Feature-Based Molecular Networking for the Exploration of the Metabolome Diversity of Common Egyptian Centaurea Species in Relation to Their Cytotoxic Activity
Abstract
1. Introduction
2. Results
2.1. Comparative Analysis of LC-MS/MS Profiles from Centaurea Species
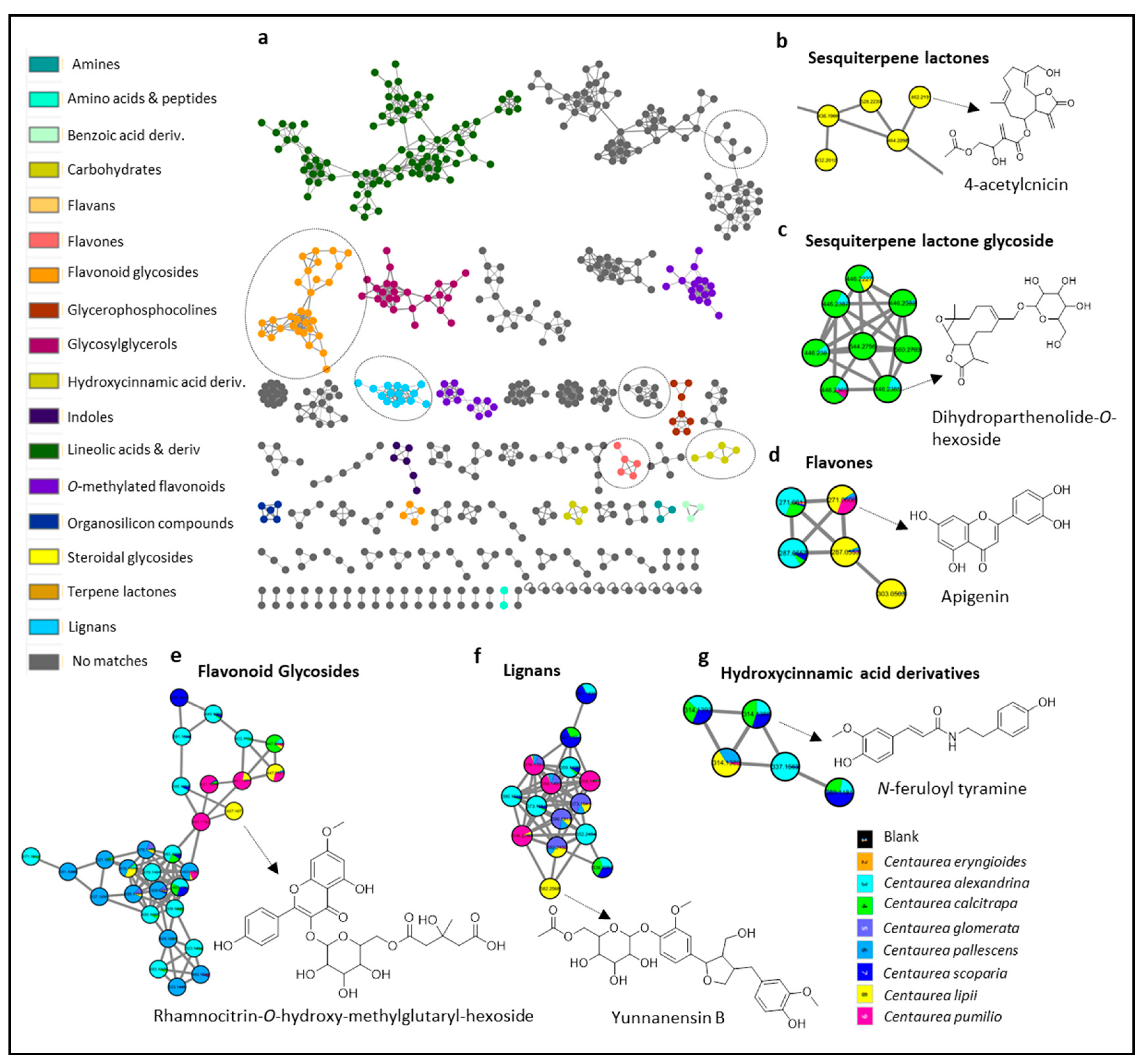
2.2. MS/MS Molecular-Networking-Based Phytochemical Investigations
2.2.1. Hydroxycinnamic Acid Derivatives
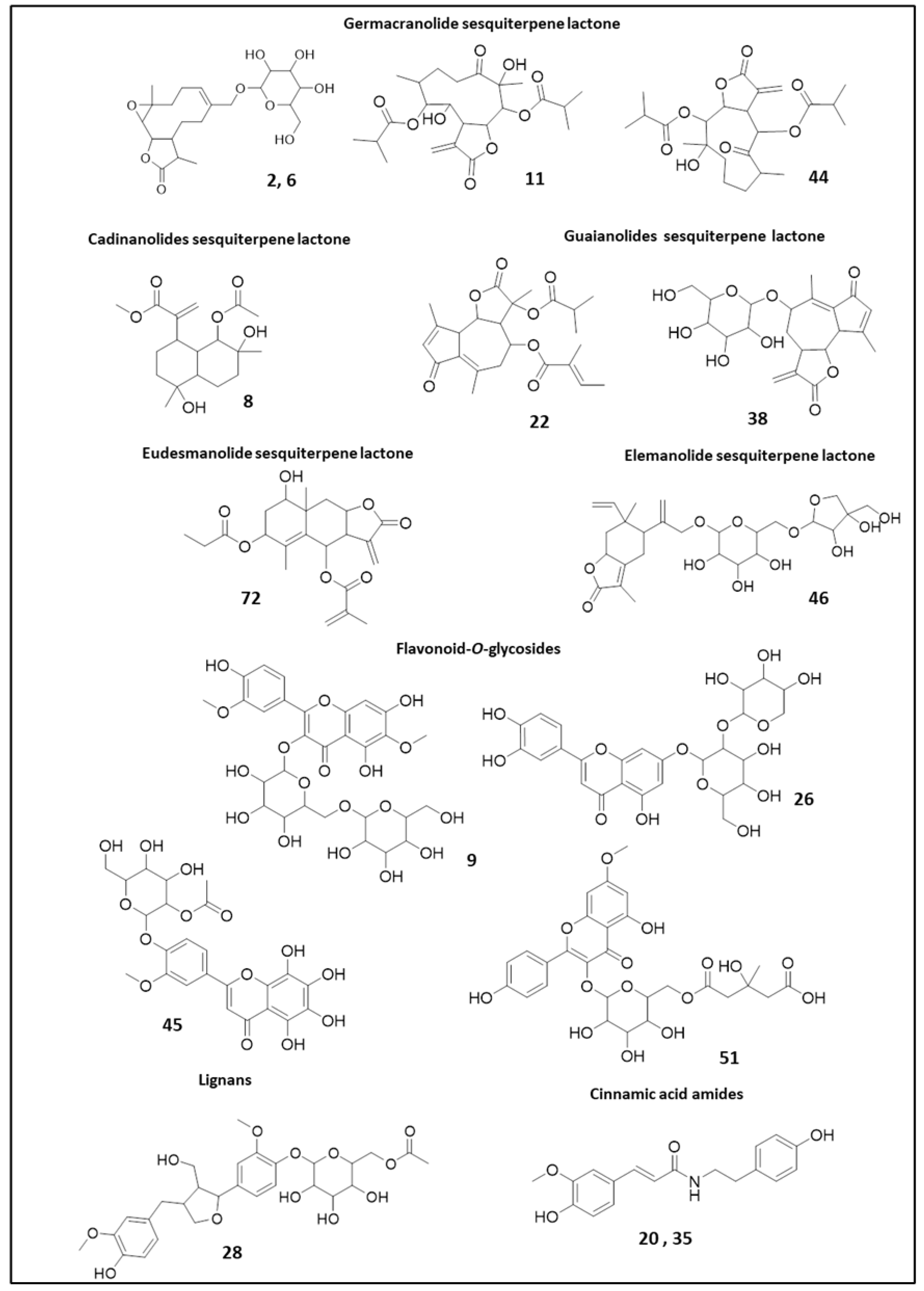
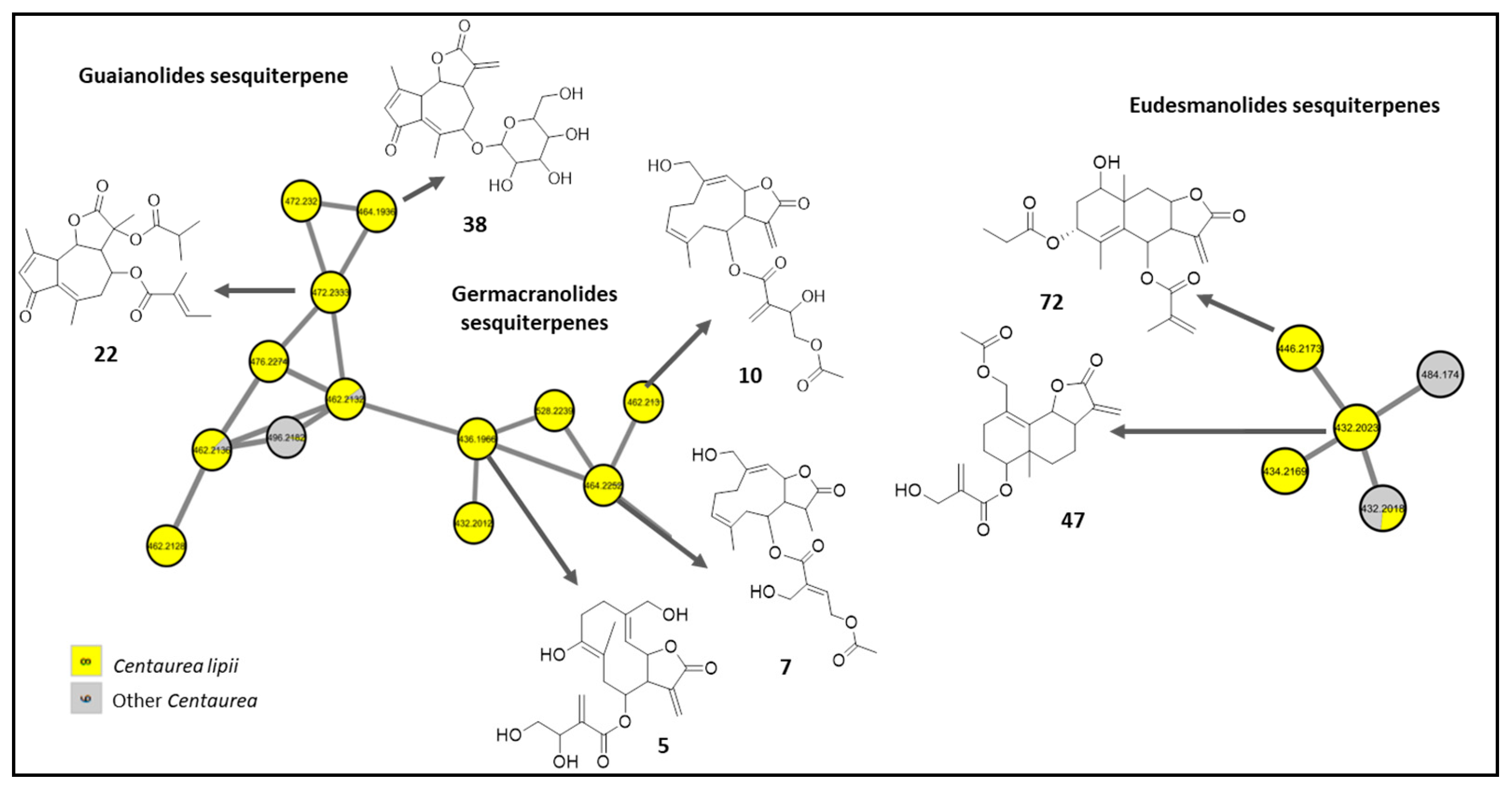
2.2.2. Sesquiterpene Lactones
2.2.3. Flavonoids
2.2.4. Lignans
2.3. Bioactivity-Guided Fractionation of C. lipii
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals
4.2.1. Chemicals and Reagents
4.2.2. Preparation of the Extracts
4.2.3. LC-MS/MS Data Acquisition
4.2.4. Data Preprocessing, Molecular Networking, and Compound Dereplication
4.2.5. Cell Culture
4.2.6. Resazurin Cytotoxicity Assay
4.2.7. Extraction, Separation, and NMR-Based Structure Elucidation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Jacas, N.; Susanna, A.; Garnatje, T.; Vilatersana, R. Generic delimitation and phylogeny of the subtribe Centaureinae (Asteraceae): A combined nuclear and chloroplast DNA analysis. Ann. Bot. 2001, 87, 503–515. [Google Scholar] [CrossRef]
- Ayad, R.; Akkal, S. Phytochemistry and biological activities of algerian Centaurea and related genera. Stud. Nat. Prod. Chem. 2019, 63, 357–414. [Google Scholar]
- Reyhan, A.; Küpeli, E.; Ergun, F. The biological activity of Centaurea L. species. Gazi Univ. J. Sci. 2004, 17, 149–164. [Google Scholar]
- Fattaheian-Dehkordi, S.; Hojjatifard, R.; Saeedi, M.; Khanavi, M. A review on antidiabetic activity of Centaurea spp.: A new approach for developing herbal remedies. Evid.-Based Complement. Altern. Med. 2021, 2021, 5587938. [Google Scholar] [CrossRef] [PubMed]
- Csupor, D.; Widowitz, U.; Blazsó, G.; Laczkó-Zöld, E.; Tatsimo, J.S.; Balogh, Á.; Boros, K.; Dankó, B.; Bauer, R.; Hohmann, J.J.P.R. Anti-inflammatory Activities of Eleven Centaurea Species Occurring in the Carpathian Basin. Phytother. Res. 2013, 27, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Younis, I.Y.; Ibrahim, R.M.; El-Halawany, A.M.; Hegazy, M.-E.F.; Efferth, T.; Mohsen, E.J.F.C. Chemometric discrimination of Hylocereus undulatus from different geographical origins via their metabolic profiling and antidiabetic activity. Food Chem. 2023, 404, 134650. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Mohamed, T.A.; Ibrahim, M.A.; Atia, M.A.; Yoneyama, T.; Umeyama, A.; Hegazy, M.E.F. Two novel oxetane containing lignans and a new megastigmane from Paronychia arabica and in silico analysis of them as prospective SARS-CoV-2 inhibitors. RSC Adv. 2021, 11, 20151–20163. