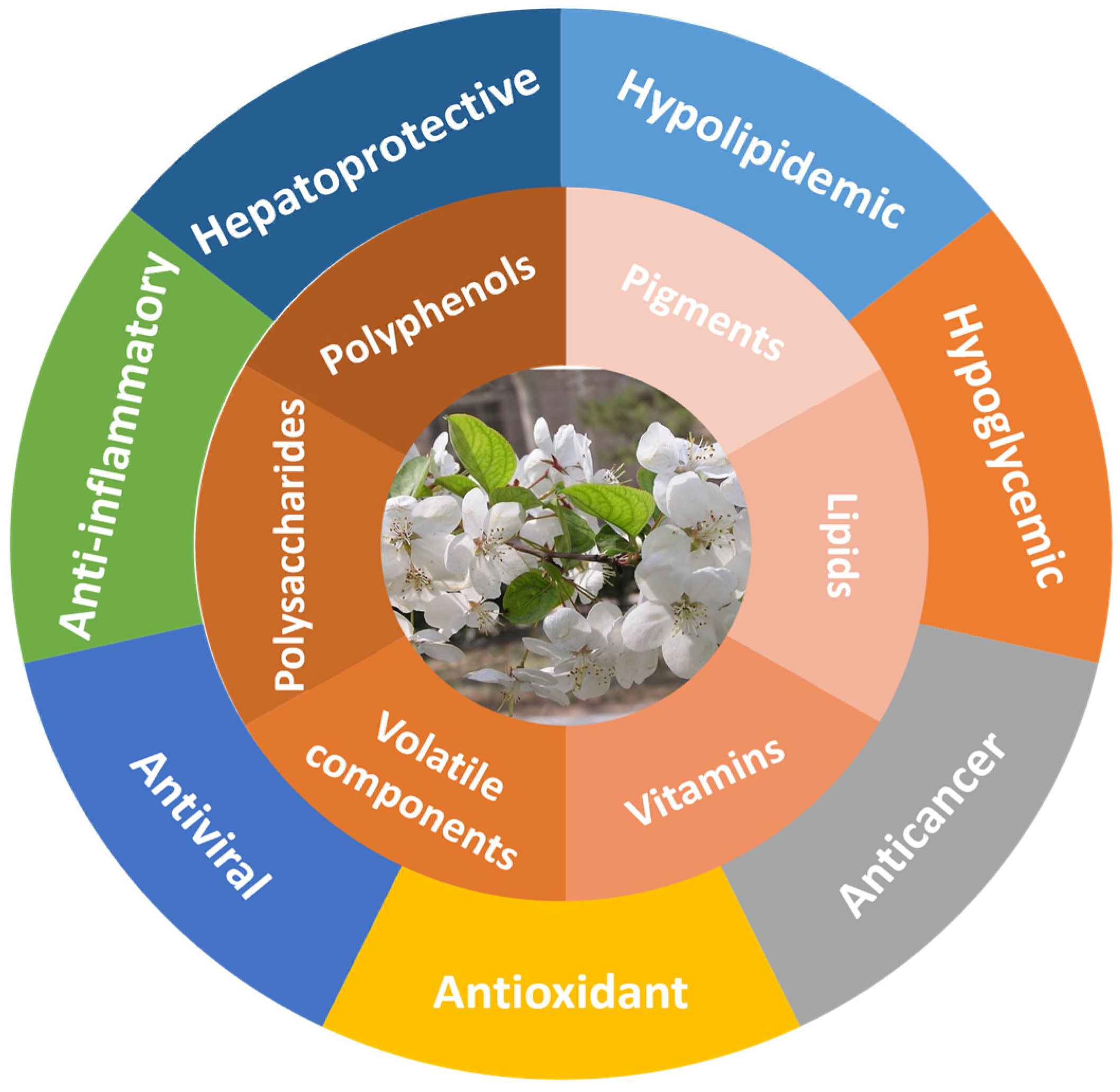
Bioactive Substances and Biological Functions in Malus hupehensis: A Review
Abstract
1. Introduction
2. Compounds in MH
2.1. Phenolic Compounds
2.2. Polysaccharides
2.3. Volatile Components
2.4. Vitamin
2.5. Other Ingredients
3. Biological Activities of MH
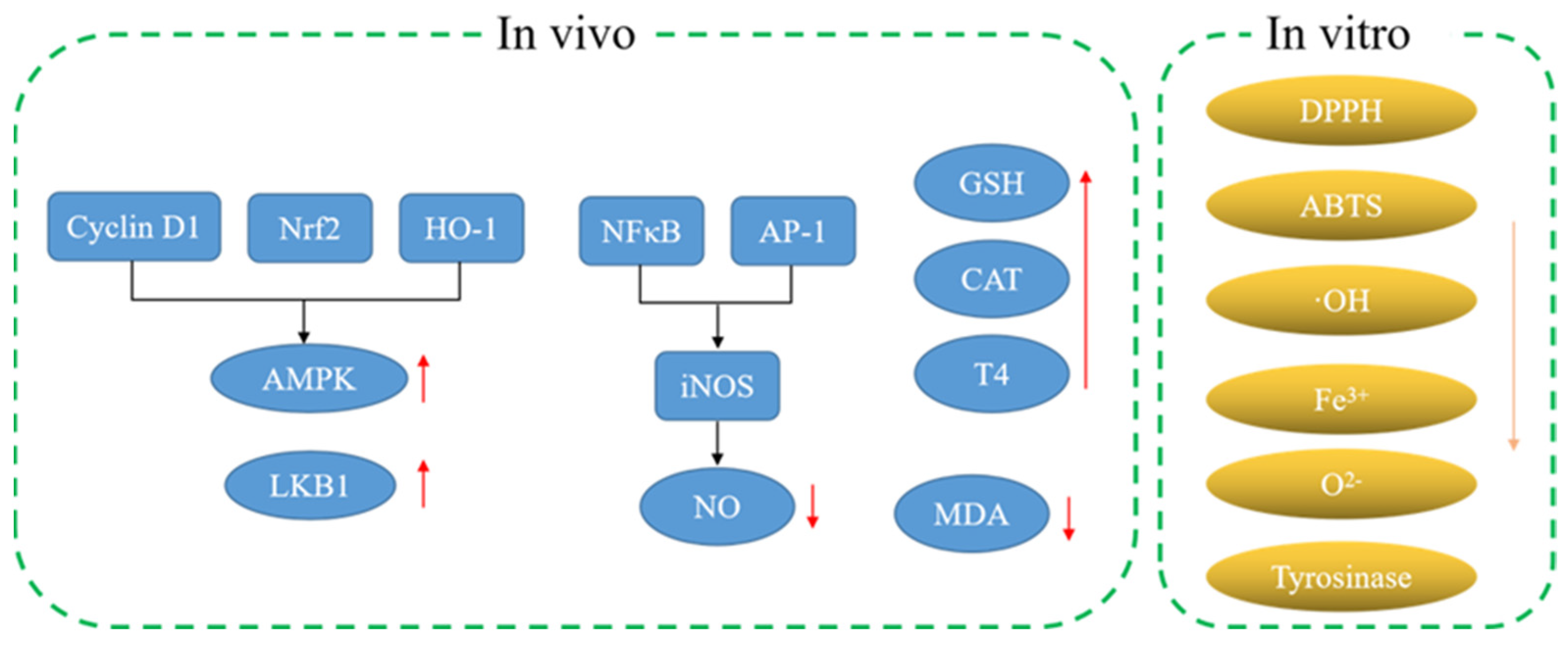
3.1. Antioxidant Activity
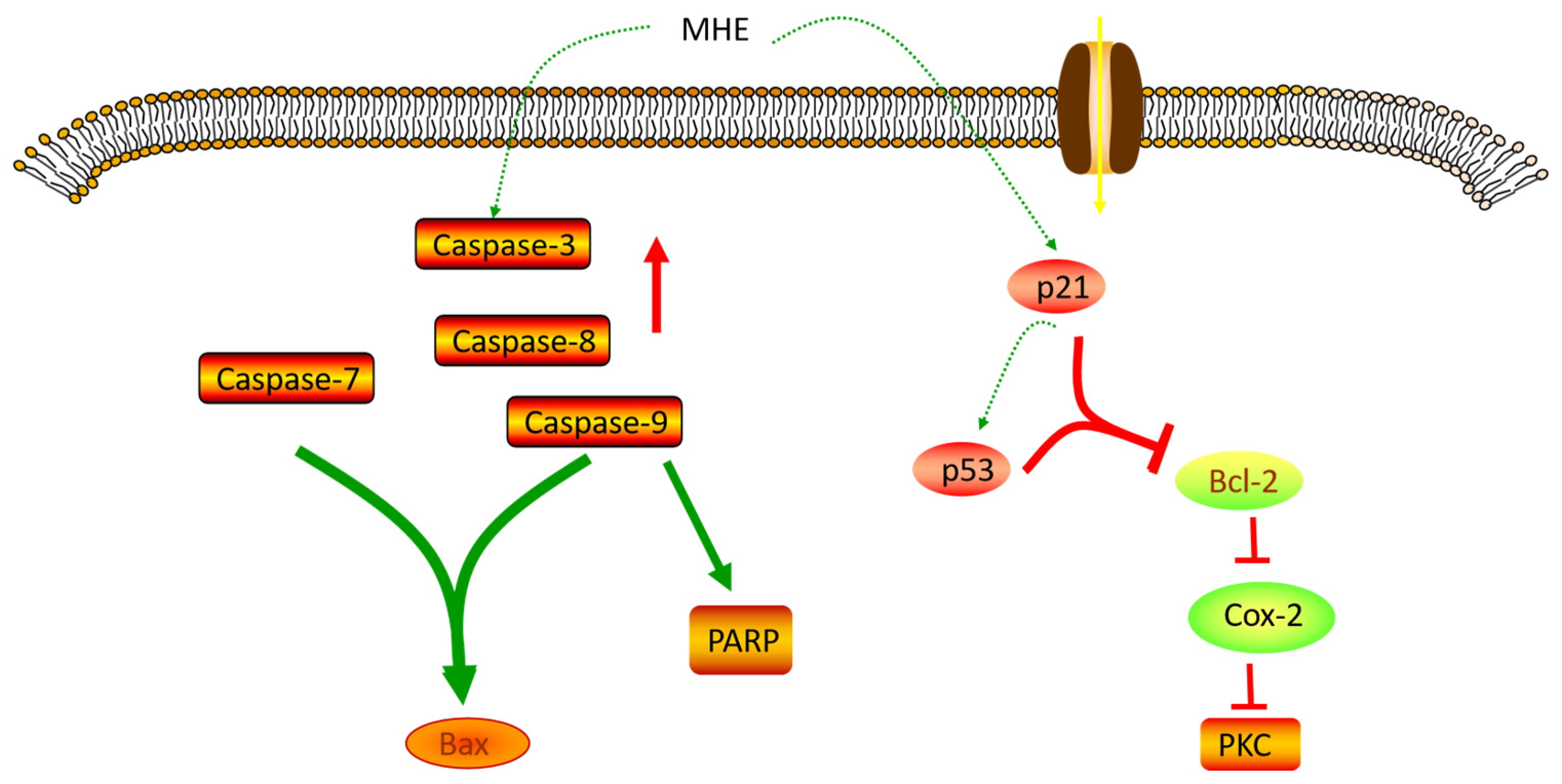
3.2. Anticancer Activity
3.3. Hypoglycemic Activity
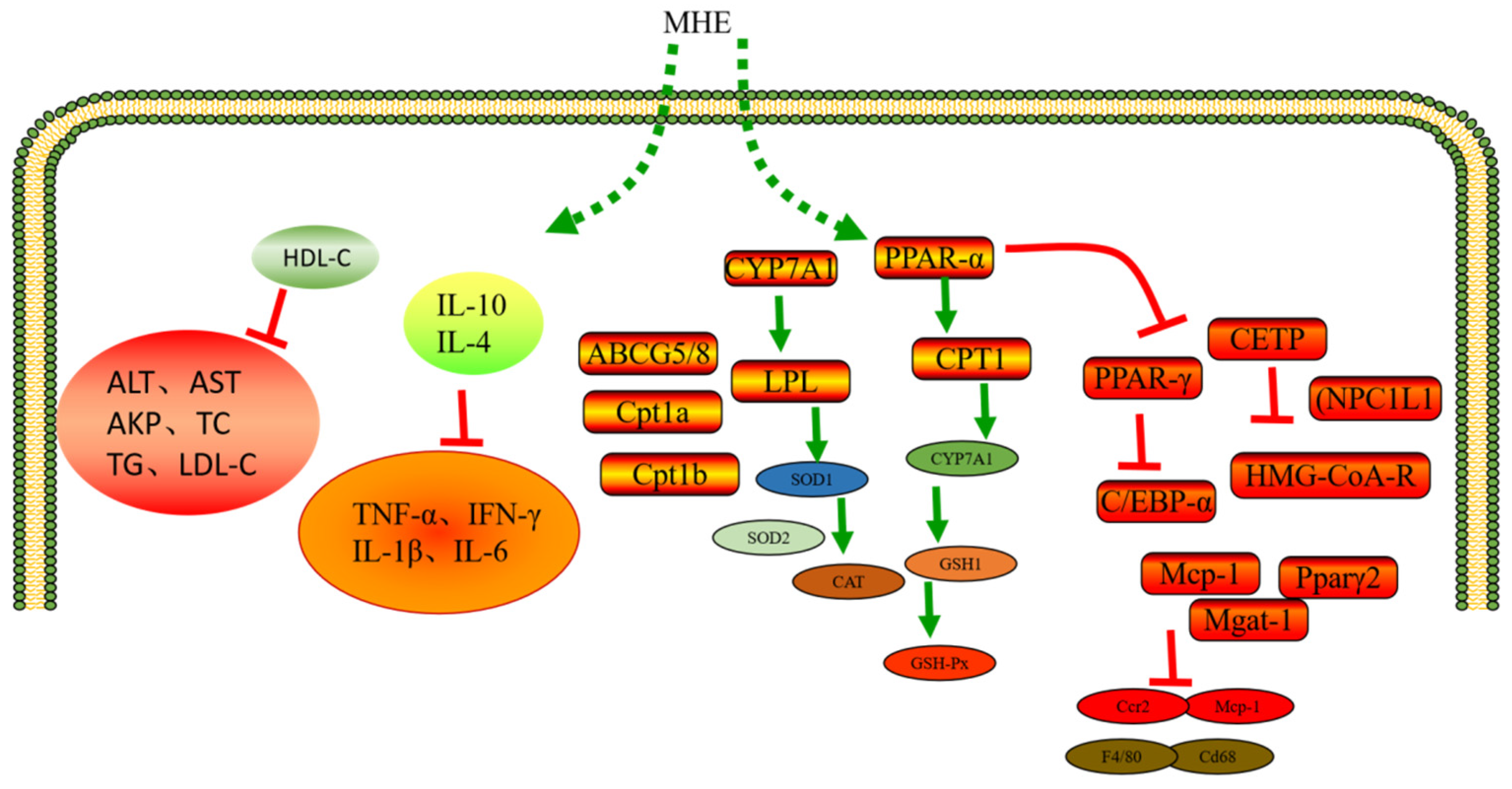
3.4. Hypolipidemic Activity
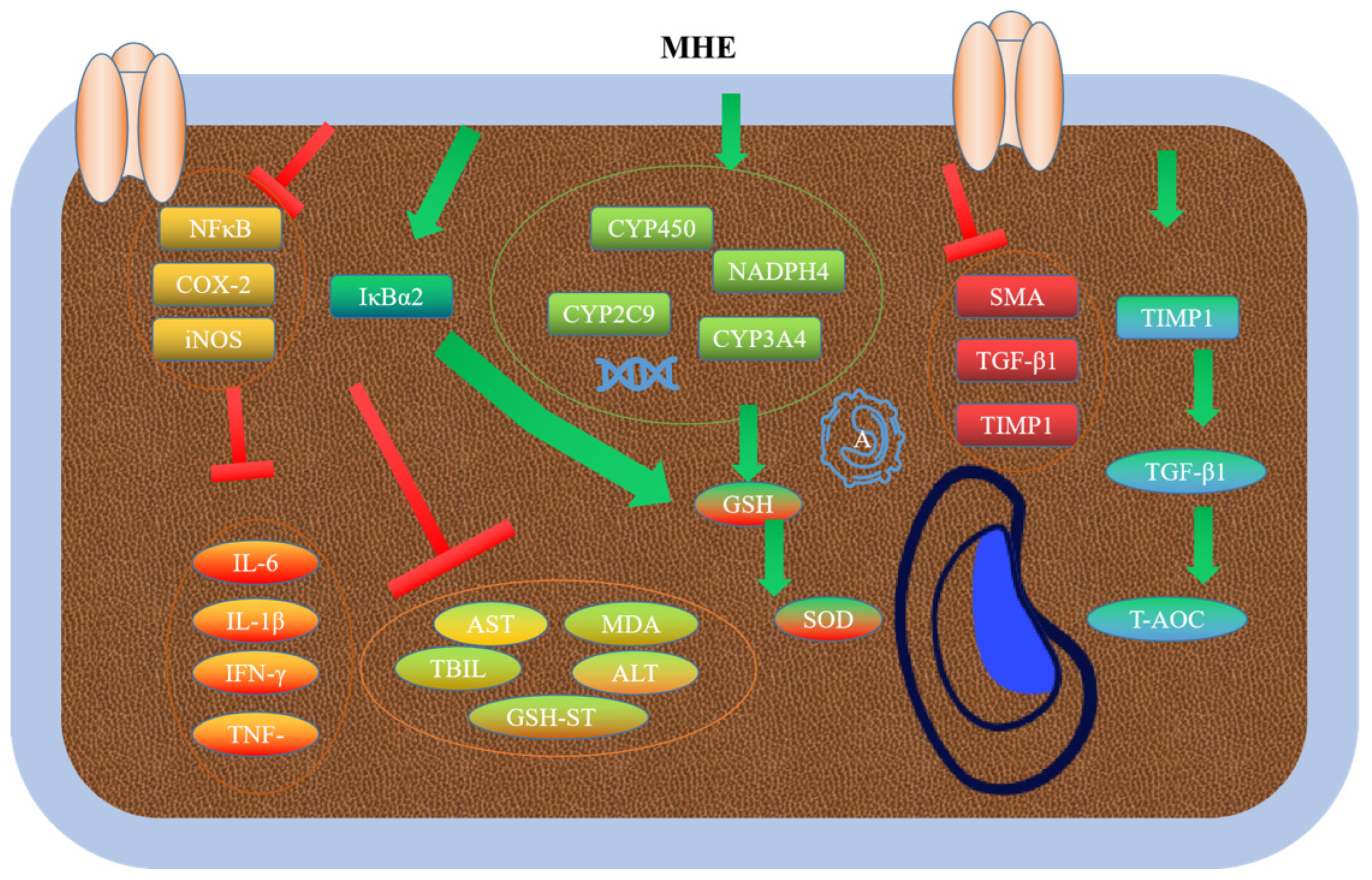
3.5. Hepatoprotective Activity
3.6. Other Biological Activities
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Duan, K.; Zhang, W. Biology and physiology of Malus hupehensis for the apogamic plant resource. Acta Hortic. 2008, 769, 441–447. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Tian, Y.; Ma, C.; Yang, S.; Wang, C. Comparative transcriptome analysis of NaCl and KCl stress response in Malus hupehensis Rehd. Provide insight into the regulation involved in Na+ and K+ homeostasis. Plant Physiol. Biochem. 2021, 164, 101–114. [Google Scholar] [CrossRef]
- Mao, J.; Niu, C.; Chen, S.; Xu, Y.; Khan, A.; Zuo, Q.; Wang, C.; Han, M.; Bao, L.; Zhang, D. Effects of exogenous methyl-jasmonate on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Sci. Hortic. 2021, 289, 110419. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Peng, Y.; Liu, C.; Zhang, X.; Zhang, Z.; Liang, W.; Ma, F.; Li, C. Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci. Hortic. 2020, 261, 108982. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Y.; Zhang, J.; Kang, W. Analysis of chemical constituents changing in physical process and nutritional components of Malus halliana Koehne tea. J. Food Qual. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Raudonis, R.; Kviklys, D.; Uselis, N.; Janulis, V. Phenolic composition and antioxidant activity of Malus domestica leaves. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Hutchinson, A.; Taper, C.D.; Towers, G.H. Studies of phloridzin in Malus. Can. J. Biochem. Physiol. 1959, 37, 901–910. [Google Scholar] [CrossRef]
- Guo, D.; Liu, J.; Fan, Y.; Cheng, J.; Shi, Y.; Zou, J.; Zhang, X. Optimization, characterization and evaluation of liposomes from Malus hupehensis (Pamp.) Rehd. extracts. J. Liposome Res. 2020, 30, 366–376. [Google Scholar] [CrossRef]
