UPLC-Q-TOF/MS Based Plasma Metabolomics for Identification of Paeonol’s Metabolic Target in Endometriosis
Abstract
1. Introduction
2. Results
2.1. Morphological Observations and Histopathological Observations
2.2. Changes in Plasma Estradiol and Progesterone Levels
2.3. Evidence of Cold Clotting and Blood Stagnation in Rats
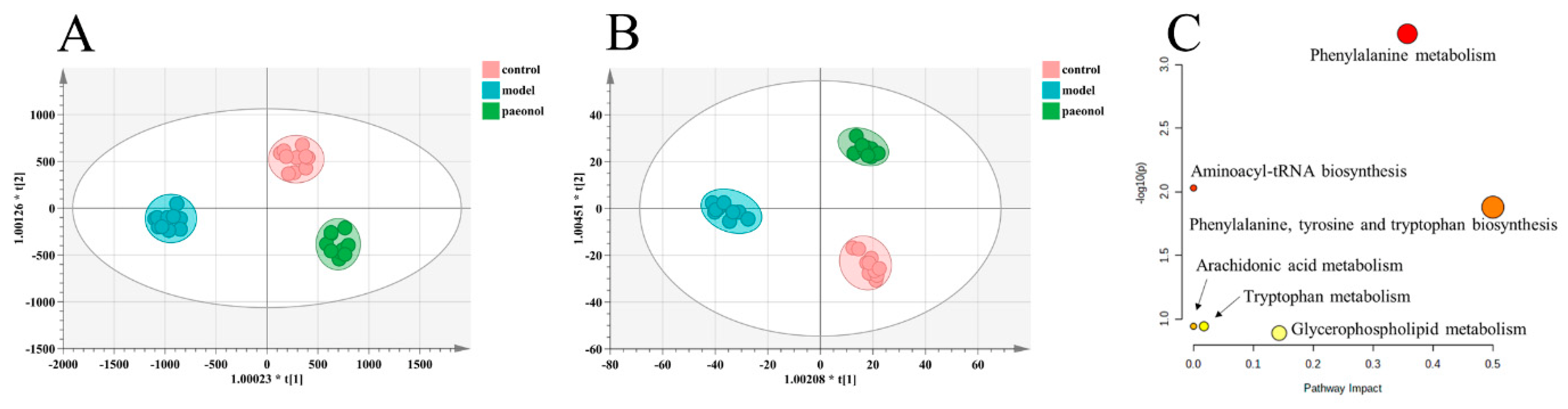
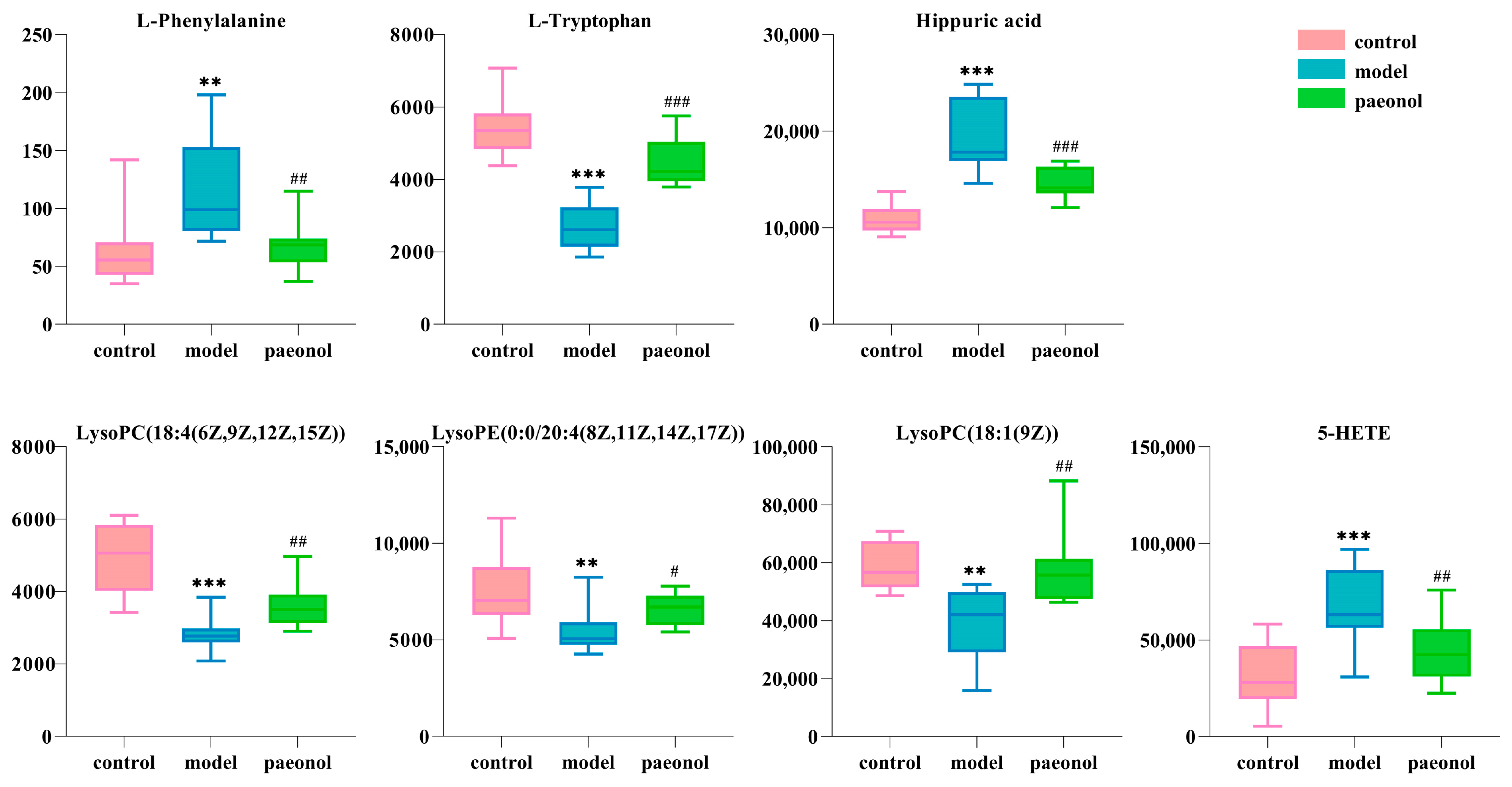
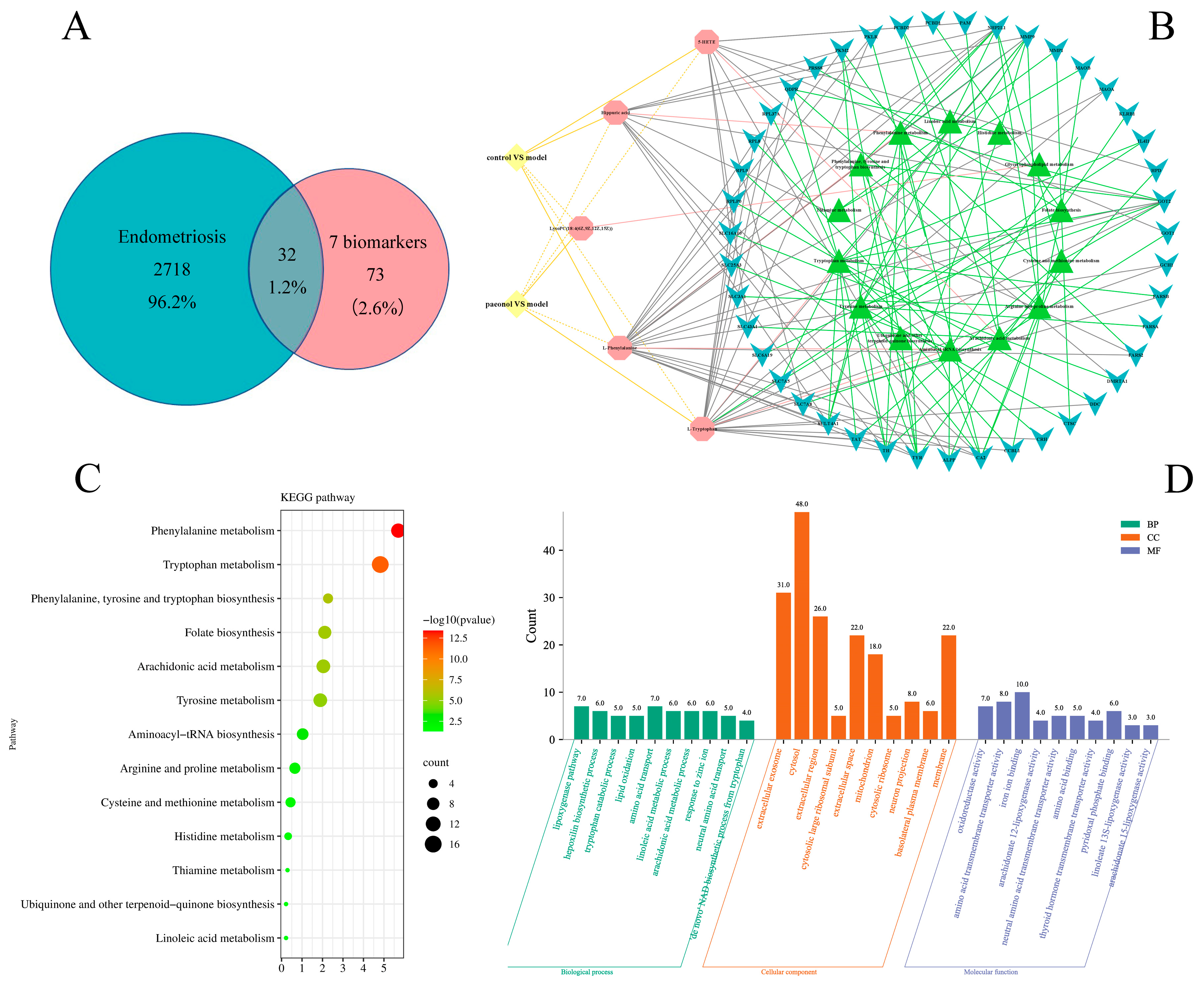
2.4. Effects of Paeonol on Potential Plasma Biomarkers in Endometriosis Rats
2.5. The Therapeutic Targets of Paeonol on Endometriosis and Related Network Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Groups
4.3. Preparation of Animal Models and Paeonol Solution
4.4. Calculation of the Volume of the Ectopic Tissue
4.5. Hematoxylin-Eosin Staining (H&E Staining)
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Preparation and Analysis of Plasma Samples
4.8. Chromatography and Mass Spectrometry Conditions
4.8.1. Chromatographic Conditions
4.8.2. Mass Spectrometry Conditions
4.9. Metabolomics Analysis
4.10. Construction and Analysis of Hey Biomarker Biological Networks
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mori, T.; Ito, F.; Koshiba, A.; Kataoka, H.; Takaoka, O.; Okimura, H.; Khan, K.N.; Kitawaki, J. Local estrogen formation and its regulation in endometriosis. Reprod. Med. Biol. 2019, 18, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.J.; Lavender, M.; Marsh, E.E.; et al. Menstruation: Science and society. Am. J. Obstet. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Ke, D.S.; Lin, C.L.; Kao, C.H. To Investigate the Risk of Herpes Zoster in Women With Endometriosis: A Taiwan National Population-Based Cohort Study. Front. Med. 2021, 8, 584322. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Szyllo, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Maddern, J.; Grundy, L.; Castro, J.; Brierley, S.M. Pain in Endometriosis. Front. Cell Neurosci. 2020, 14, 590823. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef]