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Dawood, M.; Mahmoud, N.; Elbadawi, M.; Sugimoto, Y.; Klauck, S.M.; Mohamed, N.; Efferth, T.J.P. 2α-Hydroxyalantolactone from Pulicaria undulata: Activity against multidrug-resistant tumor cells and modes of action. Phytomedicine 2021, 81, 153409. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Abdelfatah, S.; Hamed, A.R.; Mohamed, T.A.; Elshamy, A.A.; Saleh, I.A.; Reda, E.H.; Abdel-Azim, N.S.; Shams, K.A.; Sakr, M.J.P. Cytotoxicity of 40 Egyptian plant extracts targeting mechanisms of drug-resistant cancer cells. Phytomedicine 2019, 59, 152771. [Google Scholar] [CrossRef]
- Ernst, M.; Kang, K.B.; Caraballo-Rodríguez, A.M.; Nothias, L.-F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C. MolNetEnhancer: Enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef]
- Erel, S.B.; Karaalp, C.; Bedir, E.; Kaehlig, H.; Glasl, S.; Khan, S.; Krenn, L. Secondary metabolites of Centaurea calolepis and evaluation of cnicin for anti-inflammatory, antioxidant, and cytotoxic activities. Pharm. Biol. 2011, 49, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.A.; Sanz, J.F.; Sancenon, F.; Susanna, A.; Rustaiyan, A.; Saberi, M.J.P. Sesquiterpene lactones and lignans from Centaurea species. Phytochemistry 1992, 31, 3527–3530. [Google Scholar] [CrossRef]
- Sallam, A.A.; Hitotsuyanagi, Y.; Mansour, E.S.S.; Ahmed, A.F.; Gedara, S.; Fukaya, H.; Takeya, K.J.H.C.A. Sesquiterpene Lactones from Daucus Glaber. Helv. Chim. Acta 2010, 93, 48–57. [Google Scholar] [CrossRef]
- Salachna, P.; Pietrak, A.; Łopusiewicz, Ł.J.M. Antioxidant Potential of Flower Extracts from Centaurea spp. Depends on Their Content of Phenolics, Flavonoids and Free Amino Acids. Molecules 2021, 26, 7465. [Google Scholar] [CrossRef] [PubMed]
- Reda, E.H.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.-S.A.; Shams, K.A.; Mohamed, T.A.; Saleh, I.; Elshamy, A.I.; Atia, M.A.; El-Beih, A.A. Comparative Study on the Essential Oils from Five Wild Egyptian Centaurea Species: Effective Extraction Techniques, Antimicrobial Activity and In-Silico Analyses. Antibiotics 2021, 10, 252. [Google Scholar] [CrossRef]
- Szokol, L.B.; Sedlák, É.; Boldizsár, I.; Paku, S.; Preininger, É.; Gyurján, I.J.P.M. Determination of dibenzylbutyrolactone-type lignans in Centraurea species and analysis of arctigenin’s anticancer effect. Planta Med. 2010, 76, 568. [Google Scholar]