- Wen, C.; Wang, D.; Li, X.; Huang, T.; Huang, C.; Hu, K. Targeted isolation and identification of bioactive compounds lowering cholesterol in the crude extracts of crabapples using UPLC-DAD-MS-SPE/NMR based on pharmacology-guided PLS-DA. J. Pharm. Biomed. Anal. 2018, 150, 144–151. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, H.; Yi, R.; Liao, X.; Li, J.; Li, H.; Tan, F.; Zhao, X. Malus hupehensis leaves extract attenuates obesity, inflammation, and dyslipidemia by modulating lipid metabolism and oxidative stress in high-fat diet-induced obese mice. J. Food Biochem. 2020, 44, e13484. [Google Scholar] [CrossRef]
- Fang, R.; Yang, Q.; Li, L.; Xiang, J.T.; Wang, Y.Z. Determination of phloridzin in Malus hupehensis. Food Sci. Technol. 2008, 6, 195–196. [Google Scholar]
- Liu, Q.; Zeng, H.; Jiang, S.; Zhang, L.; Yang, F.; Chen, X.; Yang, H. Separation of polyphenols from leaves of Malus hupehensis (Pamp.) Rehder by off-line two-dimensional high-speed counter-current chromatography combined with recycling elution mode. Food Chem. 2015, 186, 139–145. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Zhu, X.-F.; Wang, X.-N.; Shen, T.; Xiang, F.; Lou, H.-X. Flavonoids from Malus hupehensis and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Phytochemistry 2013, 87, 119–125. [Google Scholar] [CrossRef]
- Orville, A.M.; Lipscomb, J.D.; Ohlendorf, D.H. Crystal structures of substrate and substrate analog complexes of protocatechuate 3,4-dioxygenase: Endogenous Fe3+ ligand displacement in response to substrate binding. Biochemistry 1997, 36, 10052–10066. [Google Scholar] [CrossRef]
- Lv, Q.; Lin, Y.; Tan, Z.; Jiang, B.; Xu, L.; Ren, H.; Tai, W.C.; Chan, C.; Lee, C.; Gu, Z. Dihydrochalcone-derived polyphenols from tea crab apple (Malus hupehensis) and their inhibitory effects on α-glucosidase in vitro. Food Funct. 2019, 10, 2881–2887. [Google Scholar] [CrossRef]
- Cai, X.; Xiao, M.; Tang, J.; Huang, B.; Xue, H. Rapid enrichment and separation of two novel minor phenols from Malus hupehensis utilizing liquid–liquid extraction with three-phase solvent system and high-speed counter-current chromatography based on the polarity parameter. J. Sep. Sci. 2021, 44, 1843–1851. [Google Scholar] [CrossRef]
- Cai, X.; Xiao, M.; Zou, X.; Tang, J.; Huang, B.; Xue, H. Extraction and separation of flavonoids from Malus hupehensis using high-speed countercurrent chromatography based on deep eutectic solvent. J. Chromatogr. A 2021, 1641, 461998. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, Y.-Y.; Jiao, Q.-Y.; Khan, A.; Shan, J.; Cao, G.-D.; Li, F.; Zhang, C.; Lou, H.-X. Polyphenolic compounds from Malus hupehensis and their free radical scavenging effects. Nat. Prod. Res. 2018, 32, 2152–2158. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Zhang, J.; Li, H.; Zhou, Y.; Li, Y.; Zhao, X.; Liu, W. Evaluation of in vitro bio-activities effects of WST (Wushanshencha). Appl. Sci. 2019, 9, 1325. [Google Scholar] [CrossRef]
