Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase
Abstract
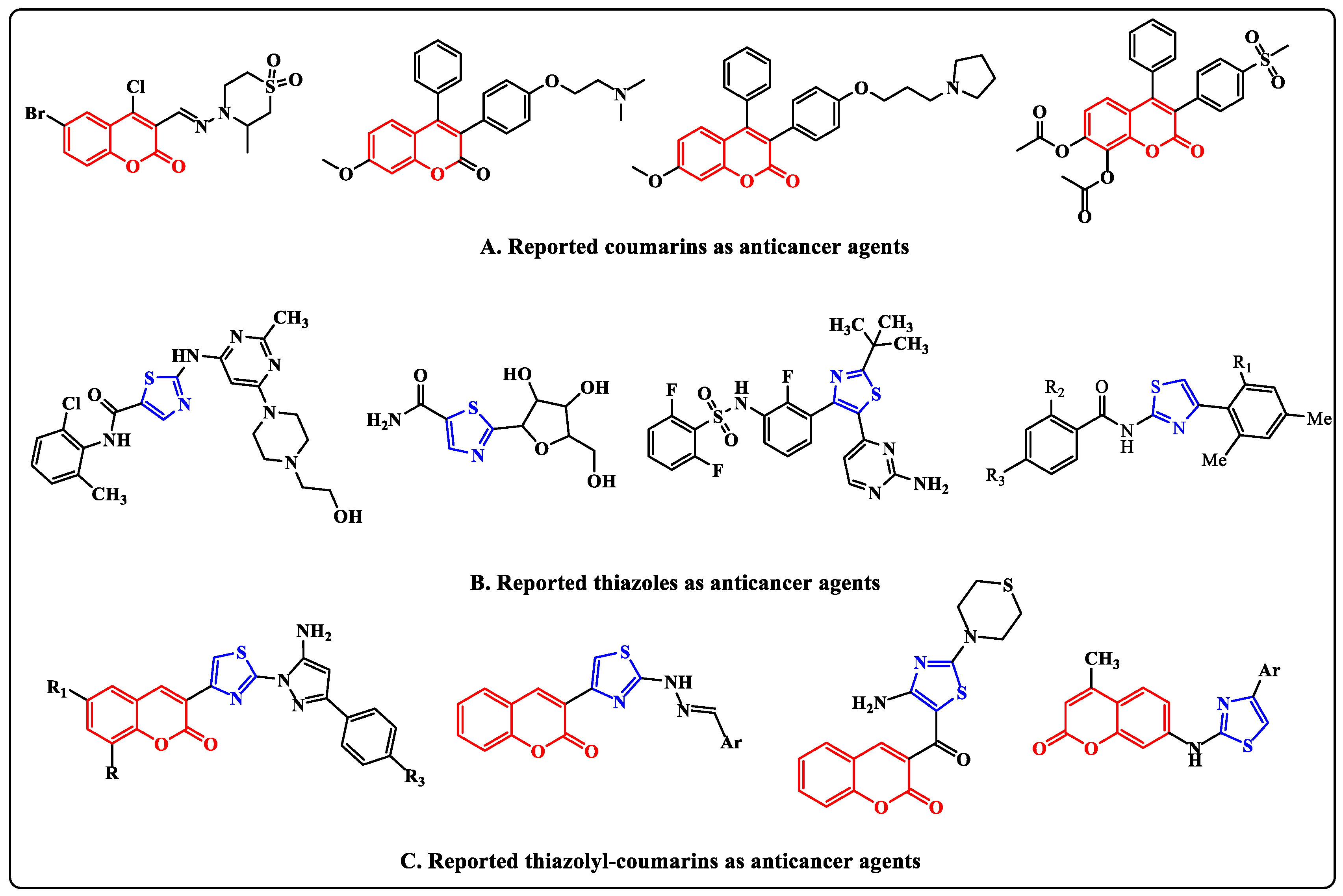
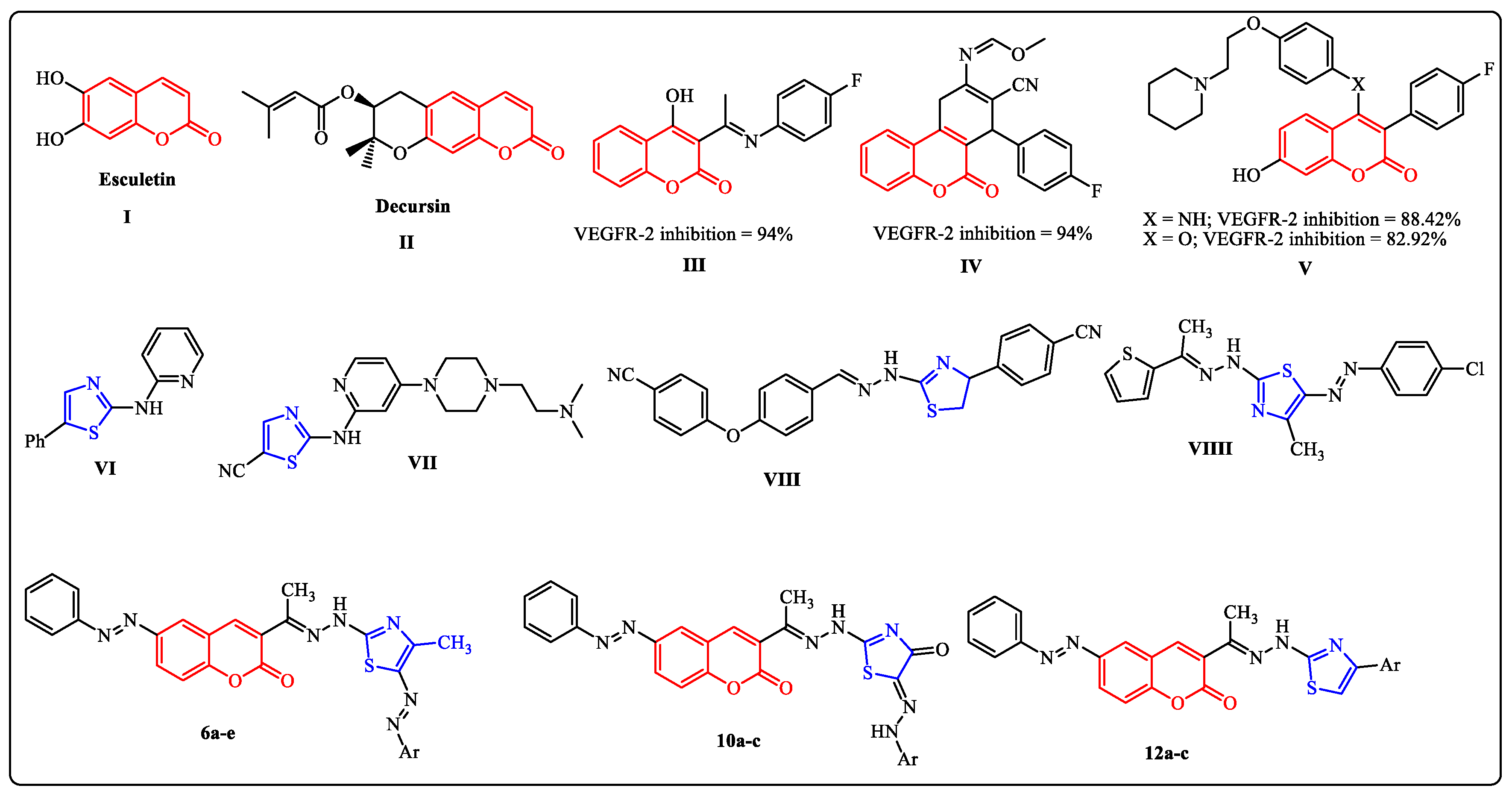
1. Introduction
2. Results and Discussion
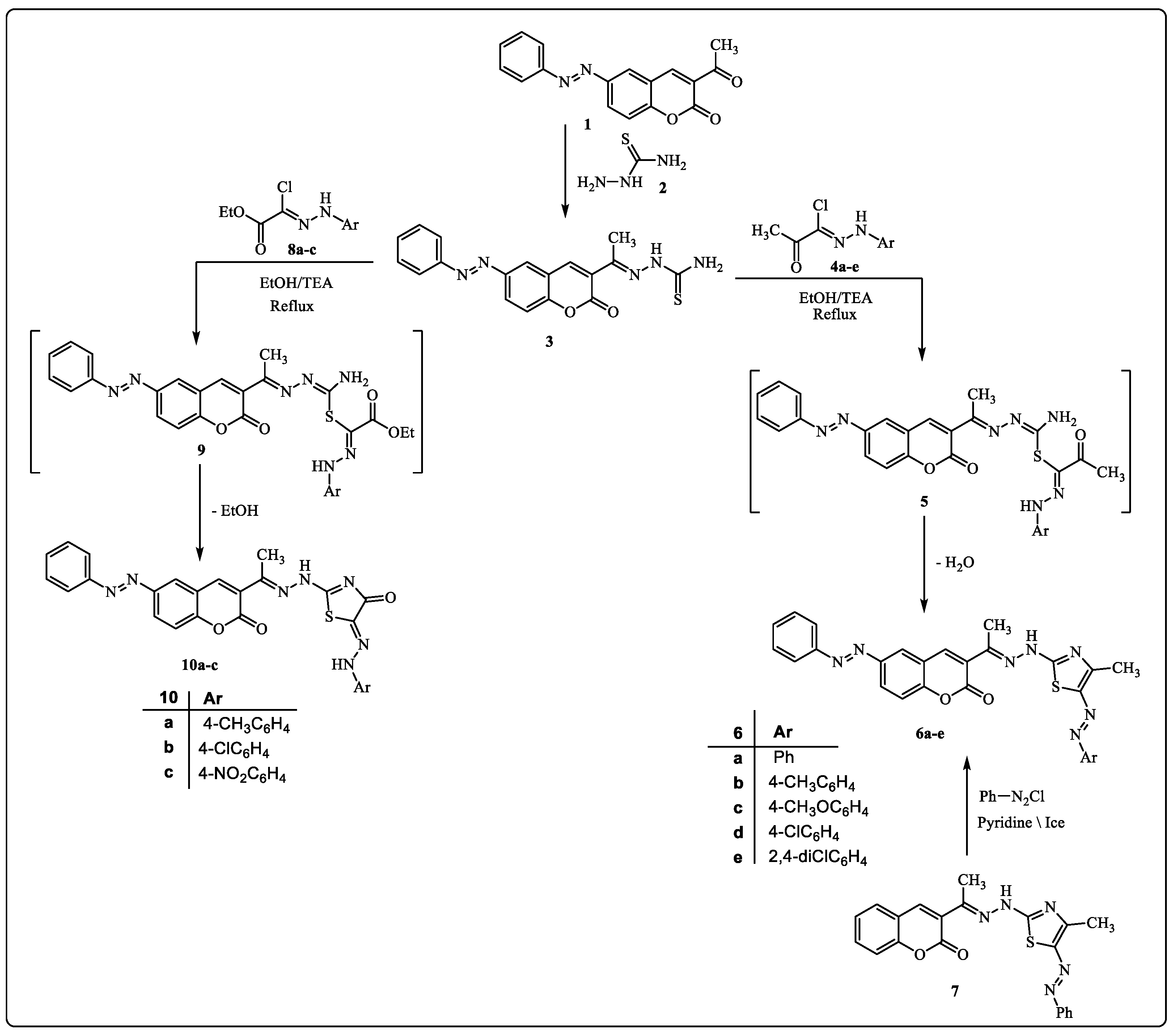
2.1. Chemistry
2.2. Molecular Docking
2.3. Cytotoxic Potential
3. Experimental Section
3.1. Molecular Docking
3.2. Cytotoxic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; World Health Organization, International Agency for Research on Cancer [IARC]: Lyon, France, 2014. [Google Scholar]
- Lee, N.C.; Wong, F.L.; Jamison, P.M.; Jones, S.F.; Galaska, L.; Brady, K.T.; Wethers, B.; Stokes-Townsend, G.A. Implementation of the National Breast and Cervical Cancer Early Detection Program. Cancer 2014, 120, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. SEER Stat Fact Sheet: Breastcancer. 2014. Available online: http://seer.cancer.gov/statfacts/html/breast.html (accessed on 22 August 2014).
- National Cancer Institute. SEER Stat Fact Sheet: Cervix Uteri Cancer. 2014. Available online: http://seer.cancer.gov/statfacts/html/cervix.html (accessed on 22 August 2014).
- Fox, S.B.; Generali, D.G.; Harris, A.L. Breast tumour angiogenesis. Breast Cancer Res. 2007, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Ryden, L.; Stendahl, M.; Jonsson, H.; Emdin, S.; Bengtsson, N.O.; Landberg, G. Tumor-specific VEGF-A and VEGFR2 in postmenopausal breast cancer patients with long-term follow-up. Implication of a link between VEGF pathway and tamoxifen response. Breast Cancer Res. Treat. 2005, 89, 135–143. [Google Scholar] [CrossRef]
- Roskoski, R.J. VEGF receptor protein-tyrosine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2008, 37, 287–291. [Google Scholar] [CrossRef]
- Zuccotto, F.; Ardini, E.; Casale, E.; Angiolini, M. Through the “gatekeeper door: Exploiting the active kinase conformation. J. Med. Chem. 2010, 53, 2681–2694. [Google Scholar] [CrossRef]
- Regan, J.; Pargellis, C.A.; Cirillo, P.F.; Gilmore, T.; Hickey, E.R.; Peet, G.W.; Proto, A.; Swinamer, A.; Moss, N. The kinetics of binding to p38MAP kinase by analogues of BIRB 796. Bioorg. Med. Chem. Lett. 2003, 13, 3101. [Google Scholar] [CrossRef]