- Bessaire, T.; Ernest, M.; Christinat, N.; Carrères, B.; Panchaud, A.; Badoud, F. High resolution mass spectrometry workflow for the analysis of food contaminants: Application to plant toxins, mycotoxins and phytoestrogens in plant-based ingredients. Food Addit. Contam. 2021, 38, 978–996. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Elso, O.G.; Borgo, J.; Laurella, L.C.; Catalán, C.A. Recent patents on sesquiterpene lactones with therapeutic application. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 69, pp. 129–194. [Google Scholar]
- Kupchan, S.M.; Eakin, M.; Thomas, A. Tumor inhibitors. 69. Structure-cytotoxicity relations among the sesquiterpene lactones. J. Med. Chem. 1971, 14, 1147–1152. [Google Scholar] [CrossRef]
- Schmidt, T.J. Toxic activities of sesquiterpene lactones: Structural and biochemical aspects. Curr. Org. Chem 1999, 3, 577–608. [Google Scholar]
- Akbar, S. Centaurea behen L.(Asteraceae/Compositae). In Handbook of 200 Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 569–571. [Google Scholar]
- Mukhametzhanova, G.; Asanova, G.; Adekenova, G.S.; Medeubayeva, B.; Kishkentayeva, A.; Adekenov, S. Chartolepis intermedia Boiss. and Centaurea ruthenica Lam.–New Medicina Plants Containing Pharmacologically Active Compounds. Open Access Maced. J. Med. Sci. 2022, 10, 56–64. [Google Scholar] [CrossRef]
- Formisano, C.; Sirignano, C.; Rigano, D.; Chianese, G.; Zengin, G.; Seo, E.-J.; Efferth, T.; Taglialatela-Scafati, O. Antiproliferative activity against leukemia cells of sesquiterpene lactones from the Turkish endemic plant Centaurea drabifolia subsp. detonsa. Fitoterapia 2017, 120, 98–102. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Busà, R.; Ercolano, G.; Formisano, C.; Allegra, M.; Taglialatela-Scafati, O.; Ianaro, A.J.P.R. Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF-κB, and Nrf-2 signaling pathways in vitro. Phytother. Res. 2021, 35, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Saeed, M.E.M.; Hegazy, M.-E.F.; Kampf, C.J.; Efferth, T. Chemopreventive property of Sencha tea extracts towards sensitive and multidrug-resistant leukemia and multiple myeloma cells. Biomolecules 2020, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Böcker, S.; Dührkop, K. Fragmentation trees reloaded. J. Cheminform. 2016, 8, 5. [Google Scholar] [CrossRef]
- Kadioglu, O.; Cao, J.; Kosyakova, N.; Mrasek, K.; Liehr, T.; Efferth, T. Genomic and transcriptomic profiling of resistant CEM/ADR-5000 and sensitive CCRF-CEM leukaemia cells for unravelling the full complexity of multi-factorial multidrug resistance. Sci. Rep. 2016, 6, 36754. [Google Scholar] [CrossRef]
- Castro, F.; Dirks, W.G.; Fähnrich, S.; Hotz-Wagenblatt, A.; Pawlita, M.; Schmitt, M. High-throughput SNP-based authentication of human cell lines. Int. J. Cancer 2013, 132, 308–314. [Google Scholar] [CrossRef]