- Janas, K.M.; Posmyk, M.M. Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol. Plant. 2013, 35, 3285–3292. [Google Scholar] [CrossRef]
- Qin, X.; Xing, Y.F.; Zhou, Z.; Yao, Y. Dihydrochalcone compounds isolated from crabapple leaves showed anticancer effects on human cancer cell lines. Molecules 2015, 20, 21193–21203. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, X.; Liu, Q.; Chen, M.; Liao, S.; Zhu, F.; Shi, S.; Yang, H.; Chen, X. Rapid screening and identification of antioxidants in the leaves of Malus hupehensis using off-line two-dimensional HPLC–UV–MS/MS coupled with a 1, 1′-diphenyl-2-picrylhydrazyl assay. J. Sep. Sci. 2018, 41, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.Y.; Li, J.; Shi, Y.Q.; Wang, J.Z. Study on the flavon ingredients of Malus hupehensis. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2011, 34, 1026–1029. [Google Scholar]
- Carabajal, M.; Teglia, C.M.; Cerutti, S.; Culzoni, M.J.; Goicoechea, H.C. Applications of liquid-phase microextraction procedures to complex samples assisted by response surface methodology for optimization. Microchem. J. 2020, 152, 104436. [Google Scholar] [CrossRef]
- Myers, R.H.; Khuri, A.I.; Carter, W.H. Response surface methodology: 1966–l988. Technometrics 1989, 31, 137–157. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef]
- Tian, J.; Han, Z.; Zhang, J.; Hu, Y.; Song, T.; Yao, Y. The balance of expression of dihydroflavonol 4-reductase and flavonol synthase regulates flavonoid biosynthesis and red foliage coloration in crabapples. Sci. Rep. 2015, 5, 12228. [Google Scholar] [CrossRef]
- Li, P.; Xue, H.; Xiao, M.; Tang, J.; Yu, H.; Su, Y.; Cai, X. Ultrasonic-Assisted Aqueous Two-Phase Extraction and Properties of Water-Soluble Polysaccharides from Malus hupehensis. Molecules 2021, 26, 2213. [Google Scholar] [CrossRef]
- Li, X.; Luan, K.; Hu, J.-J.; Li, X.-F.; Xiang, S. Aroma volatile compound analysis of SPME headspace and extract samples from crabapple (Malus sp.) fruit using GC-MS. Agric. Sci. China 2008, 7, 1451–1457. [Google Scholar] [CrossRef]
- Wang, X.; Peng, F.; Li, M.; Yang, L.; Li, G. Expression of a heterologous SnRK1 in tomato increases carbon assimilation, nitrogen uptake and modifies fruit development. J. Plant Physiol. 2012, 169, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-J.; Zhao, F.; Yang, J.-X.; Yang, H.-Q. Nitric oxide alleviates lipid peroxidation induced by osmotic stress during senescence of detached leaves of Malus hupehensis Rehd. J. Hortic. Sci. Biotechnol. 2010, 85, 367–373. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Xie, Y.; Li, M.; Chen, W.; Zhang, S.; Liang, D.; Ma, F. Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J. Pineal Res. 2014, 57, 291–307. [Google Scholar] [CrossRef]