- Patel, R.R.; Sengupta, S.; Kim, H.R.; Klein-Szanto, A.J.; Pyle, J.R.; Zhu, F.; Li, T.; Ross, E.A.; Oseni, S.; Fargnoli, J.; et al. Experimental treatment of oestrogen receptor (ER) positive breast cancer with tamoxifen and brivanib alaninate, a VEGFR-2/FGFR-1 kinase inhibitor: A potential clinical application of angiogenesis inhibitors. Eur. J. Cancer 2010, 46, 1537–1553. [Google Scholar] [CrossRef]
- Rawat, A.; Reddy, A.V.B. Recent advances on anticancer activity of coumarin derivative. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Morigi, R.; Locatelli, A.; Leoni, A.; Rambaldi, M. Recent patents on thiazole derivatives endowed with antitumor activity. Recent Pat. Anticancer. Drug Discov. 2015, 10, 280–297. [Google Scholar] [CrossRef]
- Batran, R.Z.; Dawood, D.H.; El-Seginy, S.A.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. Coumarinyl pyranopyrimidines as new neuropeptide S receptor antagonists; design, synthesis, homology and molecular docking. Bioorg. Chem. 2017, 75, 274–290. [Google Scholar] [CrossRef]
- Abdelhafez, O.M.; Amin, K.M.; Ali, H.I.; Abdalla, M.M.; Batran, R.Z. Synthesis of new 7-oxycoumarin derivatives as potent and selective monoamine oxidase A inhibitors. J. Med. Chem. 2012, 55, 10424–10436. [Google Scholar] [CrossRef]
- Batran, R.Z.; Kassem, A.F.; Abbas, E.M.H.; Elseginy, S.A.; Mounier, M.M. Design, synthesis and molecular modeling of new 4-phenylcoumarin derivatives as tubulin polymerization inhibitors targeting MCF-7 breast cancer cells. Bioorg. Med. Chem. 2018, 26, 3474–3490. [Google Scholar] [CrossRef] [PubMed]
- Alshabanah, L.A.; Al-Mutabagani, L.A.; Gomha, S.M.; Ahmed, H.A. Three-component synthesis of some new coumarin derivatives as anti-cancer agents. Front. Chem. 2022, 9, 762248. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, L.; Li, L.; Wei, Z.; Tong, B.; Jia, Y.; Kong, L.; Xia, Y.; Dai, Y. SC-III3, a novel scopoletin derivative, induces cytotoxicity in hepatocellular cancer cells through oxidative DNA damage and ataxia telangiectasia-mutated nuclear protein kinase activa-tion. BMC Cancer 2014, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Batran, R.Z.; Dawood, D.H.; El-Seginy, S.A.; Ali, M.M.