- Ferrero, S.; Barra, F.; Leone Roberti Maggiore, U. Current and Emerging Therapeutics for the Management of Endometriosis. Drugs 2018, 78, 995–1012. [Google Scholar] [CrossRef]
- Mechsner, S. Endometriosis, an Ongoing Pain-Step-by-Step Treatment. J. Clin. Med. 2022, 11, 467. [Google Scholar] [CrossRef]
- Nabi, M.Y.; Nauhria, S.; Reel, M.; Londono, S.; Vasireddi, A.; Elmiry, M.; Ramdass, P. Endometriosis and irritable bowel syndrome: A systematic review and meta-analyses. Front. Med. 2022, 9, 914356. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, Y.; Lin, H.; Dai, Y.; Yao, S. Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J. Neuroinflamm. 2020, 17, 80. [Google Scholar] [CrossRef]
- Lee, N.; Min, S.; Won, S.; Cho, Y.J.; Kim, M.; Kim, M.K.; Jung, Y.W.; Yun, B.S.; Seong, S.J.; Kim, M.L. The recurrence rate of ovarian endometrioma in women aged 40-49 years and impact of hormonal treatment after conservative surgery. Sci. Rep. 2020, 10, 16461. [Google Scholar] [CrossRef]
- Orlando, M.S.; Cadish, L.A.; Shepherd, J.P.; Falcone, T.; Chang, O.H.; Kho, R.M. Salpingo-oophorectomy or surveillance for ovarian endometrioma in asymptomatic premenopausal women: A cost-effectiveness analysis. Am. J. Obstet. Gynecol. 2022, 227, 311.e1–311.e7. [Google Scholar] [CrossRef]
- Pynna, K.; Rasanen, P.; Roine, R.P.; Vuorela, P.; Sintonen, H. Where does the money go to? Cost analysis of gynecological patients with a benign condition. PLoS ONE 2021, 16, e0254124. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zeng, W.; Zhong, Y.; Xie, D.; Zhu, W.; Chen, F.; Du, J.; Zhang, T. Establishment of DNA Methylation Profile Associated with TCM Syndrome in Endometriosis. Evid. Based Complement. Altern. Med. 2022, 2022, 4866360. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Y.; Xie, N.; Wang, H.; Wang, F.; Zhou, J.; Qu, F. A network pharmacology approach to explore active compounds and pharmacological mechanisms of a patented Chinese herbal medicine in the treatment of endometriosis. PLoS ONE 2022, 17, e0263614. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, A.; Wu, F.; Wang, X. UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis. Molecules 2022, 27, 3208. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, C.Y.; Shiu, L.Y.; Yu, Y.C.; Lai, J.C.; Chang, C.C.; Fu, C.F.; Huang, S.J. Combinatory effects of current regimens and Guizhi Fuling Wan on the development of endometriosis. Taiwan J. Obstet. Gynecol. 2022, 61, 70–74. [Google Scholar] [CrossRef]
- Wu, M.; Yu, Z.; Li, X.; Zhang, X.; Wang, S.; Yang, S.; Hu, L.; Liu, L. Paeonol for the Treatment of Atherosclerotic Cardiovascular Disease: A Pharmacological and Mechanistic Overview. Front. Cardiovasc. Med. 2021, 8, 690116. [Google Scholar] [CrossRef]
- Yu, W.; Ilyas, I.; Aktar, N.; Xu, S. A review on therapeutical potential of paeonol in atherosclerosis. Front. Pharmacol. 2022, 13, 950337. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zheng, X.; Fan, Z.; Zhou, H.; Zhu, X.; Wang, G.; Liu, Z. Paeonol alleviates primary dysmenorrhea in mice via activating CB2R in the uterus. Phytomedicine 2020, 68, 153151. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Wu, D.; Liu, X.; Xuan, Z.; Han, L.; Fang, C.; Xiao, W. Effects of different extracts of Paeonia lactiflora Pall on hemorheology and cytokines in endometriosis model rats. J. Chin. Med. Mater. 2012. [Google Scholar] [CrossRef]
- Xiuhong, W.; Fang, H.; Chengyu, P.; Dongxia, Y.; Fashan, W.; Luwen, H.; Zhang, N. High Performance Liquid Chromatography Fingerprint of Guizhi Fuling Pill. Fen Hsi K’O Hsueh Hsueh Pao 2014, 30, 100–102. [Google Scholar] [CrossRef]
- Liu, L.; Guan, L.L.; Chen, Y.; Ji, N.; Li, H.M.; Li, Z.H.; Qian, Q.J.; Yang, L.; Glatt, S.J.; Faraone, S.V.; et al. Association analyses of MAOA in Chinese Han subjects with attention-deficit/hyperactivity disorder: Family-based association test, case-control study, and quantitative traits of impulsivity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156B, 737–748. [Google Scholar] [CrossRef]
- Louca, P.; Mompeo, O.; Leeming, E.R.; Berry, S.E.; Mangino, M.; Spector, T.D.; Padmanabhan, S.; Menni, C. Dietary Influence on Systolic and Diastolic Blood Pressure in the TwinsUK Cohort. Nutrients 2020, 12, 2130. [Google Scholar] [CrossRef]