- Bai, X.; He, Y.; Quan, B.; Xia, T.; Zhang, X.; Wang, Y.; Zheng, Y.; Wang, M. Physicochemical properties, structure, and ameliorative effects of insoluble dietary fiber from tea on slow transit constipation. Food Chem. X 2022, 14, 100340. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, H.; Zhang, Y.; Li, F.; Gan, B.; Yu, Q.; Xie, J.; Chen, Y. Soluble dietary fiber from tea residues with inhibitory effects against acrylamide and 5-hydroxymethylfurfural formation in biscuits: The role of bound polyphenols. Food Res. Int. 2022, 159, 111595. [Google Scholar] [CrossRef]
- Liu, J.; Guo, D.; Fan, Y.; Sun, J.; Cheng, J.; Shi, Y. Experimental study on the antioxidant activity of Malus hupehensis (Pamp.) Rehd extracts in vitro and in vivo. J. Cell. Biochem. 2019, 120, 11878–11889. [Google Scholar] [CrossRef]
- Jin, K.-S.; Kwon, H.J.; Kim, B.W. Anti-Oxidative and Anti-Inflammatory Effects of Malus huphensis, Ophiorrhiza cantonensis, and Psychotria rubra Ethanol Extracts. Microbiol. Biotechnol. Lett. 2014, 42, 275–284. [Google Scholar] [CrossRef]
- Nithiya, T.; Udayakumar, R. In vitro antioxidant properties of phloretin—An important phytocompound. J. Biosci. Med. 2016, 4, 85. [Google Scholar]
- Oresajo, C.; Stephens, T.; Hino, P.D.; Law, R.M.; Yatskayer, M.; Foltis, P.; Pillai, S.; Pinnell, S.R. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J. Cosmet. Dermatol. 2008, 7, 290–297. [Google Scholar] [CrossRef]
- Hu, H.; Bai, X.; Xu, K.; Zhang, C.; Chen, L. Effect of phloretin on growth performance, serum biochemical parameters and antioxidant profile in heat-stressed broilers. Poult. Sci. 2021, 100, 101217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Q.; Han, L.; Pan, C.; Lei, C.; Chen, H.; Lan, X. C2C12 mouse myoblasts damage induced by oxidative stress is alleviated by the antioxidant capacity of the active substance phloretin. Front. Cell Dev. Biol. 2020, 8, 541260. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Sakurai, K.; Kawaii, S.; Soejima, J.; Murofushi, N. Antiproliferative and antioxidant properties of crabapple juices. Food Sci. Technol. Res. 2007, 10, 278–281. [Google Scholar] [CrossRef]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L.; Nabavi, S.M. Health effects of phloretin: From chemistry to medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Casarini, T.P.A.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S. Dermatological applications of the flavonoid phloretin. Eur. J. Pharmacol. 