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. New Coumarin Derivatives as Anti-Breast and Anti-Cervical Cancer Agents Targeting VEGFR-2 and p38α MAPK. Arch. Pharm. (Weinheim) 2017, 350, e1700064. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-aziz, H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles 2015, 91, 583–592. [Google Scholar] [CrossRef]
- Luo, G.; Li, X.; Zhang, G.; Wu, C.; Tang, Z.; Liu, L.; You, Q.; Xiang, H. Novel SERMs based on 3-aryl-4-aryloxy-2H-chromen-2-one skeleton—A possible way to dual ERα/VEGFR-2 ligands for treatment of breast cancer. Eur. J. Med. Chem. 2017, 140, 252–273. [Google Scholar] [CrossRef]
- Pan, R.; Dai, Y.; Gao, X.H.; Lu, D.; Xia, Y.F. Inhibition of vascular endothelial growth factorinduced angiogenesis by scopoletin through interrupting the autophosphorylation of VEGF receptor 2 and its downstream signaling pathways. Vascul. Pharmacol. 2011, 54, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Won, S.Y.; Song, J.H.; Lee, S.Y.; Kim, W.J.; Moon, S.K. Esculetin inhibits VEGF-Induced angiogenesis both in vitro and in vivo. Am. J. Chin. Med. 2016, 44, 61–76. [Google Scholar] [CrossRef]
- Franchin, M.; Rosalen, P.L.; da Cunha, M.G.; Silva, R.L.; Colon, D.F.; Bassi, G.S.; de Alenca, S.M.; Ikegaki, M.; Alves-Filho, J.C.; Cunha, F.Q.; et al. Cinnamoyloxy-mammeisin Isolated from Geopropolis Attenuates Inflammatory Process by Inhibiting Cytokine Production: Involvement of MAPK, AP-1, and NF-κB. J. Nat. Prod. 2016, 79, 1828–1833. [Google Scholar] [CrossRef]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Raveesha, R.; Anusuya, A.M.; Raghu, A.V.; Kumar, K.Y.; Kumar, M.G.D.; Prasad, S.B.B.; Prashanth, M.K. Synthesis and characterization of novel thiazole derivatives as potential anticancer agents: Molecular docking and DFT studies. Comput. Toxicol. 2022, 21, 100202–100219. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Huwaimel, B.; Zayed, M.E.M.; Abdellattif, M.H. Synthesis, molecular docking study and cytotoxic activity on MCF cells of some new thiazole clubbed thiophene scaffolds. Molecules 2022, 27, 4639. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.A.; Taher, E.S.; Lan, P.; El-Koussi, N.A.; Salem, O.I.A.; Gomaa, H.A.M.; Youssif, B.G.M. New pyrimidine/thiazole hybrids endowed with analgesic, anti-inflammatory, and lower cardiotoxic activities: Design, synthesis, and COX-2/sEH dual inhibition. Arch. Pharm. 2022, 355, e2200024. [Google Scholar] [CrossRef]
- Altıntop, M.D.; Sever, B.; Çiftçi, G.A.; Özdemir, A. Design, Synthesis, and Evaluation of a New Series of Thiazole-Based Anticancer Agents as Potent Akt Inhibitors. Molecules 2018, 23, 1318. [Google Scholar] [CrossRef]
- Hassan, A.; Badr, M.; Hassan, H.A.; Abdelhamid, D.; Abuo-Rahma, G.E.D.A. Novel 4-(piperazin-1-yl)quinolin-2(1H)-one bearing thiazoles with antiproliferative activity through VEGFR-2-TK inhibition. Bioorg. Med. Chem. 2021, 40, 116168–116181. [Google Scholar] [CrossRef]
- Perrot-Applanat, M.; Di Benedetto, M. Autocrine functions of VEGF in breast tumor cells: Adhesion, survival, migration and invasion. Cell Adh. Migr. 2012, 6, 547–553. [Google Scholar] [CrossRef]
- Song, G.; Li, Y.; Jiang, G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets. Oncol. Rep. 2012, 28, 1935–1944. [Google Scholar] [CrossRef]
- Bilodeau, M.T.; Rodman, L.D.; McGaughey, G.B.; Coll, K.E.; Koester, T.J.; Hoffman, W.F.; Thomas, K.A. The discovery of N-(1,3-thiazol-2-yl) pyridin-2-amines as potent inhibitors of KDR kinase. Bioorg. Med. Chem. Lett. 2004, 14, 2941–2945. [Google Scholar] [CrossRef]
- Bilodeau, M.T.; Balitza, A.E.; Koester, T.J.; Manley, P.J.; Rodman, L.D.; Buser-Doepner, C.; Hartman, G.D. Potent N-(1,3-thiazol-2-yl)pyridin-2-amine vascular endothelial growth factor receptor tyrosine kinase inhibitors with excellent pharmacokinetics and low affinity for the hERG ion channel. J. Med. Chem. 2004, 47, 6363–6372. [Google Scholar] [CrossRef]
- Sisko, J.T.; Tucker, T.J.; Bilodeau, M.T.; Buser, C.A.; Ciecko, P.A.; Coll, K.E.; Hartman, G.D. Potent 2-[(pyrimidin-4-yl)amine}-1,3-thiazole-5-carbonitrile-based inhibitors of VEGFR-2 (KDR) kinase. Bioorg. Med. Chem. Lett. 2006, 16, 1146–1150. [Google Scholar] [CrossRef]