- Kotsos, M.P.; Aligiannis, N.; Myrianthopoulos, V.; Mitaku, S.; Skaltsounis, L. Sesquiterpene lactones from Staehelina fruticosa. J. Nat. Prod. 2008, 71, 847–851. [Google Scholar] [CrossRef]
- Tastan, P.; Hajdú, Z.; Kúsz, N.; Zupkó, I.; Sinka, I.; Kivcak, B.; Hohmann, J. Sesquiterpene lactones and flavonoids from Psephellus pyrrhoblepharus with antiproliferative activity on human gynecological cancer cell lines. Molecules 2019, 24, 3165. [Google Scholar] [CrossRef]
- Ćirić, A.; Karioti, A.; Glamočlija, J.; Soković, M.; Skaltsa, H. Antimicrobial activity of secondary metabolites isolated from Centaurea spruneri Boiss. & Heldr. J. Serb. Chem. Soc. 2011, 76, 27–34. [Google Scholar]
- Saroglou, V.; Karioti, A.; Demetzos, C.; Dimas, K.; Skaltsa, H. Sesquiterpene Lactones from Centaurea spinosa and their antibacterial and cytotoxic activities. J. Nat. Prod. 2005, 68, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Djeddi, S.; Karioti, A.; Sokovic, M.; Stojkovic, D.; Seridi, R.; Skaltsa, H. Minor sesquiterpene lactones from Centaurea pullata and their antimicrobial activity. J. Nat. Prod. 2007, 70, 1796–1799. [Google Scholar] [CrossRef]
- Bordoloi, M.; Barua, N.C.; Ghosh, A.C. An artemisinic acid analogue from Tithonia diversifolia. Phytochemistry 1996, 41, 557–559. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Bücherl, D.; Nikolov, S.; Heilmann, J.; Nedialkov, P.T. Flavonol glycosides from Chenopodium foliosum Asch. Phytochem. Lett. 2011, 4, 367–371. [Google Scholar] [CrossRef]
- Skaltsa, H.; Lazari, D.; Panagouleas, C.; Georgiadou, E.; Garcia, B.; Sokovic, M. Sesquiterpene lactones from Centaurea thessala and Centaurea attica. Antifungal activity. Phytochemistry 2000, 55, 903–908. [Google Scholar] [CrossRef]
- Gao, X.; Lin, C.-J.; Jia, Z.-J. Cytotoxic germacranolides and acyclic diterpenoides from the seeds of Carpesium triste. J. Nat. Prod. 2007, 70, 830–834. [Google Scholar] [CrossRef]
- Flamini, G.; Bulleri, C.; Morelli, I. Secondary constituents from Centaurea horrida and their evolutionary meaning. Biochem. Syst. Ecol. 2002, 30, 1051–1054. [Google Scholar] [CrossRef]
- Akkal, S.; Benayache, F.; Medjroubi, K.; Tillequin, F.; Seguin, E. Flavonoids from Centaurea furfuracea (Asteraceae). Biochem. Syst. Ecol. 2003, 31, 641–643. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.M.; Raccuglia, R.A.; Simmonds, M.S.; Arnold, N.A.; Bruno, M. Guaianolides from the aerial parts of Centaurea hololeuca. Nat. Prod. Commun. 2006, 1, 281–285. [Google Scholar] [CrossRef]
- Sen, A.; Gurbuz, B.; Gurer, U.S.; Bulut, G.; Bitis, L. Flavonoids and biological activities of Centaurea stenolepis. Chem. Nat. Compd. 2014, 50, 128–129. [Google Scholar] [CrossRef]