2020, 889, 173593. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Li, B.; Zhang, Z.; Yu, F.; Li, X.; Cai, Q.; Gao, H.; Shen, L. Beneficial effects of phlorizin on diabetic nephropathy in diabetic db/db mice. J. Diabetes Complicat. 2014, 28, 596–603. [Google Scholar] [CrossRef]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary intake of phloridzin from natural occurrence in foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, X.; Wang, Z.; Gao, Z.; Liu, G.; Hu, H.; Zou, L.; Li, X. Insulin sensitivity-enhancing activity of phlorizin is associated with lipopolysaccharide decrease and gut microbiota changes in obese and type 2 diabetes (db/db) mice. J. Agric. Food Chem. 2016, 64, 7502–7511. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Bian, Y.; Deng, G.-G.; Wang, Y.; Yan, H.-L.; Zhang, X.-L.; Huang, Y.-M.; Li, A.; Liao, X.-Y.; Feng, T.-Y. Effects of phloridzin on blood glucose and key enzyme G-6-Pase of gluconeogenesis in mice. J. Food Biochem. 2021, 45, e13956. [Google Scholar] [CrossRef]
- Xi, L.; Jian, J.; Zha, D.; Zhao, X.; Wang, J.; Li, J.; Jiang, Z.; Zhang, T. Establishment of high-resolution bioassay profiling platform to screen α-glucosidase inhibitors from Malus hupehensis. Acta Pharm. Sin. 2021, 12, 2419–2425. [Google Scholar]
- Kamdi, S.P.; Badwaik, H.R.; Raval, A.; Nakhate, K.T. Ameliorative potential of phloridzin in type 2 diabetes-induced memory deficits in rats. Eur. J. Pharmacol. 2021, 913, 174645. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin ameliorates type 2 diabetes-induced depression in mice by mitigating oxidative stress and modulating brain-derived neurotrophic factor. J. Diabetes Metab. Disord. 2021, 20, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, S.; Fu, H.; Shu, G.; Tang, H.; Zhao, X.; Chen, Y.; Huang, X.; Zhao, L.; Yin, L. Hypoglycemic and hypolipidemic activities of phlorizin from Lithocarpus polystachyus Rehd in diabetes rats. Food Sci. Nutr. 2021, 9, 1989–1996. [Google Scholar] [CrossRef]
- Liang, Z.H.; Liu, X.H.; Gong, S.M. Evaluation of anti-hyperglycemic activities of phloridzin in diabetic mice. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 209–218. [Google Scholar] [CrossRef]
- Cai, Q.; Li, B.; Yu, F.; Lu, W.; Zhang, Z.; Yin, M.; Gao, H. Investigation of the protective effects of phlorizin on diabetic cardiomyopathy in db/db mice by quantitative proteomics. J. Diabetes Res. 2013, 2013, 263845. [Google Scholar] [CrossRef]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef]
- Rossetti, L.; Shulman, G.I.; Zawalich, W.; DeFronzo, R.A. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J. Clin. Investig. 1987, 80, 1037–1044. [Google Scholar] [CrossRef]