- Kiselyov, A.S.; Piatnitski, E.; Semenov, V.V. N-(aryl)-4-(azolylethyl)thiazole-5-carboxamides: Novel potent inhibitors of VEGF receptors I and II. Bioorg. Med. Chem. Lett. 2006, 16, 602–606. [Google Scholar] [CrossRef]
- Wickens, P.; Kluender, H.; Dixon, J.; Brennan, C.; Achebe, F.; Bacchiocchi, A.; Levy, J. SAR of a novel “anthranilamide like” series of VEGFR-2, multi protein kinase inhibitors for the treatment of cancer. Bioorg. Med. Chem. Lett. 2007, 17, 4378–4381. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Eldehna, W.M.; Ali, M.M.; Abou El Ella, D.A. 1-piperazinylphthalazines as potential VEGFR-2 inhibitors and anticancer agents: Synthesis and in vitro biological evaluation. Eur. J. Med. Chem. 2016, 107, 165–179. [Google Scholar] [CrossRef]
- El-Miligy, M.M.; Abd El Razik, H.A.; Abu-Serie, M.M. Synthesis of piperazine-based thiazolidinones as VEGFR2 tyrosine kinase inhibitors inducing apoptosis. Fut. Med. Chem. 2017, 9, 1709–1729. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdelhady, H.A.; Hassain, D.Z.H.; Abdelmonsef, A.H.; El-Naggar, M.; Elaasser, M.M.; Mahmoud, H.K. Thiazole based thiosemicarbazones: Synthesis, cytotoxicity evaluation and molecular docking study. Drug Des. Dev. Ther. 2021, 15, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, G.F.; Abolibda, T.Z.; Alhilal, M.; Al-Humaidi, J.Y.; Alhilal, S.; Ahmed, H.A.; Gomha, S.M. Novel thiadiazole-thiazole hybrids: Synthesis, molecular docking, and cytotoxicity evaluation against liver cancer cell lines. J. Taibah Uni. Sci. 2022, 16, 1005–1015. [Google Scholar] [CrossRef]
- Abouzied, A.S.; Al-Humaidi, J.Y.; Bazaid, A.S.; Qanash, H.; Binsaleh, N.K.; Alamri, A.; Ibrahim, S.M.; Gomha, S.M. Synthesis, molecular docking study, and cytotoxicity evaluation of some novel 1,3,4-thiadiazole as well as 1,3-thiazole derivatives bearing a pyridine moiety. Molecules 2022, 27, 6368. [Google Scholar] [CrossRef]