- Bandyukova, V.A.; Sergeeva, N.V.; Dzhumyrko, S.F. Luteolin glycosides in some plants of the family Compositae. Chem. Nat. Compd. 1970, 6, 483. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Kamel, E.M. Cytotoxic activities of flavonoids from Centaurea scoparia. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gökçen, T.A.N.; Erel, Ş.B.; Demir, S.; Akgün, İ.; Bedir, E.; Karaalp, C. Secondary Metabolites of Centaurea Cyanus L. Ank. Üniversitesi Eczacılık Fakültesi Derg. 2007, 37, 285–294. [Google Scholar]
- Wang, S.; Suh, J.H.; Zheng, X.; Wang, Y.; Ho, C.T. Identification and quantification of potential anti-inflammatory hydroxycinnamic acid amides from wolfberry. J. Agric. Food Chem. 2017, 65, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Hodaj, E.; Tsiftsoglou, O.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Lignans and indole alkaloids from the seeds of Centaurea vlachorum Hartvig (Asteraceae), growing wild in Albania and their biological activity. Nat. Prod. Res. 2017, 31, 1195–1200. [Google Scholar] [CrossRef]
- Luca, S.V.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Jumabayeva, A.; Sakipova, Z.; Xiao, J.; Skalicka-Woźniak, K. Liquid-Liquid Chromatography Separation of Guaiane-Type Sesquiterpene Lactones from Ferula penninervis Regel & Schmalh. and Evaluation of Their In Vitro Cytotoxic and Melanin Inhibitory Potential. Int. J. Mol. Sci. 2021, 22, 10717. [Google Scholar]
- Öksüz, S.; Serin, S.; Topçu, G. Sesquiterpene lactones from Centaurea hermannii. Phytochemistry 1994, 35, 435–438. [Google Scholar] [CrossRef]
- Labed, F.; Masullo, M.; Mirra, V.; Nazzaro, F.; Benayache, F.; Benayache, S.; Piacente, S. Amino acid-sesquiterpene lactone conjugates from the aerial parts of Centaurea pungens and evaluation of their antimicrobial activity. Fitoterapia 2019, 133, 51–55. [Google Scholar] [CrossRef]
- Kamanzi, K.; Raynaud, J.; Voirin, B. Flavonoid O-heterosides from flowers of Centaurea solstitialis L (Compositae). Plantes Med. Et Phytother. 1983, 17, 57–60. [Google Scholar]
- Shang, S.; Chen, H.; Liang, C.; Gao, Z.; Du, X.; Wang, R.; Shi, Y.; Zheng, Y.; Xiao, W.; Sun, H.D. Phenolic constituents from Parakmeria yunnanensis and their anti-HIV-1 activity. Arch. Pharmacal Res. 2013, 36, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, T.; Matsuda, H.; Morikawa, T.; Yoshikawa, M.; Tani, T. Bioactive constituents from Chinese natural medicines. XV. Inhibitory effect on aldose reductase and structures of saussureosides A and B from Saussurea medusa. Chem. Pharm. Bull. 2005, 53, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Pardini, M.; Morelli, I. A flavonoid sulphate and other compounds from the roots of Centaurea bracteata. Phytochemistry 2001, 58, 1229–1233. [Google Scholar] [CrossRef]
