A {Co9}-Added Polyoxometalate for Efficient Visible-Light-Driven Hydrogen Evolution
Abstract
1. Introduction
2. Experimental Section
2.1. General Procedure
2.2. Synthesis of 1
2.3. X-ray Crystallography
3. Result and Discussion
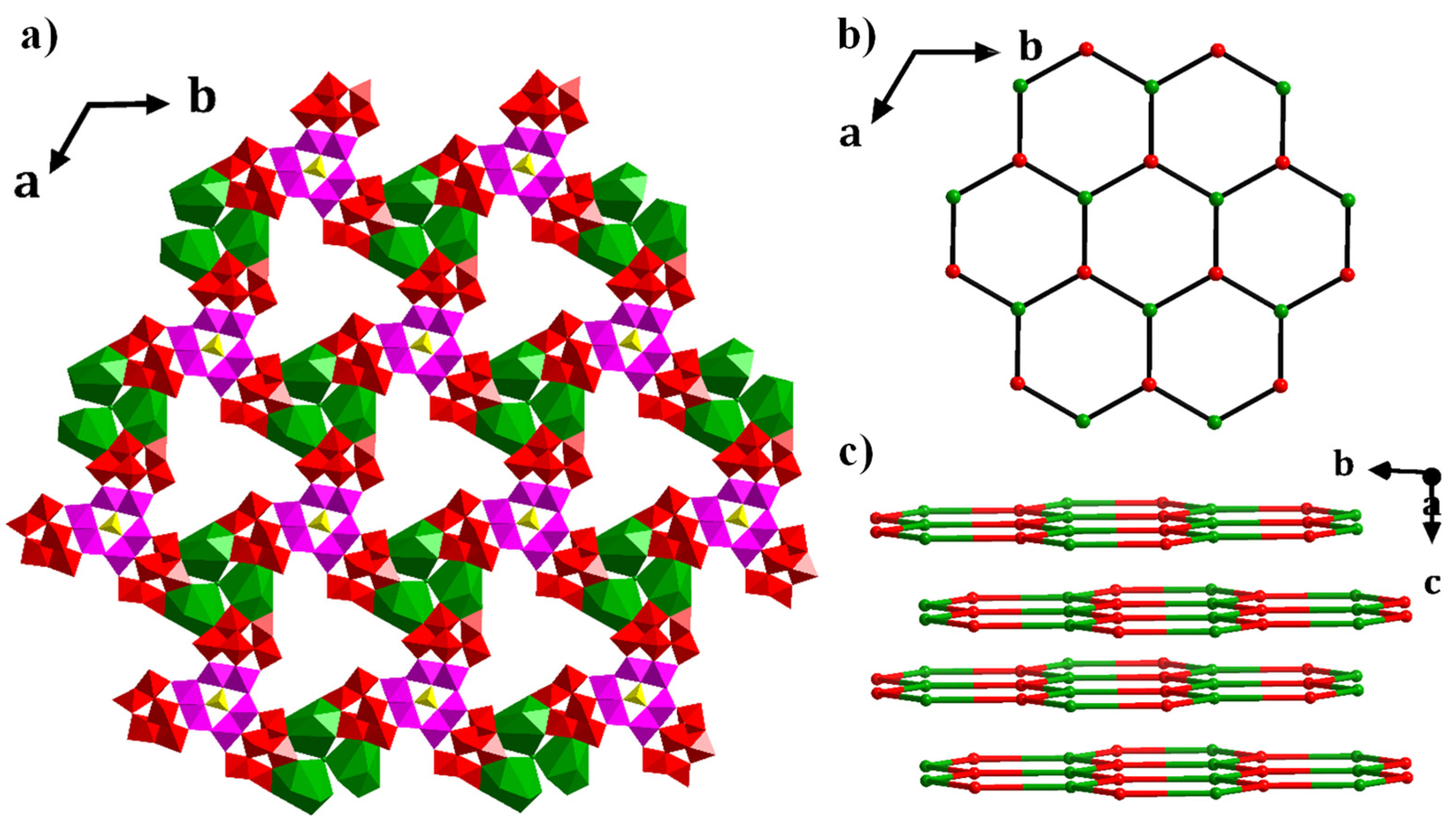
3.1. Structure of 1
3.2. IR Spectrum and Optical Band Gap
3.3. PXRD and Thermogravimetric Analysis
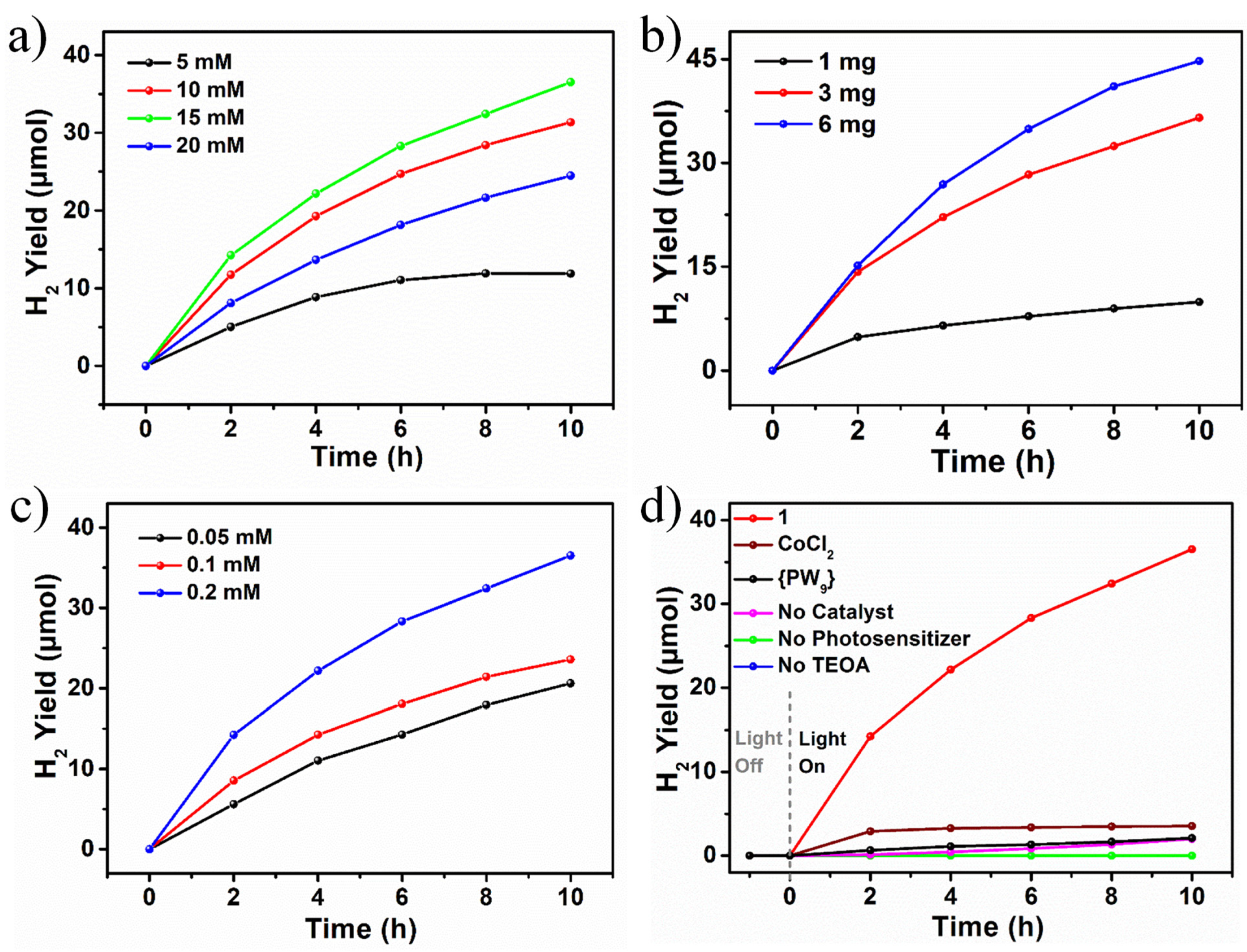
3.4. Photocatalytic H2 Evolution Performance
3.5. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, J.X.; Zhang, X.B.; Li, Y.L.; Huang, S.L.; Yang, G.Y. Polyoxometalate Functionalized Architectures. Coord. Chem. Rev. 2020, 414, 213260. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, G.Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chen, Q.; Wan, G.; Yang, Y.; Yu, H.; Li, B.; Wu, L. Hyaluronic Acid-Enwrapped Polyoxometalate Complex for Synergistic Near Infrared-II Photothermal/Chemo-Therapy and Chemodynamic Therapy. Biomacromolecules 2022, 23, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Lydon, C.; Sabi, M.M.; Symes, M.D.; Long, D.L.; Murrie, M.; Yoshii, S.; Nojiri, H.; Cronin, L. Directed Assembly of Nanoscale Co(II)-Substituted {Co9[P2W15]3} and {Co14[P2W15]4} Polyoxometalates. Chem. Commun. 2012, 48, 9819–9821. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Lan, Y.; Bassil, B.S.; Xiang, Y.; Suchopar, A.; Powell, A.K.; Kortz, U. Hexadecacobalt(II)-Containing Polyoxometalate -Based Single-Molecule Magnet. Angew. Chem. Int. Ed. 2011, 50, 4708–4711. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, G.; Li, B.; Wu, L. An Integrated Giant Polyoxometalate Complex for Photothermally Enhanced Catalytic Oxidation. Sci. Adv. 2021, 7, eabf8413. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, A.; Wang, G.; Wei, M.; Liu, N.; Li, B.; Wu, L. Reinforced Catalytic Oxidation of Polyoxometalate@charge Transfer Complex by On-Site Heating from Photothermal Conversion. Chem. Eng. J. 2022, 446, 137134. [Google Scholar] [CrossRef]