- Nayl, A.A.; Arafa, W.A.A.; Ahmed, M.; Abd-Elhamid, A.I.; El-Fakharany, E.M.; Abdelgawad, M.A.; Gomha, S.M.; Ibrahim, H.M.; Aly, A.A.; Bräse, S.; et al. Novel pyridinium based ionic liquid promoter for aqueous knoevenagel condensation: Green and efficient synthesis of new derivatives with their anticancer evaluation. Molecules 2022, 27, 2940. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Edrees, M.M.; Muhammad, Z.A.; El-Reedy, A.A. 5-(Thiophen-2-yl)-1,3,4-thiadiazole derivatives: Synthesis, molecular docking and in-vitro cytotoxicity evaluation as potential anti-cancer agents. Drug Des. Devel. Ther. 2018, 12, 1511–1523. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Faty, R.A.M.; Muhammad, Z.A.; Mabkhot, Y.N. Microwave-assisted one pot three-component synthesis of some novel pyrazole scaffolds as potent anticancer agents. Chem. Central J. 2017, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdelaziz, M.R.; Kheder, N.A.; Abdel-aziz, H.M.; Alterary, S.; Mabkhot, Y.N. A Facile access and evaluation of some novel thiazole and 1,3,4-thiadiazole derivatives incorporating thiazole moiety as potent anticancer agents. Chem. Central J. 2017, 11, 105. [Google Scholar] [CrossRef]
- Edrees, M.M.; Abu-Melha, S.; Saad, A.M.; Kheder, N.A.; Gomha, S.M.; Muhammad, Z.A. Eco-friendly synthesis, characterization and biological evaluation of some new pyrazolines containing thiazole moiety as potential anticancer and antimicrobial agents. Molecules 2018, 23, 2970. [Google Scholar] [CrossRef]
- Sivaguru, P.; Sandhiya, R.; Adhiyaman, M.; Lalitha, A. Synthesis and antioxidant properties of novel 2H-chromene-3-carboxylate and 3-acetyl-2H-chromene derivatives. Tetrahedron Lett. 2016, 57, 2496–2501. [Google Scholar] [CrossRef]
- Badrey, M.G.; Gomha, S.M. 3-Amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno[2,3-d]pyrimidine: An efficient key precursor for novel synthesis of some interesting triazines and triazepines as potential anti-tumor agents. Molecules 2012, 17, 11538–11553. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Khalil, K.D. A Convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.; Solowiej, J.; Kania, R.S. Molecular Conformations, Interactions, and Properties Associated with Drug Efficiency and Clinical Performance Among Vegfr Tk Inhibitors molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among Vegfr Tk Inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar]