- Michalska, K.; Szneler, E.; Kisiel, W.J.P. Sesquiterpene lactones from Lactuca canadensis and their chemotaxonomic significance. Phytochemistry 2013, 90, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Mishio, T.; Honma, T.; Iwashina, T. Yellow flavonoids in Centaurea ruthenica as flower pigments. Biochem. Syst. Ecol. 2006, 2, 180–184. [Google Scholar] [CrossRef]
- Gülcemal, D.; Alankuş-Çalışkan, Ö.; Karaalp, C.; Örs, A.U.; Ballar, P.; Bedir, E. Phenolic glycosides with antiproteasomal activity from Centaurea urvillei DC. subsp. urvillei. Carbohydr. Res. 2010, 345, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Baatouche, S.; Cheriet, T.; Sarri, D.; Mekkiou, R.; Boumaza, O.; Benayache, S.; Benayache, F.; Brouard, I.; León, F.; Seghiri, R. Centaurea microcarpa Coss. & Dur.(Asteraceae) extracts: New cyanogenic glucoside and other constituents. Nat. Prod. Res. 2019, 33, 3070–3076. [Google Scholar] [PubMed]
- Zhu, N.; Tang, C.; Xu, C.; Ke, C.; Lin, G.; Jenis, J.; Ye, Y. Cytotoxic germacrane-type sesquiterpene lactones from the whole plant of Carpesium lipskyi. J. Nat. Prod. 2019, 82, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Li, J.; Yu, S.; Li, Y.; Luo, Y. Hepatoprotective Sesquiterpene Glycosides from Sarcandra g labra. J. Nat. Prod. 2006, 69, 616–620. [Google Scholar] [CrossRef]
- Massanet, G.M.; Collado, I.G.; Macías, F.A.; Bohlmann, F.; Jakupovic, J. Structural determination of clementein, a new guaianolide isolated from Centaurea clementei. Tetrahedron Lett. 1983, 24, 1641–1642. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Elshamy, A.I.; Abd-ElGawad, A.M.; Hussien, T.A.; El-Toumy, S.A.; Efferth, T.; Hegazy, M.E.F. Cytotoxic and chemotaxonomic study of isolated metabolites from Centaurea aegyptiaca. J. Chin. Chem. Soc. 2021, 68, 159–168. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Que, S.; Tu, G.; Wan, D.; Cheng, W.; Liang, H.; Ye, J.; Zhang, Q. 3-Hydroxy-3-methylglutaryl flavonol glycosides from Oxytropis falcata. J. Nat. Prod. 2012, 75, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, L.; Seghiri, R.; Benayache, S.; Mosset, P.; Lobstein, A.; Chaabi, M.; León, F.; Brouard, I.; Bermejo, J.; Benayache, F. A new flavonoid and other constituents from Centaurea nicaeensis All. var. walliana M. Nat. Prod. Res. 2012, 26, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Al-Easa, H.S.; Kamel, A.; Rizk, A.-F.M. Flavonoids from Centaurea sinaica. Fitoterapia 1992, 63, 468–469. [Google Scholar]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Gornostai, T.Y.G.; Selyutina, I.Y.; Zilfikarov, I.N. Effect of low temperature cultivation on the phytochemical profile and bioactivity of Arctic plants: A case of Dracocephalum palmatum. Int. J. Mol. Sci. 2017, 18, 2579. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I. New isorhamnetin glycosides and other phenolic compounds from Calendula officinalis. Chem. Nat. Compd. 2013, 49, 833–840. [Google Scholar] [CrossRef]