- Miras, H.N.; Yan, J.; Long, D.-L.; Cronin, L. Engineering Polyoxometalates with Emergent Properties. Chem. Soc. Rev. 2012, 41, 7403–7430. [Google Scholar] [CrossRef]
- Hill, C.L. Introduction: PolyoxometalatesMulticomponent Molecular Vehicles to Probe Fundamental Issues and Practical Problems. Chem. Rev. 1998, 98, 1–2. [Google Scholar] [CrossRef]
- Chen, W.; Li, H.; Song, J.; Zhao, Y.; Ma, P.; Niu, J.; Wang, J. Binuclear Ru(III)-Containing Polyoxometalate with Efficient Photocatalytic Activity for Oxidative Coupling of Amines to Imines. Inorg. Chem. 2022, 61, 2076–2085. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Zhang, C.N.; Li, S.; Liu, G.C.; Wang, X.L. An excellent multifunctional photocatalyst with a polyoxometalate-viologen framework for CEES oxidation, Cr(VI) reduction and dye decolorization under different light regimes. Inorg. Chem. Front. 2022, 9, 4824–4833. [Google Scholar] [CrossRef]
- Xu, B.; Xu, Q.; Wang, Q.; Liu, Z.; Zhao, R.; Li, D.; Ma, P.; Wang, J.; Niu, J. A Copper-Containing Polyoxometalate-Based Metal–Organic Framework as an Efficient Catalyst for Selective Catalytic Oxidation of Alkylbenzenes. Inorg. Chem. 2021, 60, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Narkhede, N.; Singh, S.; Pathan, S. Keggin-Type Lacunary and Transition Metal Substituted Polyoxometalates as Heterogeneous Catalysts: A Recent Progress. Catal. Rev. 2016, 58, 337–370. [Google Scholar] [CrossRef]
- Wang, S.S.; Kong, X.Y.; Wu, W.; Wu, X.Y.; Cai, S.; Lu, C.Z. Synergic Coordination of Multicomponents for the Formation of a {Ni30} Cluster Substituted Polyoxometalate and Its In-Situ Assembly. Inorg. Chem. Front. 2022, 9, 4350–4358. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhao, Q.; Chen, C.A.; Sun, J.J.; Lv, H.; Yang, G.Y. Chiral {Ni6PW9} Cluster–Organic Framework: Synthesis, Structure, and Properties. Inorg. Chem. 2022, 61, 7477–7483. [Google Scholar] [CrossRef]