- Feinstein, D.L.; Heneka, M.T.; Gavrilyuk, V.; Dello Russo, C.; Weinberg, G.; Galea, E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 2002, 41, 357–365. [Google Scholar] [CrossRef]
- Jia, S.; Chen, G.; Liang, Y.; Liang, X.; Meng, C. GCH1-regulated miRNAs are potential targets for microglial activation in neuropathic pain. Biosci. Rep. 2021, 41, BSR20210051. [Google Scholar] [CrossRef]
- Sarkar, S.; Murphy, M.A.; Dammer, E.B.; Olsen, A.L.; Rangaraju, S.; Fraenkel, E.; Feany, M.B. Comparative proteomic analysis highlights metabolic dysfunction in alpha-synucleinopathy. NPJ Park. Dis. 2020, 6, 40. [Google Scholar] [CrossRef]
- Brandes, R.P. Stay in Shape with BH4: Loss of Endothelial Tetrahydrobiopterin Promotes Aortic Aneurysm Development in Mice. Hypertension 2018, 72, 61–62. [Google Scholar] [CrossRef]
- Strasser, B.; Sperner-Unterweger, B.; Fuchs, D.; Gostner, J.M. Mechanisms of Inflammation-Associated Depression: Immune Influences on Tryptophan and Phenylalanine Metabolisms. Curr. Top Behav. Neurosci. 2017, 31, 95–115. [Google Scholar] [CrossRef]
- Harada, T.; Kagamiyama, H.; Hatakeyama, K. Feedback regulation mechanisms for the control of GTP cyclohydrolase I activity. Science 1993, 260, 1507–1510. [Google Scholar] [CrossRef]
- Wei, J.L.; Wu, S.Y.; Yang, Y.S.; Xiao, Y.; Jin, X.; Xu, X.E.; Hu, X.; Li, D.Q.; Jiang, Y.Z.; Shao, Z.M. GCH1 induces immunosuppression through metabolic reprogramming and IDO1 upregulation in triple-negative breast cancer. J. Immunother. Cancer 2021, 9, e002383. [Google Scholar] [CrossRef]
- Fanet, H.; Capuron, L.; Castanon, N.; Calon, F.; Vancassel, S. Tetrahydrobioterin (BH4) Pathway: From Metabolism to Neuropsychiatry. Curr. Neuropharmacol. 2021, 19, 591–609. [Google Scholar] [CrossRef]
- Lin, S.; Liu, H.; Kanawati, B.; Liu, L.; Dong, J.; Li, M.; Huang, J.; Schmitt-Kopplin, P.; Cai, Z. Hippocampal metabolomics using ultrahigh-resolution mass spectrometry reveals neuroinflammation from Alzheimer’s disease in CRND8 mice. Anal. Bioanal. Chem. 2013, 405, 5105–5117. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, S.; Kong, D.; Wang, H.; Shi, Y.; Wang, Z.; Zheng, H. Integrative multi-omics analysis of identified ferroptosis-marker RPL8 as a candidate oncogene correlates with poor prognosis and immune infiltration in liver cancer. Comb. Chem. High Throughput Screen 2022. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Yamashita, S.; Hashimoto, K.; Sawada, I.; Ogawa, M.; Nakatsuka, E.; Kawano, M.; Kinose, Y.; Kodama, M.; Sawada, K.; Kimura, T. Endometrial galectin-3 causes endometriosis by supporting eutopic endometrial cell survival and engraftment in the peritoneal cavity. Am. J. Reprod. Immunol. 2022, 87, e13533. [Google Scholar] [CrossRef]
- Defrere, S.; Lousse, J.C.; Gonzalez-Ramos, R.; Colette, S.; Donnez, J.; Van Langendonckt, A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol. Hum. Reprod. 2008, 14, 377–385. [Google Scholar] [CrossRef]
- Li, G.; Lin, Y.; Zhang, Y.; Gu, N.; Yang, B.; Shan, S.; Liu, N.; Ouyang, J.; Yang, Y.; Sun, F.; et al. Endometrial stromal cell ferroptosis promotes angiogenesis in endometriosis. Cell Death Discov. 2022, 8, 29. [Google Scholar] [CrossRef]
- Cho, M.K.; Jin, L.; Han, J.H.; Jin, J.S.; Cheon, S.Y.; Shin, S.; Bae, S.J.; Park, J.K.; Ha, K.T. Water-Extracted Prunella vulgaris Alleviates Endometriosis by Reducing Aerobic Glycolysis. Front. Pharmacol. 2022, 13, 872810. [Google Scholar] [CrossRef] [PubMed]
- Illera, M.J.; Juan, L.; Stewart, C.L.; Cullinan, E.; Ruman, J.; Lessey, B.A. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil. Steril. 2000, 74, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Noordin, L.; San, G.T.; Singh, H.J.; Othman, M.S.; Hafizah, W. Pyruvate reduces in vitro the embryotoxic effect of peritoneal fluid from infertile women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Loo, J.M.; Mital, R.; Weinberg, E.M.; Man, F.Y.; Zeng, Z.; Paty, P.B.; Saltz, L.; Janjigian, Y.Y.; de Stanchina, E.; et al. PKLR promotes colorectal cancer liver colonization through induction of glutathione synthesis. J. Clin. Investig. 2016, 126, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Tosti, C.; Pinzauti, S.; Santulli, P.; Chapron, C.; Petraglia, F. Pathogenetic Mechanisms of Deep Infiltrating Endometriosis. Reprod. Sci. 2015, 22, 1053–1059. [Google Scholar] [CrossRef]