- Othman, I.M.M.; Alamshany, Z.M.; Tashkandi, N.Y.; Gad-Elkareem, M.A.; Anwar, M.M.; Nossier, E.S. New pyrimidine and pyrazole-based compounds as potential EGFR inhibitors: Synthesis, anticancer, antimicrobial evaluation and computational studies. Bioorg. Chem. 2021, 114, 105078. [Google Scholar] [CrossRef]
- Labute, P. Protonate3D: Assignment of Ionization States and Hydrogen Coordinates to Macromolecular Structures. Proteins 2008, 75, 187–205. [Google Scholar] [CrossRef]
- Kattan, S.W.; Nafie, M.S.; Elmgeed, G.A.; Alelwani, W.; Badar, M.; Tantawy, M.A. Molecular docking, anti-proliferative activity and induction of apoptosis in human liver cancer cells treated with androstane derivatives: Implication of PI3K/AKT/mTOR pathway. J. Steroid Biochem. Mol. Biol. 2020, 198, 105604. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Sroor, F.M.; Mohamed, M.F.; El-Naggar, M.E.; Saleh, F.M.; Hassaneen, H.M.; Abdelhamid, I.A. Molecular Docking Study, Cytotoxicity, Cell Cycle Arrest and Apoptotic Induction of Novel Chalcones Incorporating Thiadiazolyl Isoquinoline in Cervical Cancer. Anti-Cancer Agents Med. Chem. 2020, 20, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of different drug design tools to predict the mode of action of steroidal derivatives as anti-cancer agents. Steroids 2019, 152, 108485. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-grafted-poly(vinylpyridine) as basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]

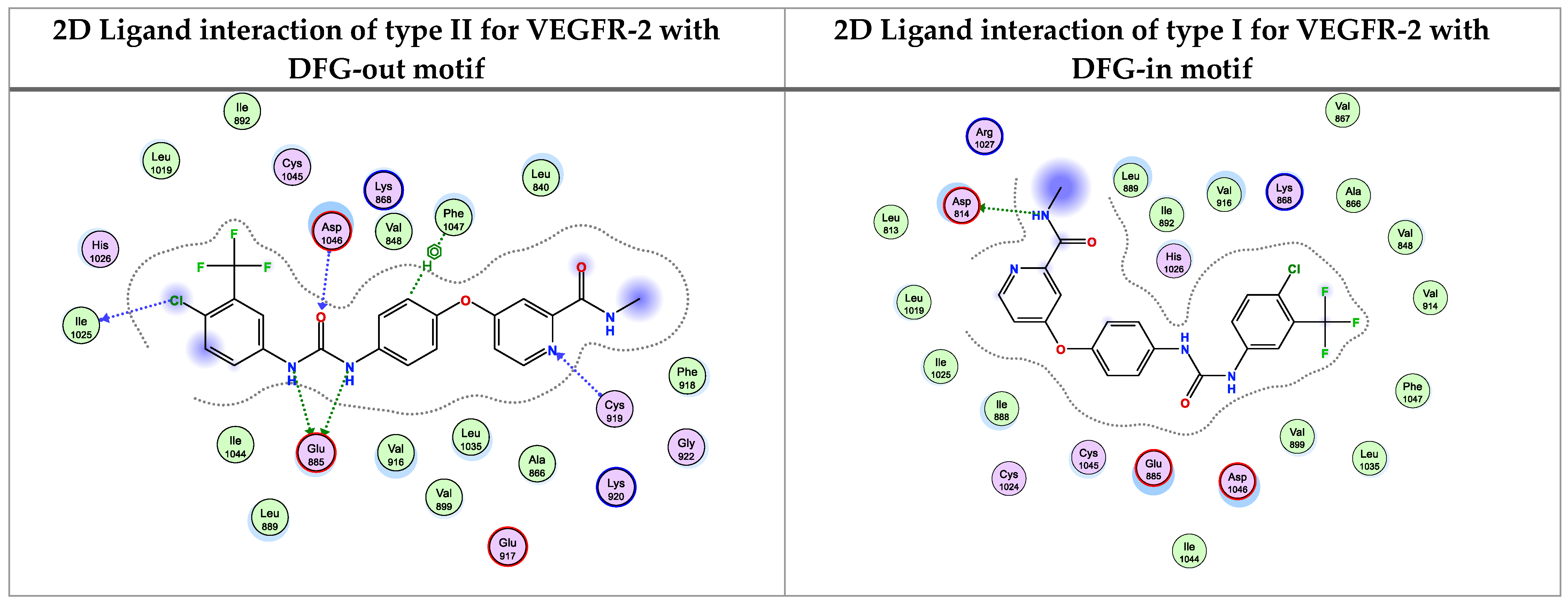
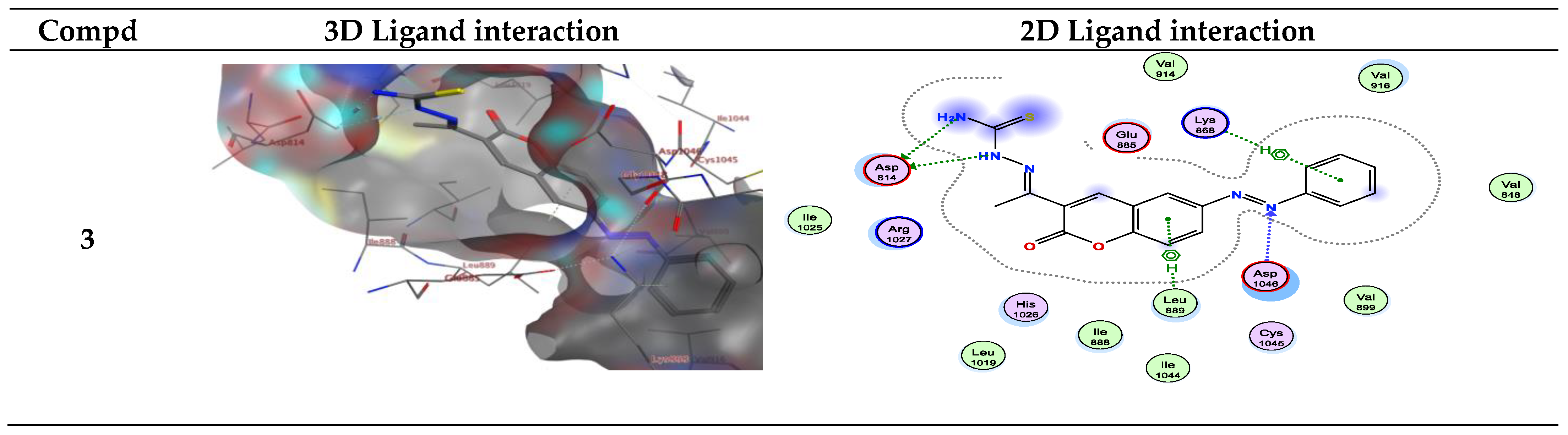
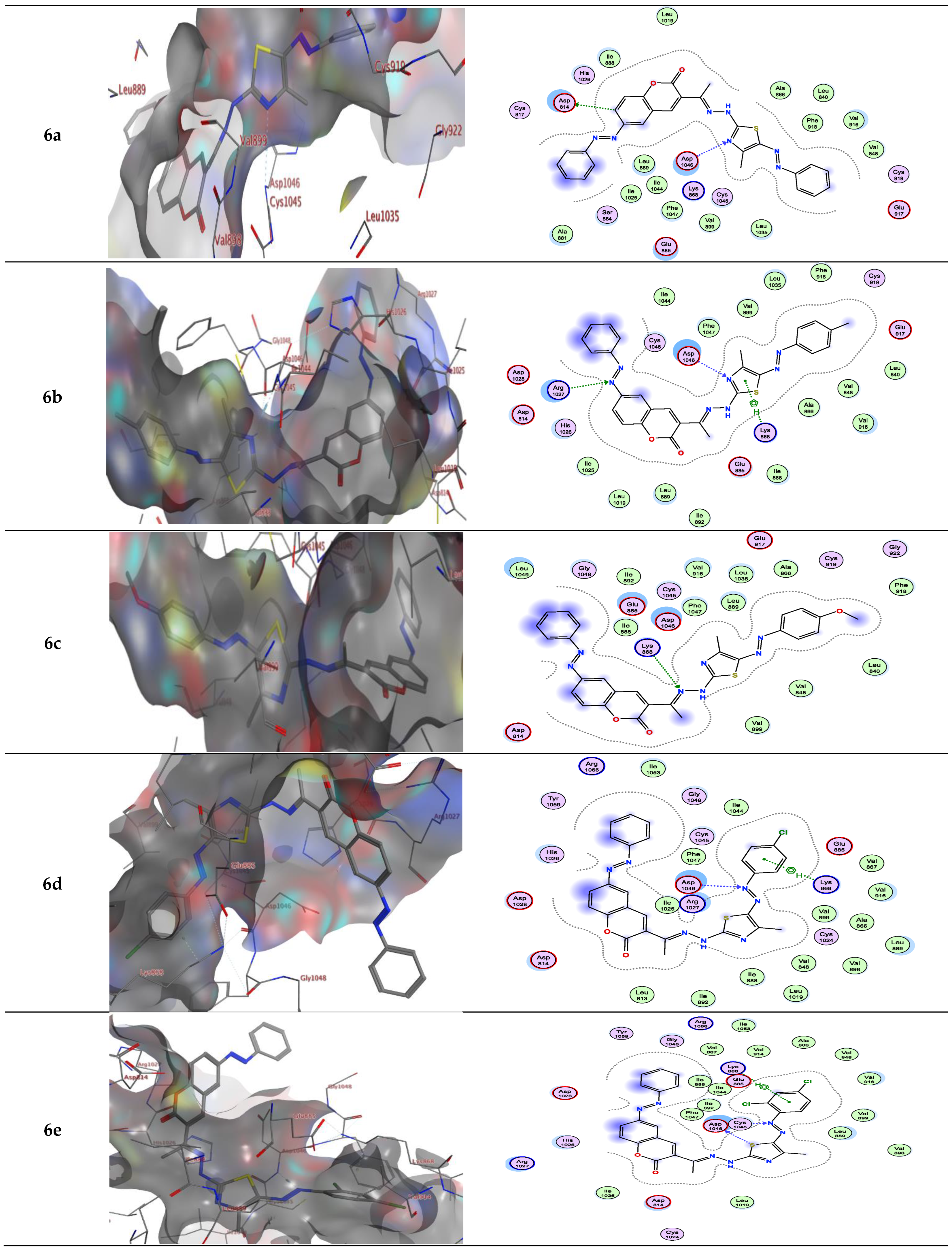
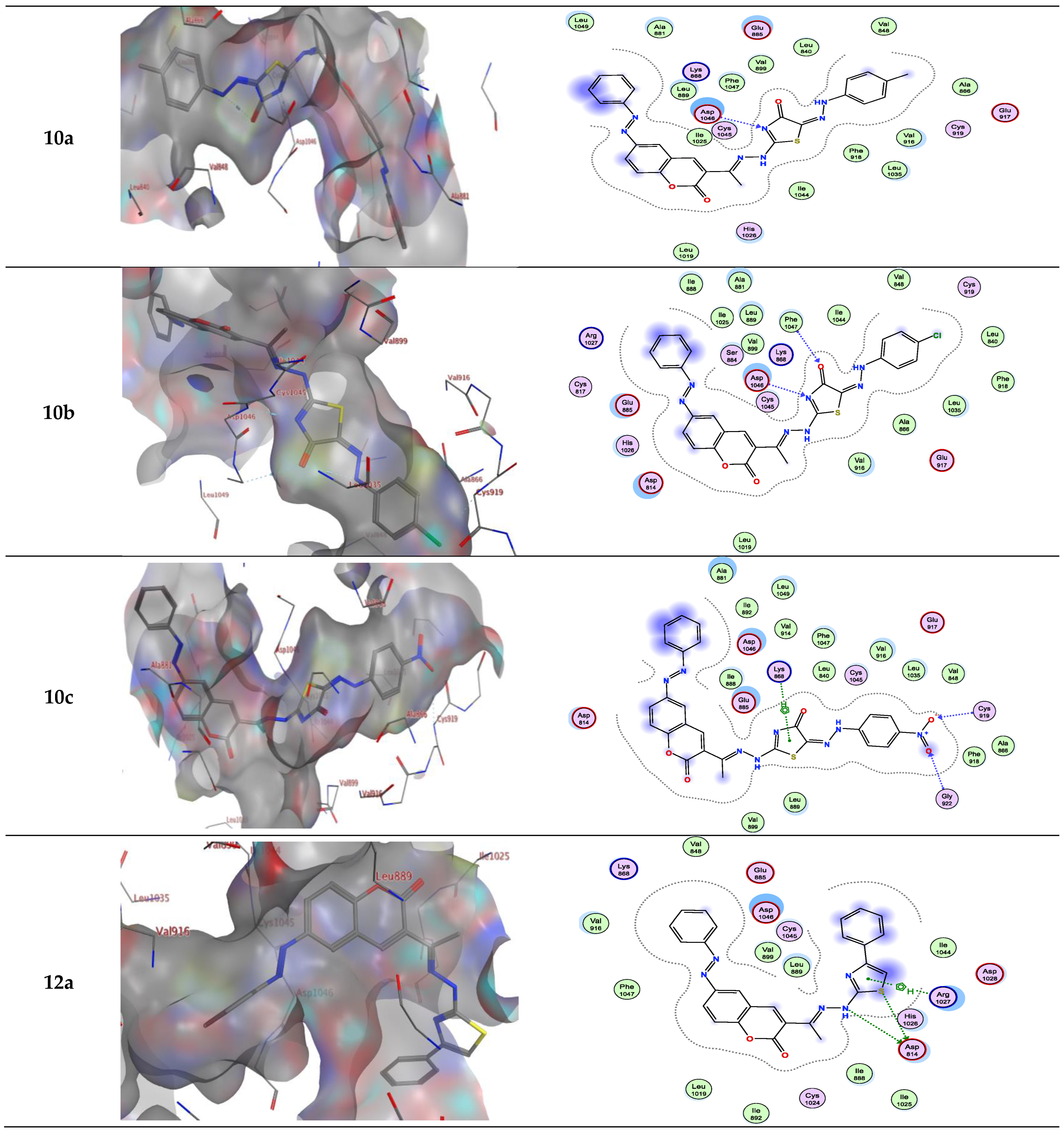
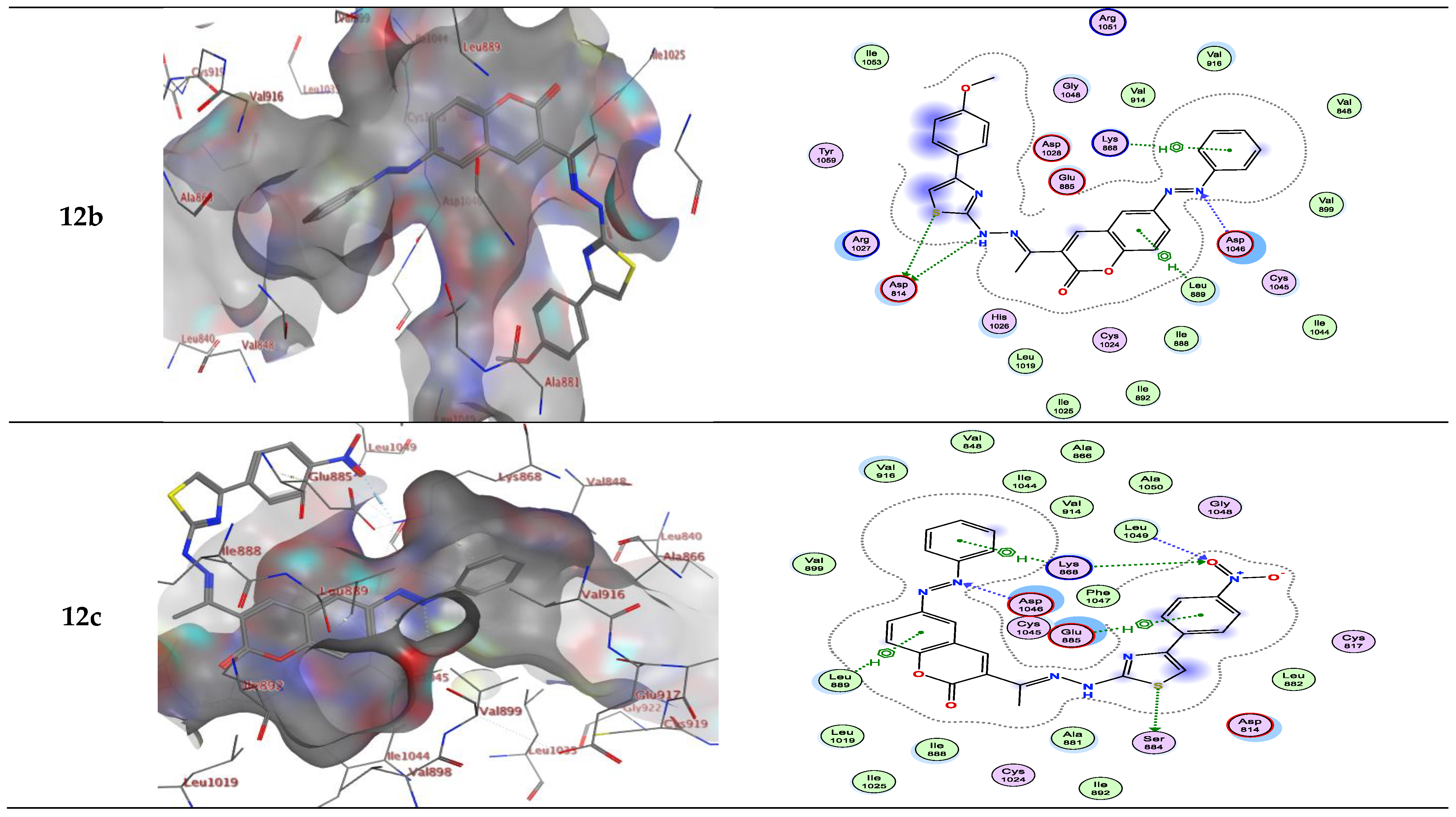
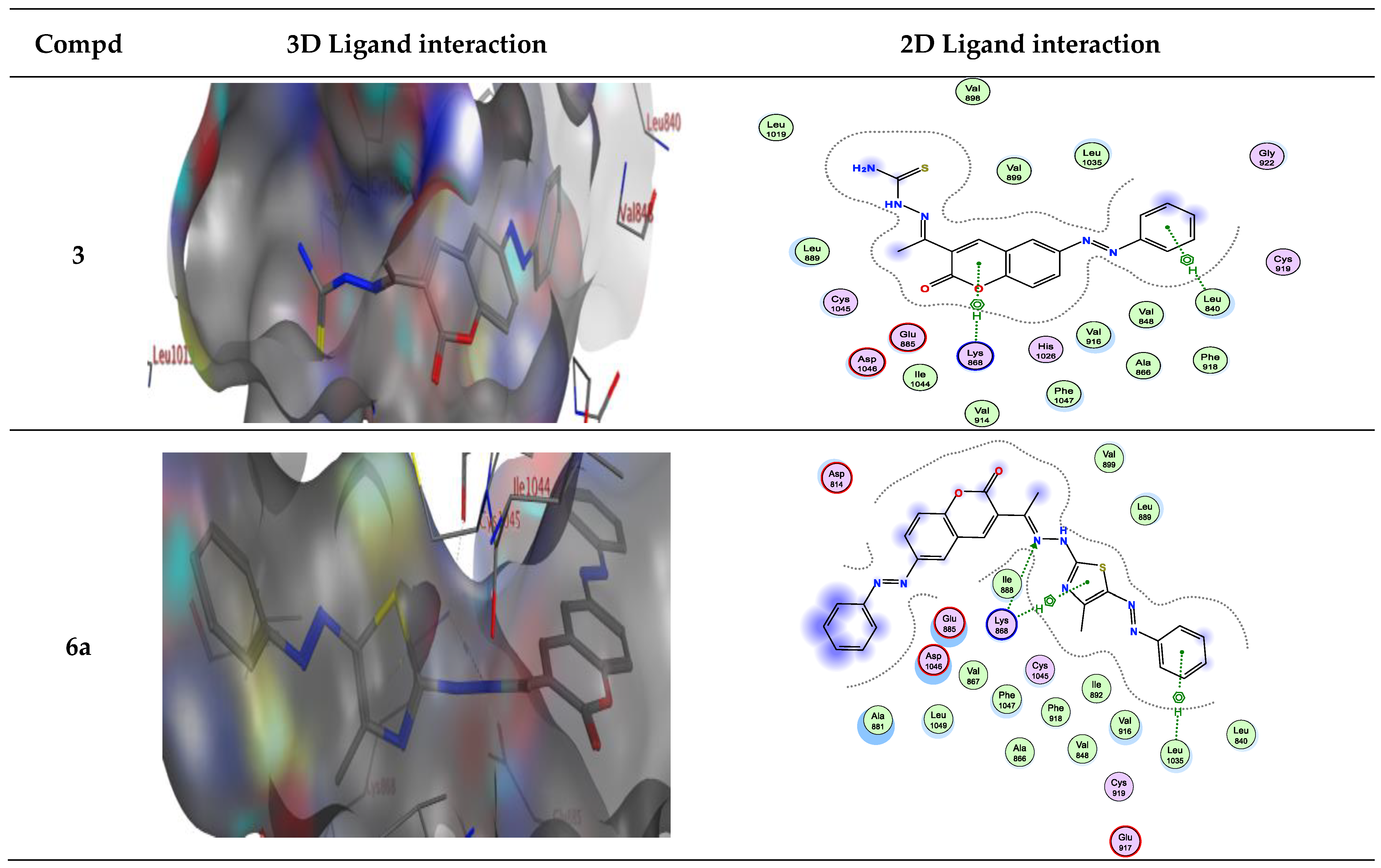
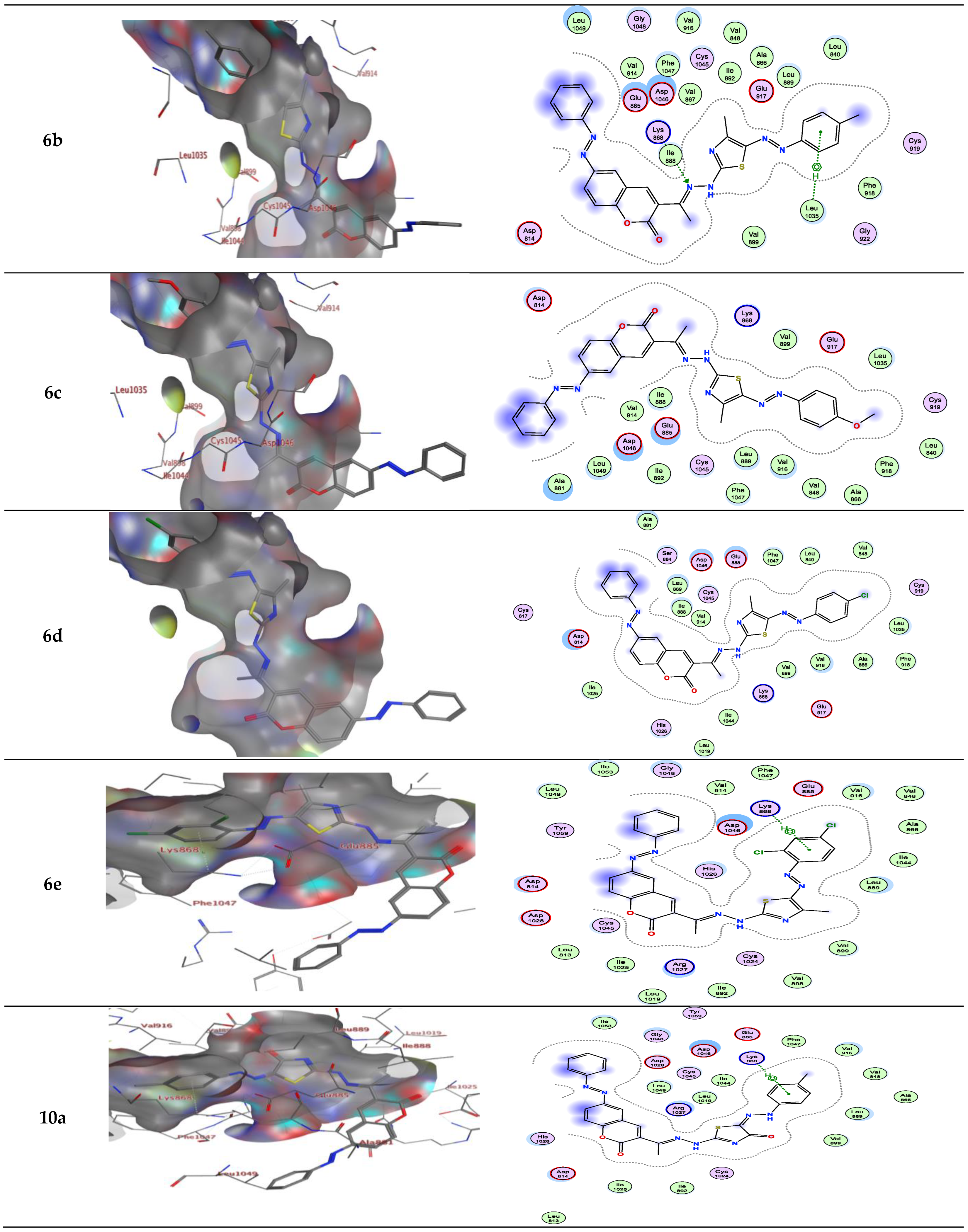
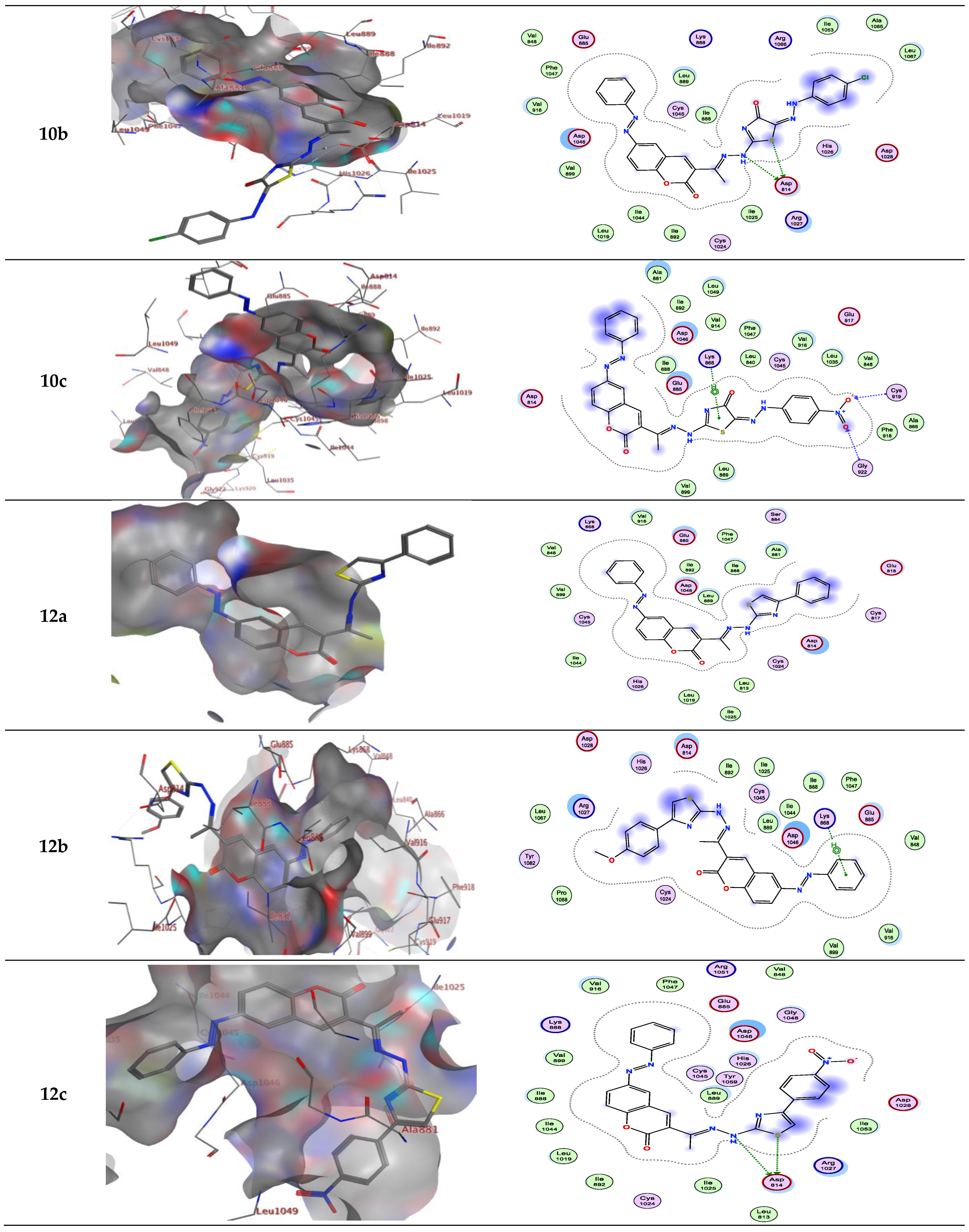

| Compound | Energy Score (S) (kcal/mol) | Interacting Residues |
|---|---|---|
| 3 | −7.72 | Asp814, Asp1046, Lys868, and Leu889 |
| 6a | −9.15 | Leu1035 and Lys868 |
| 6b | −9.81 | Asp1046, Arg1027 and Lys868 |