- Zheng, S.T.; Yuan, D.Q.; Jia, H.P.; Zhang, J.; Yang, G.Y. Combination between Lacunary Polyoxometalates and High-Nuclear Transition Metal Clusters under Hydrothermal Conditions: I. from Isolated Cluster to 1-D Chain. Chem. Commun. 2007, 18, 1858–1860. [Google Scholar] [CrossRef]
- Zheng, S.T.; Zhang, J.; Clemente-Juan, J.M.; Yuan, D.Q.; Yang, G.Y. Poly(Polyoxotungstate)s with 20 Nickel Centers: From Nanoclusters to One-Dimensional Chains. Angew. Chem. Int. Ed. 2009, 48, 7176–7179. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, J.; Cheng, L.; Yang, G.Y. Poly(Polyoxometalate)s Assembled by {Ni6PW9} Units: From Ring-Shaped Ni24-Tetramers to Rod-Shaped Ni40-Octamers. Chem. Commun. 2012, 48, 9658–9660. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, M.; Lian, C.; Lang, Z.L.; Lv, H.J.; Yang, G.Y. Ring-Shaped Polyoxometalate Built by {Mn4PW9} and PO4 Units for Efficient Visible-Light-Driven Hydrogen Evolution. CCS Chem. 2021, 3, 2095–2103. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.W.; Li, X.X.; Zheng, S.T.; Yang, G.Y. Multicomponent Cooperative Assembly of Nanoscale Boron-Rich Polyoxotungstates with 22 and 30 Boron Atomsa. CCS Chem. 2021, 4, 1305–1314. [Google Scholar] [CrossRef]
- Huang, L.; Wang, S.S.; Zhao, J.W.; Cheng, L.; Yang, G.Y. Synergistic Combination of Multi-ZrIV Cations and Lacunary Keggin Germanotungstates Leading to a Gigantic Zr24-Cluster-Substituted Polyoxometalate. J. Am. Chem. Soc. 2014, 136, 7637–7642. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Guo, W.; Wu, K.; Chen, Z.; Bacsa, J.; Musaev, D.G.; Geletii, Y.V.; Lauinger, S.M.; Lian, T.; Hill, C.L. A Noble-Metal-Free, Tetra-Nickel Polyoxotungstate Catalyst for Efficient Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2014, 136, 14015–14018. [Google Scholar] [CrossRef] [PubMed]