- Dudash, J., Jr.; Zhang, X.; Zeck, R.E.; Johnson, S.G.; Cox, G.G.; Conway, B.R.; Rybczynski, P.J.; Demarest, K.T. Glycosylated dihydrochalcones as potent and selective sodium glucose co-transporter 2 (SGLT2) inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 5121–5125. [Google Scholar] [CrossRef]
- Malatiali, S.; Francis, I.; Barac-Nieto, M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp. Diabetes Res. 2008, 2008, 305403. [Google Scholar] [CrossRef]
- Seufert, J. SGLT2 inhibitors—An insulin-independent therapeutic approach for treatment of type 2 diabetes: Focus on canagliflozin. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 543. [Google Scholar] [CrossRef]
- Neumiller, J.J.; White, J.R.; Campbell, R.K. Sodium-glucose co-transport inhibitors. Drugs 2010, 70, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sultana, M.; Raina, R.; Pankaj, N.K.; Verma, P.K.; Prawez, S. Hypoglycemic, hypolipidemic, and wound healing potential of quercetin in streptozotocin-induced diabetic rats. Pharmacogn. Mag. 2017, 13, S633. [Google Scholar] [PubMed]
- Takeno, A.; Kanazawa, I.; Tanaka, K.; Notsu, M.; Sugimoto, T. Phloretin suppresses bone morphogenetic protein-2-induced osteoblastogenesis and mineralization via inhibition of phosphatidylinositol 3-kinases/Akt pathway. Int. J. Mol. Sci. 2019, 20, 2481. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ji, Y.; Guo, Y.; Wang, H.; Wu, Z.; Li, H.; Wang, H. Dietary supplementation of apple phlorizin attenuates the redox state related to gut microbiota homeostasis in c57bl/6j mice fed with a high-fat diet. J. Agric. Food Chem. 2020, 69, 198–211. [Google Scholar] [CrossRef]
- Lang, L.-J.; Wang, M.; Lei, C.; Shen, Y.; Zhu, Q.-J.; Diao, H.-M.; Chen, H.; Shen, L.; Dong, X.; Jiang, B. Phloridzin highly accumulated in Malus rockii Rehder and its structure revision and hypolipidemic activity. Planta Med. 2022, 88, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Z.; Riaz, S.; Shen, T.-T.; Fan, Z.-C.; Liu, D. Apple phlorizin reduce plasma cholesterol by down-regulating hepatic HMG-CoA reductase and enhancing the excretion of fecal sterols. J. Funct. Foods 2019, 62, 103548. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, S.-J.; Jung, U.J.; Ryu, R.; Choi, M.-S. Phlorizin supplementation attenuates obesity, inflammation, and hyperglycemia in diet-induced obese mice fed a high-fat diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef]