| 6c | −9.58 | Lys868 |
| 6d | −9.90 | Asp1046 and Lys868 |
| 6e | −9.37 | Asp1046 and Lys868 |
| 10a | −9.67 | Asp1046 |
| 10b | −9.42 | Asp1046 and Phe1047 |
| 10c | −9.69 | Cys919, Gly922 and Lys868 |
| 12a | −8.52 | Asp814 and Arg1027 |
| 12b | −8.90 | Asp814, Asp1046, Lys868 and Leu889 |
| 12c | −8.95 | Asp814 |
| Sorafenib | −8.65 | Cys1045, Asp1046, Glu885 and Cys919 |
| Compound | Energy Score (S) (kcal/mol) | Receptor Interactions |
|---|---|---|
| 3 | −7.319 | Leu840/π-H Lys868/π-H |
| 6a | −9.155 | Lys868/H-bond acceptor Lys868/π-H Leu1035/π-H |
| 6b | −9.089 | Lys868/H-bond acceptor Leu1035/π-H |
| 6c | −9.580 | - |
| 6d | −9.857 | - |
| 6e | −9.254 | Lys868/π-H |
| 10a | −9.205 | Lys868/π-H |
| 10b | −8.728 | Asp814/H-bond donor |
| 10c | −9.572 | Cyss919/H-bond acceptor Cly922/H-bond acceptor Lys868/π-H |
| 12a | −8.135 | - |
| 12b | −8.363 | Lys868/π-H |
| 12c | −8.735 | Asp814/H-bond donor |
| Sorafenib | −8.214 | Asp814 |
| Tested Compounds | MCF-7IC50 (μM) * | LLC-MK2 * CC50 (μM) |
|---|---|---|
| 6b | 11.2 ± 0.80 | 152.3 ± 9.30 |
| 6c | 14.1 ± 0.63 | 141.0 ± 5.15 |
| 6d | 10.5 ± 0.71 | 132.2 ± 8.26 |
| 10a | 28.6 ± 0.64 | 140.3 ± 10.29 |
| 10c | 15.7 ± 0.67 | 163.1 ± 9.12 |
| Sorafenib | 5.10 ± 0.49 | 135.3 ± 4.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abolibda, T.Z.; Fathalla, M.; Farag, B.; Zaki, M.E.A.; Gomha, S.M. Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase. Molecules 2023, 28, 689. https://doi.org/10.3390/molecules28020689
Abolibda TZ, Fathalla M, Farag B, Zaki MEA, Gomha SM. Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase. Molecules. 2023; 28(2):689. https://doi.org/10.3390/molecules28020689
Chicago/Turabian StyleAbolibda, Tariq Z., Maher Fathalla, Basant Farag, Magdi E. A. Zaki, and Sobhi M. Gomha. 2023. "Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase" Molecules 28, no. 2: 689. https://doi.org/10.3390/molecules28020689
APA StyleAbolibda, T. Z., Fathalla, M., Farag, B., Zaki, M. E. A., & Gomha, S. M. (2023). Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase. Molecules, 28(2), 689. https://doi.org/10.3390/molecules28020689