- Amthor, S.; Knoll, S.; Heiland, M.; Zedler, L.; Li, C.; Nauroozi, D.; Tobaschus, W.; Mengele, A.K.; Anjass, M.; Schubert, U.S.; et al. A Photosensitizer–Polyoxometalate Dyad That Enables the Decoupling of Light and Dark Reactions for Delayed on-Demand Solar Hydrogen Production. Nat. Chem. 2022, 14, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Huang, Q.; He, C.T.; Chen, Y.; Liu, J.; Shen, F.C.; Lan, Y.Q. Oriented Electron Transmission in Polyoxometalate-Metalloporphyrin Organic Framework for Highly Selective Electroreduction of CO2. Nat Commun. 2018, 9, 4466. [Google Scholar] [CrossRef]
- Jiao, L.; Dong, Y.; Xin, X.; Qin, L.; Lv, H. Facile Integration of Ni-Substituted Polyoxometalate Catalysts into Mesoporous Light-Responsive Metal-Organic Framework for Effective Photogeneration of Hydrogen. Appl. Catal. B 2021, 291, 120091. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, J.; Zhang, M.; Meng, X.; Chen, M.; Feng, Y.; Chen, X.; Ding, Y. Recent Advances and Perspectives in Cobalt-Based Heterogeneous Catalysts for Photocatalytic Water Splitting, CO2 Reduction, and N2 Fixation. Chin. J. Catal. 2022, 43, 2273–2300. [Google Scholar] [CrossRef]
- Ginsberg, A.P. Inorganic Syntheses; John Wiley & Sons: New York, NY, USA, 1990; p. 108. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.a.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. Sect. C: Struct. Chem. C. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.W.; Pan, R.; Yang, B.F.; Yang, G.Y.; Liu, H.S. Diverse Ligand-Functionalized Mixed-Valent Hexa-Manganese Sandwiched Silicotungstates with Single-Molecule Magnet Behavior. Chem. Eur. J. 2016, 22, 12322–12331. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhao, C.; Yao, L.Y.; Dou, H.; Zhang, M.; Xie, J.; Weng, T.C.; Lv, H.; Yang, G.Y. Efficient Photogeneration of Hydrogen Boosted by Long-Lived Dye-Modified Ir(III) Photosensitizers and Polyoxometalate Catalyst. CCS Chem. 2022, 4, 259–271. [Google Scholar] [CrossRef]
- Ding, Y.S.; Wang, H.Y.; Ding, Y. Visible-Light-Driven Hydrogen Evolution Using a Polyoxometalate-Based Copper Molecular Catalyst. Dalton Trans. 2020, 49, 3457–3462. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Li, Y.; Wang, S.; Wang, E. Visible-Light Photocatalytic H2 Evolution over a Series of Transition Metal Substituted Keggin-Structure Heteropoly Blues. Chin. Sci. Bull. 2012, 57, 2265–2268. [Google Scholar] [CrossRef]