- Letsiou, S.; Peterse, D.P.; Fassbender, A.; Hendriks, M.M.; van den Broek, N.J.; Berger, R.; Dorien, F.O.; Vanhie, A.; Vodolazkaia, A.; Van Langendonckt, A.; et al. Endometriosis is associated with aberrant metabolite profiles in plasma. Fertil. Steril. 2017, 107, 699–706.e6. [Google Scholar] [CrossRef]
- Li, M.; Haixia, Y.; Kang, M.; An, P.; Wu, X.; Dang, H.; Xu, X. The Arachidonic Acid Metabolism Mechanism Based on UPLC-MS/MS Metabolomics in Recurrent Spontaneous Abortion Rats. Front. Endocrinol. 2021, 12, 652807. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Alammari, A.H.; Shoieb, S.M.; Maayah, Z.H.; El-Kadi, A.O.S. Fluconazole Represses Cytochrome P450 1B1 and Its Associated Arachidonic Acid Metabolites in the Heart and Protects Against Angiotensin II-Induced Cardiac Hypertrophy. J. Pharm. Sci. 2020, 109, 2321–2335. [Google Scholar] [CrossRef]
- Reis, F.M.; Reis, A.M. Angiotensin-converting enzyme 2 (ACE2), angiotensin-(1-7) and Mas receptor in gonadal and reproductive functions. Clin. Sci. 2020, 134, 2929–2941. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, D.; Li, D.; Ding, H.; Zhang, T.; Xu, T.; Zhang, Y. Luteolin inhibits angiotensin II-induced human umbilical vein endothelial cell proliferation and migration through downregulation of Src and Akt phosphorylation. Circ. J. 2013, 77, 772–779. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.C.; Liu, L.F. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 2019, 72, 413–421. [Google Scholar] [CrossRef]
- Zhu, X.L.; Chen, J.J.; Han, F.; Pan, C.; Zhuang, T.T.; Cai, Y.F.; Lu, Y.P. Novel antidepressant effects of Paeonol alleviate neuronal injury with concomitant alterations in BDNF, Rac1 and RhoA levels in chronic unpredictable mild stress rats. Psychopharmacology 2018, 235, 2177–2191. [Google Scholar] [CrossRef]