- Menet, J.-M.; Thiebaut, D. Countercurrent Chromatography; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Demiroz, T.; Nalbantsoy, A.; Kose, F.A.; Baykan, S. Phytochemical composition and antioxidant, cytotoxic and anti-inflammatory properties of Psephellus goeksunensis (Aytaç & H. Duman) Greuter & Raab-Straube. South Afr. J. Bot. 2020, 130, 1–7. [Google Scholar]
- Zaghloul, A.M.; Salama, O.M.; Halim, A.F. Chemical investigation of Centaurea glomerata vahl. Mansoura J. Pharm. Sci. 1990, 6, 61–68. [Google Scholar]
- Seghiri, R.; Boumaza, O.; Mekkiou, R.; Benayache, S.; Mosset, P.; Quintana, J.; Estevez, F.; Leon, F.; Bermejo, J.; Benayache, F. A flavonoid with cytotoxic activity and other constituents from Centaurea africana. Phytochem. Lett. 2009, 2, 114–118. [Google Scholar] [CrossRef]
- Flamini, G.; Antognoli, E.; Morelli, I. Two flavonoids and other compounds from the aerial parts of Centaurea bracteata from Italy. Phytochemistry 2001, 57, 559–564. [Google Scholar] [CrossRef]
- Kitouni, R.; Benayache, F.; Benayache, S. Flavonoids of the exudate of Centaurea calcitrapa. Chem. Nat. Compd. 2015, 51, 762–763. [Google Scholar] [CrossRef]
- Dayrit, F.M.; Lapid, M.R.J.; Cagampang, J.V.; Lagurin, L.G. Phytochemical studies on the leaves of Vitex negundo L. (Lagundi), 1: Investigations of the bronchial relaxing constituents [Philippines]. Philipp. J. Sci. 1987, 116, 403–470. [Google Scholar]
- Radan, M.; Carev, I.; Tešević, V.; Politeo, O.; Čulić, V.Č. Qualitative HPLC-DAD/ESI-TOF-MS Analysis, Cytotoxic, and Apoptotic Effects of Croatian Endemic Centaurea ragusina L. Aqueous Extracts. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- Şekerler, T.; Şen, A.; Bitiş, L.; Şener, A. In vitro antihepatocellular carcinoma activity of secondary metabolites of Centaurea kilaea Boiss. J. Res. Pharm. 2020, 24, 479–486. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. Eudesmanolides and kaurene derivatives from Wedelia hookeriana. Phytochemistry 1982, 21, 2329–2333. [Google Scholar] [CrossRef]
- S Tuzun, B.; Hajdu, Z.; Orban-Gyapai, O.; P Zomborszki, Z.; Jedlinszki, N.; Forgo, P.; Kıvcak, B.; Hohmann, J. Isolation of chemical constituents of Centaurea virgata lam. and xanthine oxidase inhibitory activity of the plant extract and compounds. Med. Chem. 2017, 13, 498–502. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Phytochemical analysis and bioactivity screening of three medicinal plants of Saudi Arabia. Trop. J. Pharm. Res. 2020, 19, 371–376. [Google Scholar] [CrossRef]