- Alsanea, S.; Gao, M.; Liu, D. Phloretin prevents high-fat diet-induced obesity and improves metabolic homeostasis. AAPS J. 2017, 19, 797–805. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Broncel, M.; Podsędek, A.; Koter-Michalak, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2012, 51, 435–443. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, C.; Xing, M.; Li, Y.; Zhu, L.; Wang, D.; Yang, X.; Liu, L.; Yao, P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem. Toxicol. 2012, 50, 1194–1200. [Google Scholar] [CrossRef]
- Padma, V.V.; Lalitha, G.; Shirony, N.P.; Baskaran, R. Effect of quercetin against lindane induced alterations in the serum and hepatic tissue lipids in wistar rats. Asian Pac. J. Trop. Biomed. 2012, 2, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Kang, M.-J.; Choi, H.-N.; Kim, J.-H.; Kim, J.-I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, Y.; Yang, J.; Suo, Y.; Xu, H.; Liu, P.; Wang, J.; Deng, G.; Feng, T. Hepatoprotective effects of Malus hupehensis tea against isoniazid-and rifampicin-induced liver injury by regulating cytochrome P450 in mice. J. Funct. Foods 2021, 84, 104580. [Google Scholar] [CrossRef]
- Sha, J.; Song, J.; Yu, M.; Zhao, X.; Wang, H.; Zhang, Y.; Suo, H. Polyphenolic extracts from Wushan tea leaves attenuate hepatic injury in CCl4-treated mice. J. Funct. Foods 2020, 66, 103826. [Google Scholar] [CrossRef]
- Zuo, A.-R.; Yu, Y.-Y.; Shu, Q.-L.; Zheng, L.-X.; Wang, X.-M.; Peng, S.-H.; Xie, Y.-F.; Cao, S.-W. Hepatoprotective effects and antioxidant, antityrosinase activities of phloretin and phloretin isonicotinyl hydrazone. J. Chin. Med. Assoc. 2014, 77, 290–301. [Google Scholar] [CrossRef]
- Deng, G.; Wang, J.; Zhang, Q.; He, H.; Wu, F.; Feng, T.; Zhou, J.; Zou, K.; Hattori, M. Hepatoprotective effects of phloridzin on hepatic fibrosis induced by carbon tetrachloride against oxidative stress-triggered damage and fibrosis in rats. Biol. Pharm. Bull. 2012, 35, 1118–1125. [Google Scholar] [CrossRef]
- Boccia, M.M.; Kopf, S.R.; Baratti, C.M. Phlorizin, a competitive inhibitor of glucose transport, facilitates memory storage in mice. Neurobiol. Learn. Mem. 1999, 71, 104–112. [Google Scholar] [CrossRef]
| Number | Chemical Compound | Structure | Reference |
|---|---|---|---|
| MH1 | Phlorizin | 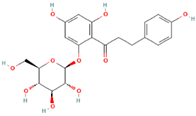 | [12] |
| MH2 | Phloretin | 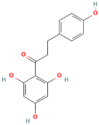 | [12] |
| MH3 | Avicularin |  | [12] |
| MH4 | Kaempferol-3-O-β-D glucoside | 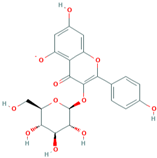 | [12] |
| MH5 | Retinoin | 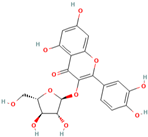 | [12] |
| MH6 | Quercetin | 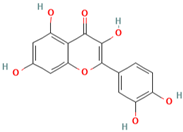 | [13] |
| MH7 | Acacetin | 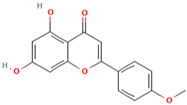 | [13] |
| MH8 | Chrysin |  | [13] |
