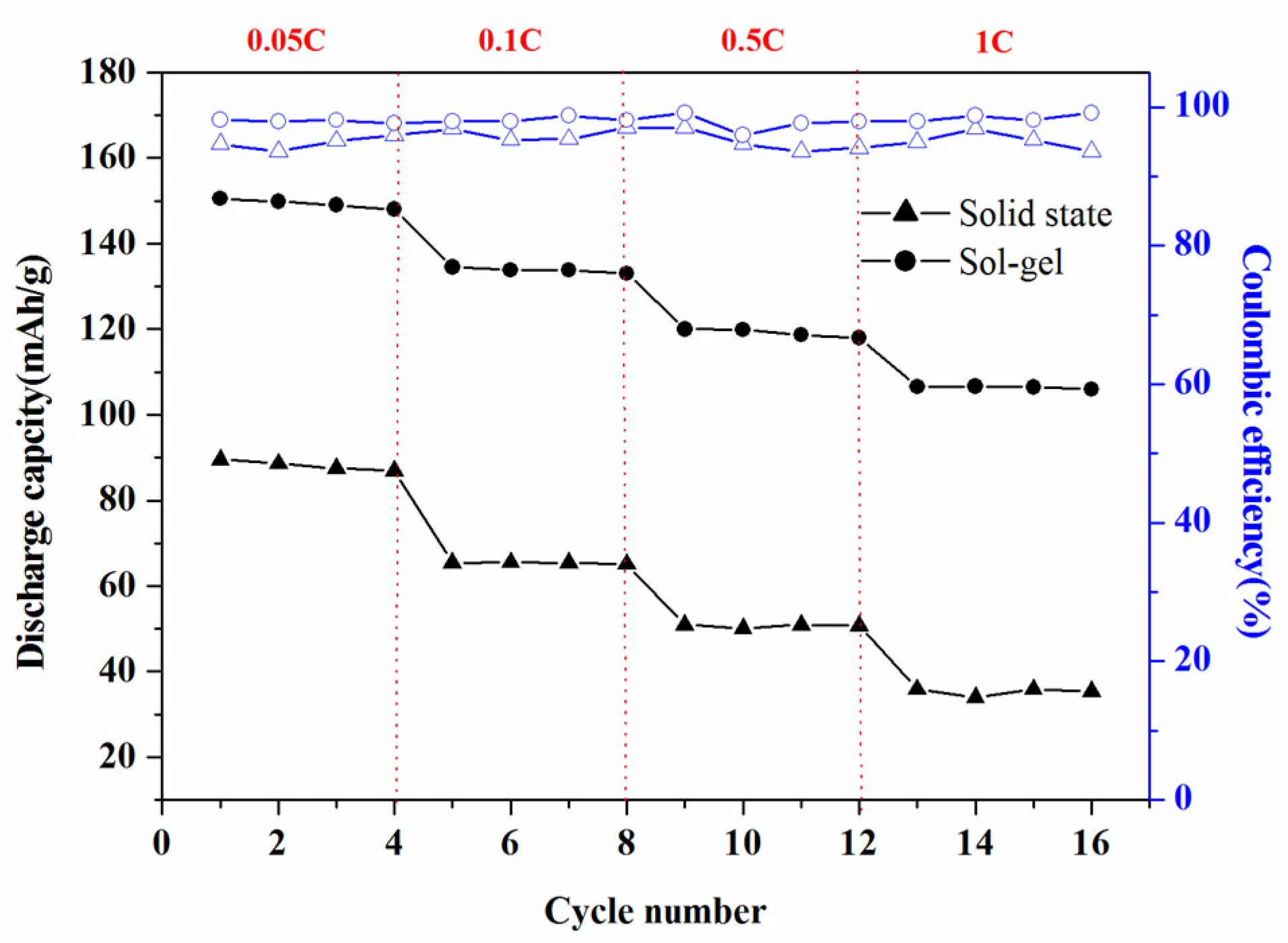
3.5. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhao, H.; Lam, W.Y.A.; Sheng, L.; Wang, L.; Bai, P.; Yang, Y.; Ren, D.S.; Xu, H.; He, X.M. Cobalt-Free Cathode Materials: Families and their Prospects. Adv. Energy Mater. 2022, 12, 2103894. [Google Scholar] [CrossRef]
- Darbar, D.; Malkowski, T.; Self, E.C.; Bhattacharya, I.; Reddy, M.V.V.; Nanda, J. An overview of cobalt-free, nickel-containing cathodes for Li-ion batteries. Mater. Today Energy 2022, 30, 101173. [Google Scholar] [CrossRef]
- Nie, L.; Wang, Z.Y.; Zhao, X.W.; Chen, S.J.; He, Y.J.; Zhao, H.J.; Gao, T.Y.; Zhang, Y.; Dong, L.; Kim, F.; et al. Cation/Anion Codoped and Cobalt-Free Li-Rich Layered Cathode for High-Performance Li-Ion Batteries. Nano Lett. 2021, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhang, D.Y.; Zhang, M.L.; Yan, Y.X.; Li, Z.M.; Wang, P.P.; Murakami, R.I. Understanding the Stability of LiNi0.5Mn0.5O2 as a Co-Free Positive Electrode. J. Phys. Chem. Lett. 2022, 13, 6181–6186. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Wan, L.; Cao, C.B. A high-rate and long cycling life cathode for rechargeable lithium-ion batteries: Hollow LiNi0.5Mn0.5O2 nano/micro hierarchical microspheres. Electrochim. Acta 2016, 191, 974–979. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiNi1/2Mn1/2O2: A possible alternative to LiCoO2 for advanced lithium-ion batteries. Chem. Lett. 2001, 30, 744–745. [Google Scholar] [CrossRef]
- Koyama, Y.; Makimura, Y.; Tanaka, I.; Adachi, H.; Ohzuku, T. Systematic research on insertion materials based on superlattice models in a phase triangle of LiCoO2-LiNiO2-LiMnO2. I. First-principles calculation on electronic and crystal structures, phase stability and new LiNi1/2Mn1/2O2 material. J. Electrochem. Soc. 2004, 151, A1499–A1506. [Google Scholar] [CrossRef]
- Breger, J.; Meng, Y.S.; Hinuma, Y.; Kumar, S.; Kang, K.; Shao-Horn, Y.; Ceder, G.; Grey, C.P. Effect of high voltage on the structure and electrochemistry of LiNi0.5Mn0.5O2: A joint experimental and theoretical study. Chem. Mater. 2006, 18, 4768–4781. [Google Scholar] [CrossRef]
- Phattharasupakun, N.; Cormier, M.M.E.; Lyle, E.; Zsoldos, E.; Liu, A.R.; Geng, C.X.; Liu, Y.L.; Li, H.Y.; Sawangphruk, M.; Dahn, J.R. Correlating Cation Mixing with Li Kinetics: Electrochemical and Li Diffusion Measurements on Li-Deficient LiNiO2 and Li-Excess LiNi0.5Mn0.5O2. J. Electrochem. Soc. 2021, 168, 090535. [Google Scholar] [CrossRef]
- Gwon, H.; Kim, S.W.; Park, Y.U.; Hong, J.; Ceder, G.; Jeon, S.; Kang, K. Ion-Exchange Mechanism of Layered Transition-Metal Oxides: Case Study of LiNi0.5Mn0.5O2. Inorg. Chem. 2014, 53, 8083–8087. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Muta, T.; Noguchi, H. Electrochemical characteristics of LiNi0.5Mn0.5-xTixO2 prepared by solid state method. J. Power Sources 2004, 135, 262–266. [Google Scholar] [CrossRef]