- Hu, X.; Ding, L.; Cao, S.; Cheng, L.; Wang, K.; Guang, C.; Li, W.; Koike, K.; Qiu, F. Pharmacokinetics, Tissue Distribution and Excretion of Paeonol and Its Major Metabolites in Rats Provide a Further Insight Into Paeonol Effectiveness. Front. Pharmacol. 2020, 11, 190. [Google Scholar] [CrossRef]
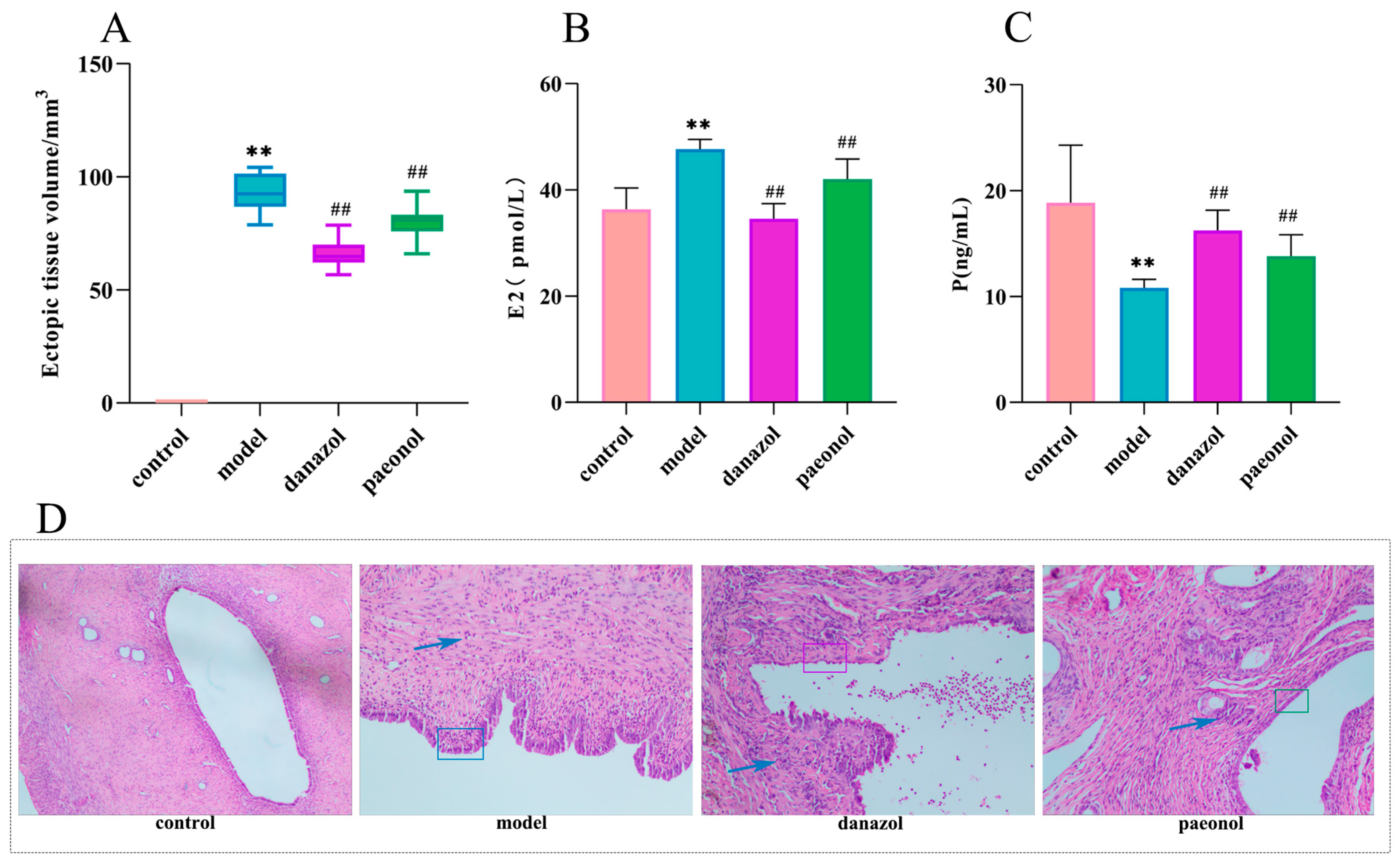
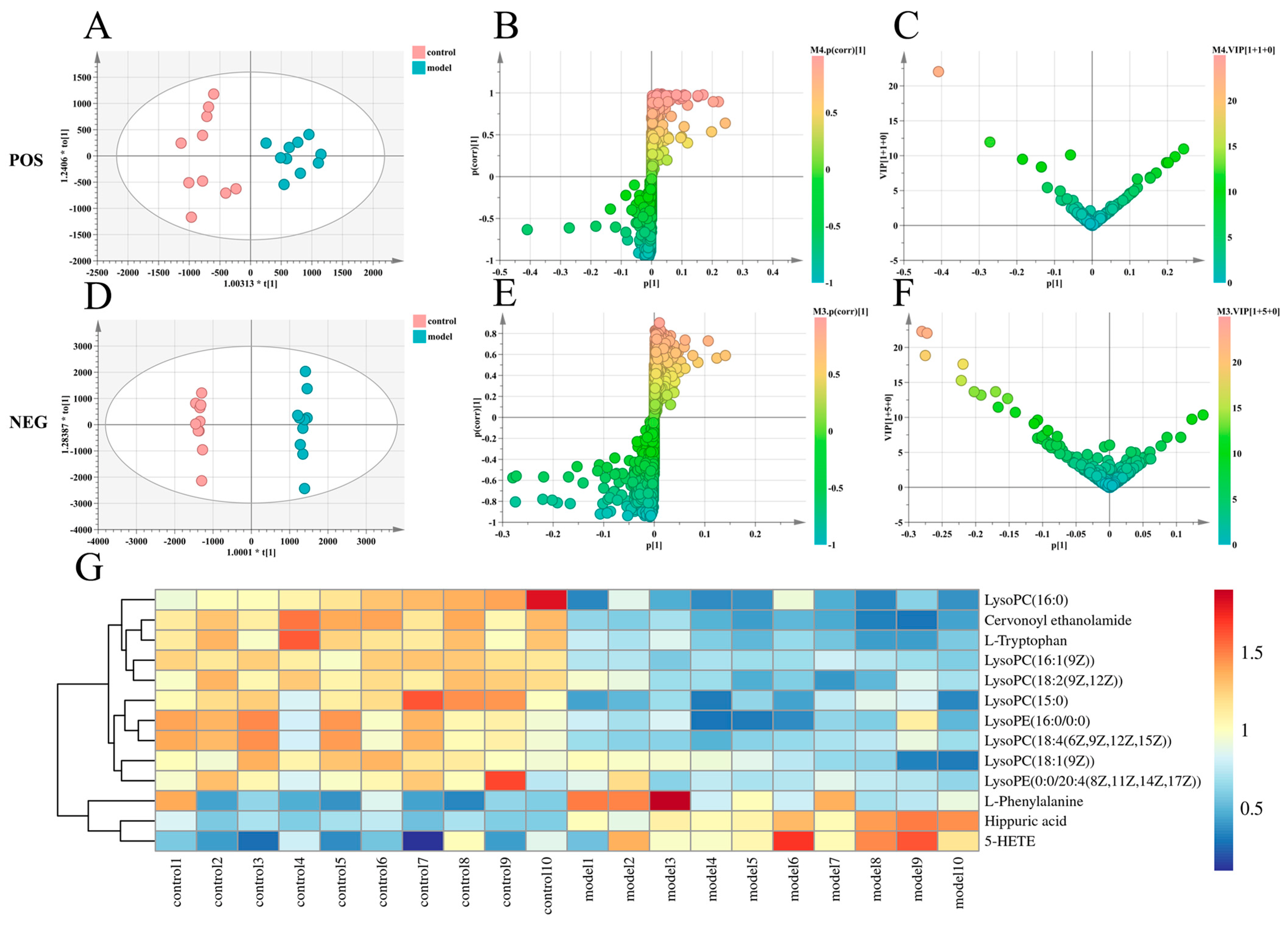

| Group | Ectopic Tissue Volume/mm3 | p (ng/mL) | E2 (p·mol/L) |
|---|---|---|---|
| Control group | 0.00 ± 0.00 | 18.86 ± 5.43 | 36.36 ± 4.02 |
| Model group | 92.87 ± 8.54 ** | 10.80 ± 0.83 ** | 47.68 ± 1.84 ** |
| Danazol group | 66.08 ± 6.36 ## | 16.25 ± 1.91 ## | 34.57 ± 2.85 ## |
| Paeonol group | 80.07 ± 7.12 ## | 13.80 ± 2.04 ## | 42.03 ± 3.78 ## |
| No. | Rt min | Determined Mass | Calc Mass | [M−H]−/[M+H]+ | ppm | Proposed Composition | Postulated Identity | HMDB Number | Trend in Model | Paeonol |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.36 | 166.0869 | 166.0868 | [M+H]+ | 0.6 | C9H11NO2 | L-Phenylalanine | HMDB0000159 | ↑ ** | ↓ ## |
| 2 | 1.73 | 203.0827 | 203.0821 | [M−H]− | 3.0 | C11H12N2O2 | L-Tryptophan | HMDB0000929 | ↓ *** | ↑ ### |
| 3 | 2.20 | 178.0504 | 178.0504 | [M−H]− | 0.0 | C9H9NO3 | Hippuric acid | HMDB0000714 | ↑ *** | ↓ ### |
| 4 | 4.79 | 514.2930 | 514.2934 | [M−H]− | −1.0 | C26H46NO7P | LysoPC(18:4(6Z,9Z,12Z,15Z)) | HMDB0010389 | ↓ *** | ↑ ## |
| 5 | 7.71 | 500.2790 | 500.2777 | [M−H]− | 2.4 | C25H44NO7P | LysoPE(0:0/20:4(8Z,11Z,14Z,17Z)) | HMDB0011488 | ↓ ** | ↑ # |
| 6 | 8.64 | 522.3563 | 522.3560 | [M+H]+ | 0.6 | C26H52NO7P | LysoPC(18:1(9Z)) | HMDB0002815 | ↓ ** | ↑ ## |
| 7 | 8.99 | 319.2278 | 319.2273 | [M−H]− | 1.6 | C20H32O3 | 5-HETE | HMDB0011134 | ↑ *** | ↓ ## |
| Pathway | Total | Hits | Raw p | FDR | Impact |
|---|---|---|---|---|---|
| Phenylalanine metabolism | 12 | 2 | 0.000572 | 0.04808 | 0.35714 |
| Aminoacyl-tRNA biosynthesis | 48 | 2 | 0.009322 | 0.36963 | 0.00000 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 4 | 1 | 0.013201 | 0.36963 | 0.50000 |
| Arachidonic acid metabolism | 36 | 1 | 0.113870 | 1.00000 | 0.00000 |
| Glycerophospholipid metabolism | 36 | 1 | 0.113870 | 1.00000 | 0.01736 |
| Tryptophan metabolism | 41 | 1 | 0.128830 | 1.00000 | 0.14305 |