| MH9 | 5,7-dihydroxychromone-7-O-β-d-glucoside | 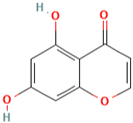 | [13] |
| MH10 | Quercetin-3-O-β-d-glucopyranoside | 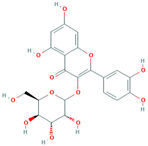 | [13] |
| MH11 | Luteolin-5-O-β-d-glucopyranoside | NO | [13] |
| MH12 | Phloretin-2′,4′-di-O-β-d-glucopyranoside | NO | [13] |
| MH13 | Epipinoresinol-4-β-d-glucoside | NO | [13] |
| MH14 | Protocatechuic acid |  | [14] |
| MH15 | 3-hydroxyphloridzin |  | [15] |
| MH16 | Polyphenol |  | [15] |
| MH17 | 3-O-coumaroylquinic acid | 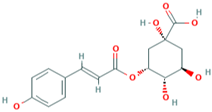 | [15] |
| MH18 | β-hydroxypropiovanillone | 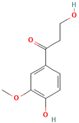 | [15] |
| MH19 | Huperolides A | NO | [15] |
| MH20 | Huperolides B | NO | [15] |
| MH21 | Huperolides C | NO | [15] |
| MH22 | 6″-O-coumaroyl-2′-O-glucopyranosylphloretin | NO | [16] |
| MH23 | 3‴-methoxy-6″-O-feruloy-2′-glucopyranosylphloretin | NO | [17] |
| MH24 | 5-O-β-d-glucopyranoside-4-chromanone | NO | [18] |
| MH25 | Quercitrin | 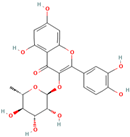 | [19] |
| MH26 | Isoquercitrin |  | [19] |
| MH27 | Chlorogenic acid | 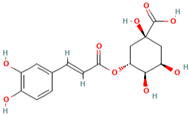 | [19] |
| MH28 | Neosperidin Dihydrochalcone |  | [19] |
| MH29 | 4-hydroxycinnamic acid |  | [19] |
| MH30 | Taxifolin | 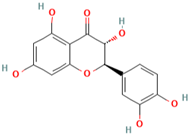 | [19] |
| MH31 | Rosmarinic acid |  | [19] |
| MH32 | Myricetin | 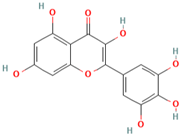 | [19] |
| MH33 | Baicalin | 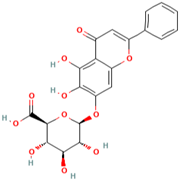 | [19] |
| MH34 | Melatonin | 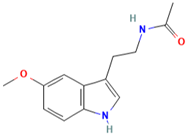 | [20] |
| MH35 | Trilobatin |  | [21] |
| MH36 | 6″-O-coumaroyl-4′-O-glucopyranosylphloretin | NO | [21] |
| MH37 | 3‴-methoxy-6″-O-feruloy-4′-O-glucopyranosyl-phloretin | NO | [21] |
| MH38 | Phloretin rutinoside | NO | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Tan, J.; Xiao, M.; Cai, X.; Xue, H.; Yu, H. Bioactive Substances and Biological Functions in Malus hupehensis: A Review. Molecules 2023, 28, 658. https://doi.org/10.3390/molecules28020658
Li P, Tan J, Xiao M, Cai X, Xue H, Yu H. Bioactive Substances and Biological Functions in Malus hupehensis: A Review. Molecules. 2023; 28(2):658. https://doi.org/10.3390/molecules28020658
Chicago/Turabian StyleLi, Pengcheng, Jiaqi Tan, Mi Xiao, Xu Cai, Hongkun Xue, and Hansong Yu. 2023. "Bioactive Substances and Biological Functions in Malus hupehensis: A Review" Molecules 28, no. 2: 658. https://doi.org/10.3390/molecules28020658
APA StyleLi, P., Tan, J., Xiao, M., Cai, X., Xue, H., & Yu, H. (2023). Bioactive Substances and Biological Functions in Malus hupehensis: A Review. Molecules, 28(2), 658. https://doi.org/10.3390/molecules28020658