- Wu, T.; Wan, Y.H.; Huang, Q.; Xia, H. One-step hydrothermal synthesis and characterization of LiNi0.5Mn0.5O2 nanoparticles. Mater. Technol. 2015, 30, 176–180. [Google Scholar] [CrossRef]
- Lu, H.Q.; Wu, F.; Su, Y.F.; Li, N.; Chen, S.; Bao, L.Y. Electrochemical Performance of LiNi0.5Mn0.5O2 as Cathode Material for Lithium-Ion Batteries Prepared by Oxalate Co-Precipitation Method. Acta Phys.-Chim. Sin. 2010, 26, 51–56. [Google Scholar]
- Noda, Y.; Koga, N. Phenomenological Kinetics of the Carbonation Reaction of Lithium Hydroxide Monohydrate: Role of Surface Product Layer and Possible Existence of a Liquid Phase. J. Phys. Chem. C 2014, 118, 5424–5436. [Google Scholar] [CrossRef]
- Terayama, K.; Ikeda, M. Study on Thermal-Decomposition of MnO2 and Mn2O3 by Thermal-Analysis. Trans. Jpn. Inst. Met. 1983, 24, 754–758. [Google Scholar]
- Pei, Y.; Xu, C.Y.; Xiao, Y.C.; Chen, Q.; Huang, B.; Li, B.; Li, S.; Zhen, L.; Cao, G.Z. Phase Transition Induced Synthesis of Layered/Spinel Heterostructure with Enhanced Electrochemical Properties. Adv. Funct Mater. 2017, 27, 1604349. [Google Scholar] [CrossRef]
- Li, D.; Lian, F.; Hou, X.M.; Chou, K.C. Reaction mechanisms for 0.5Li2MnO3 center dot 0.5LiMn0.5Ni0.5O2 precursor prepared by low-heating solid state reaction. Int. J. Min. Met. Mater. 2012, 19, 856–862. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzynski, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2011, 104, 731–735. [Google Scholar] [CrossRef]
- Abdul Aziz, N.A.; Abdullah, T.K.; Mohamad, A.A. Synthesis of LiCoO2 via sol-gel method for aqueous rechargeable lithium batteries. Ionics 2018, 24, 403–412. [Google Scholar] [CrossRef]
- Kos, J.; Mousavihashemi, S.; Wilson, B.P.; Rautama, E.L.; Kallio, T. Comparative analysis on the thermal, structural, and electrochemical properties of Al-doped Li7La3Zr2O12 solid electrolytes through solid state and sol-gel routes. Solid State Ionics 2022, 380, 115943. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-Y.; Fung, K.-Z. Synthesis Routes on Electrochemical Behavior of Co-Free Layered LiNi0.5Mn0.5O2 Cathode for Li-Ion Batteries. Molecules 2023, 28, 794. https://doi.org/10.3390/molecules28020794
Tsai S-Y, Fung K-Z. Synthesis Routes on Electrochemical Behavior of Co-Free Layered LiNi0.5Mn0.5O2 Cathode for Li-Ion Batteries. Molecules. 2023; 28(2):794. https://doi.org/10.3390/molecules28020794
Chicago/Turabian StyleTsai, Shu-Yi, and Kuan-Zong Fung. 2023. "Synthesis Routes on Electrochemical Behavior of Co-Free Layered LiNi0.5Mn0.5O2 Cathode for Li-Ion Batteries" Molecules 28, no. 2: 794. https://doi.org/10.3390/molecules28020794
APA StyleTsai, S.-Y., & Fung, K.-Z. (2023). Synthesis Routes on Electrochemical Behavior of Co-Free Layered LiNi0.5Mn0.5O2 Cathode for Li-Ion Batteries. Molecules, 28(2), 794. https://doi.org/10.3390/molecules28020794