| Species | Sample Code | Voucher ID | Collection Site | Latitude (N) | Longitude (E) |
|---|---|---|---|---|---|
| C. alexandrina | Ce.Alex | M/2282 | Marsa Matrouh | 31°23′37.81″ | 27°01′7.64″ |
| C. calcitrapa | Ce.Co | M/2279 | Marsa Matrouh | 31°03′41.10″ | 28°12′31.6″ |
| C. eryngioides | CE | M/2284 | Saint Catherine | 28°33′20.83″ | 33°56′9.13″ |
| C. glomerata | Ce.G | M/2280 | Rashid | 30°56′52.51″ | 30°58′33.1″ |
| C. lipii | CL | M/2281 | Egyptian north coast | 29°38′16.55″ | 32°18′23.72″ |
| C. pallescens | Ce.PA | M/2283 | Marsa Matrouh | 31°22′37.01″ | 31°03′41.16″ |
| C. pumilio | CP | M/2285 | Egyptian north coast | 30°54′9.06″ | 29°26′8.63″ |
| C. scoparia | Ce.Sco | M/2278 | Red Sea Coast | 31°03′41.16″ | 31°03′41.16″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reda, E.H.; Hegazi, N.M.; Marzouk, M.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.-S.A.; Mohamed, T.A.; Ibrahim, M.A.A.; Shams, K.A.; Abdel-Azim, N.S.; et al. Feature-Based Molecular Networking for the Exploration of the Metabolome Diversity of Common Egyptian Centaurea Species in Relation to Their Cytotoxic Activity. Molecules 2023, 28, 674. https://doi.org/10.3390/molecules28020674
Reda EH, Hegazi NM, Marzouk M, Shakour ZTA, El-Halawany AM, El-Kashoury E-SA, Mohamed TA, Ibrahim MAA, Shams KA, Abdel-Azim NS, et al. Feature-Based Molecular Networking for the Exploration of the Metabolome Diversity of Common Egyptian Centaurea Species in Relation to Their Cytotoxic Activity. Molecules. 2023; 28(2):674. https://doi.org/10.3390/molecules28020674
Chicago/Turabian StyleReda, Eman H., Nesrine M. Hegazi, Mona Marzouk, Zienab T. Abdel Shakour, Ali M. El-Halawany, El-Sayeda A. El-Kashoury, Tarik A. Mohamed, Mahmoud A. A. Ibrahim, Khaled A. Shams, Nahla S. Abdel-Azim, and et al. 2023. "Feature-Based Molecular Networking for the Exploration of the Metabolome Diversity of Common Egyptian Centaurea Species in Relation to Their Cytotoxic Activity" Molecules 28, no. 2: 674. https://doi.org/10.3390/molecules28020674
APA StyleReda, E. H., Hegazi, N. M., Marzouk, M., Shakour, Z. T. A., El-Halawany, A. M., El-Kashoury, E.-S. A., Mohamed, T. A., Ibrahim, M. A. A., Shams, K. A., Abdel-Azim, N. S., Kampf, C. J., Efferth, T., Paré, P. W., & Hegazy, M.-E. F. (2023). Feature-Based Molecular Networking for the Exploration of the Metabolome Diversity of Common Egyptian Centaurea Species in Relation to Their Cytotoxic Activity. Molecules, 28(2), 674. https://doi.org/10.3390/molecules28020674
