- Shang, X.; Liu, R.; Zhang, G.; Zhang, S.; Cao, H.; Gu, Z. Artificial Photosynthesis for Solar Hydrogen Generation over Transition-Metal Substituted Keggin-Type Titanium Tungstate. New J. Chem. 2014, 38, 1315–1320. [Google Scholar] [CrossRef]
- Sun, J.J.; Wang, W.D.; Li, X.Y.; Yang, B.F.; Yang, G.Y. {Cu8} Cluster-Sandwiched Polyoxotungstates and Their Polymers: Syntheses, Structures, and Properties. Inorg. Chem. 2021, 60, 10459–10467. [Google Scholar] [CrossRef] [PubMed]


| 1 | |
|---|---|
| Formula | H103Na8ClCs3Co9O158P5W27 |
| Mr | 8898.75 |
| Crystal system | Hexagonal |
| Space group | P63/m |
| a [Å] | 20.7840(8) |
| b [Å] | 20.7840(8) |
| c [Å] | 20.6385(9) |
| α [º] | 90 |
| β [º] | 90 |
| γ [º] | 120 |
| V [Å3] | 7721.1 |
| Z, Dc [g cm−3] | 2 |
| F(000) | 7316.0 |
| GOF on F2 | 1.036 |
| Final R indices [I > 2σ(I)] | R1 = 0.0762 wR2 = 0.2026 |
| R indices [all data] a | R1 = 0.01328 wR2 = 0.2464 |
| CCDC number | 2213769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-W.; Yang, G.-Y. A {Co9}-Added Polyoxometalate for Efficient Visible-Light-Driven Hydrogen Evolution. Molecules 2023, 28, 664. https://doi.org/10.3390/molecules28020664
Wang Z-W, Yang G-Y. A {Co9}-Added Polyoxometalate for Efficient Visible-Light-Driven Hydrogen Evolution. Molecules. 2023; 28(2):664. https://doi.org/10.3390/molecules28020664
Chicago/Turabian StyleWang, Zhen-Wen, and Guo-Yu Yang. 2023. "A {Co9}-Added Polyoxometalate for Efficient Visible-Light-Driven Hydrogen Evolution" Molecules 28, no. 2: 664. https://doi.org/10.3390/molecules28020664
APA StyleWang, Z.-W., & Yang, G.-Y. (2023). A {Co9}-Added Polyoxometalate for Efficient Visible-Light-Driven Hydrogen Evolution. Molecules, 28(2), 664. https://doi.org/10.3390/molecules28020664