| Pathway | PVal.Z | p Value | Hits |
|---|---|---|---|
| Phenylalanine metabolism | 5.700 | 4.08 × 10−14 | 11 |
| Tryptophan metabolism | 4.820 | 3.50 × 10−12 | 16 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 2.270 | 1.52 × 10−6 | 5 |
| Folate biosynthesis | 2.110 | 3.31 × 10−6 | 9 |
| Arachidonic acid metabolism | 2.040 | 4.73 × 10−6 | 10 |
| Tyrosine metabolism | 1.890 | 1.01 × 10−5 | 10 |
| Aminoacyl-tRNA biosynthesis | 1.030 | 0.000794 | 7 |
| Arginine and proline metabolism | 0.649 | 0.005540 | 6 |
| Cysteine and methionine metabolism | 0.437 | 0.016300 | 5 |
| Histidine metabolism | 0.321 | 0.029300 | 3 |
| Thiamine metabolism | 0.290 | 0.034400 | 2 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 0.218 | 0.049400 | 2 |
| Linoleic acid metabolism | 0.218 | 0.049400 | 2 |
| Paeonol | Target (pdb Code) | Combination of Free Energy |
| GCH1 (6Z89) | −5.300 kcal/mol | |
| RPL8 (4CCM) | −6.101 kcal/mol | |
| PKLR (7QDN) | −7.397 kcal/mol | |
| MAOA (2Z5Y) | −8.013 kcal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yang, D.; Piao, C.; Wang, X.; Sun, X.; Li, Y.; Zhang, S.; Wu, X. UPLC-Q-TOF/MS Based Plasma Metabolomics for Identification of Paeonol’s Metabolic Target in Endometriosis. Molecules 2023, 28, 653. https://doi.org/10.3390/molecules28020653
Liu J, Yang D, Piao C, Wang X, Sun X, Li Y, Zhang S, Wu X. UPLC-Q-TOF/MS Based Plasma Metabolomics for Identification of Paeonol’s Metabolic Target in Endometriosis. Molecules. 2023; 28(2):653. https://doi.org/10.3390/molecules28020653
Chicago/Turabian StyleLiu, Jing, Dongxia Yang, Chengyu Piao, Xu Wang, Xiaolan Sun, Yongyan Li, Shuxiang Zhang, and Xiuhong Wu. 2023. "UPLC-Q-TOF/MS Based Plasma Metabolomics for Identification of Paeonol’s Metabolic Target in Endometriosis" Molecules 28, no. 2: 653. https://doi.org/10.3390/molecules28020653
APA StyleLiu, J., Yang, D., Piao, C., Wang, X., Sun, X., Li, Y., Zhang, S., & Wu, X. (2023). UPLC-Q-TOF/MS Based Plasma Metabolomics for Identification of Paeonol’s Metabolic Target in Endometriosis. Molecules, 28(2), 653. https://doi.org/10.3390/molecules28020653





