Effects of Losartan, Atorvastatin, and Aspirin on Blood Pressure and Gut Microbiota in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Results
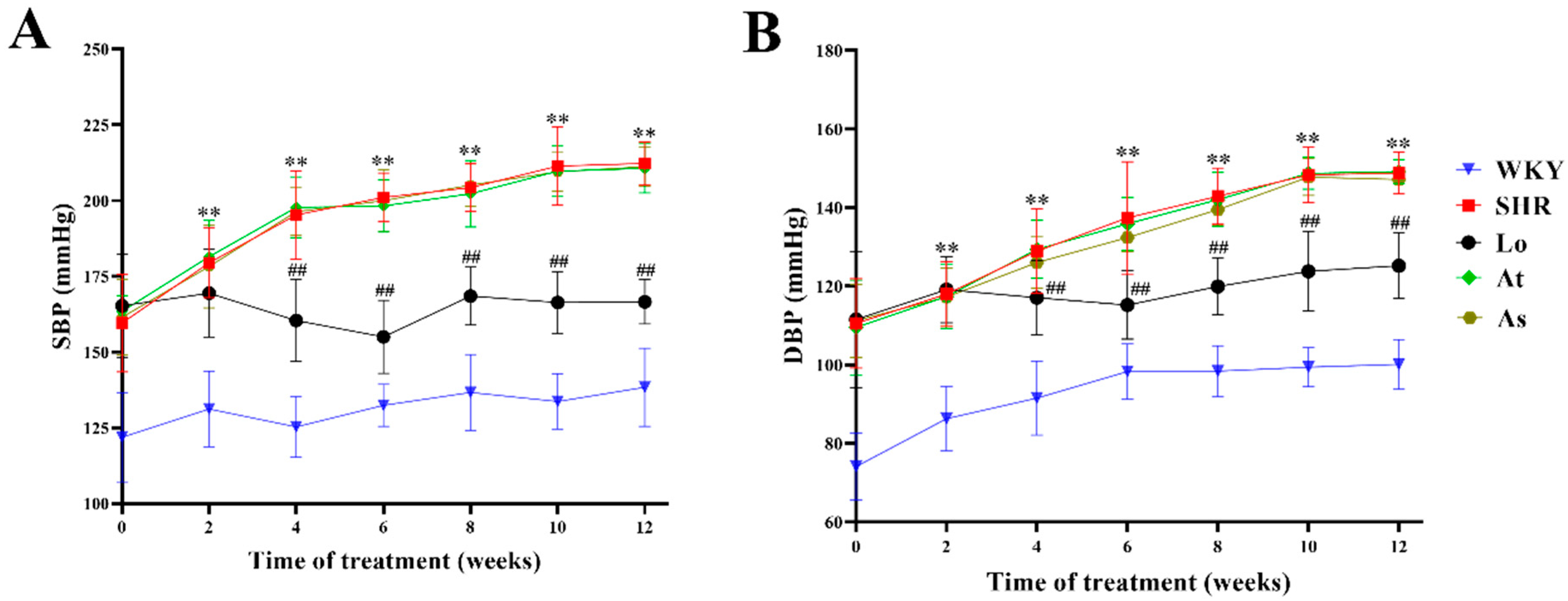
2.1. Blood Pressure
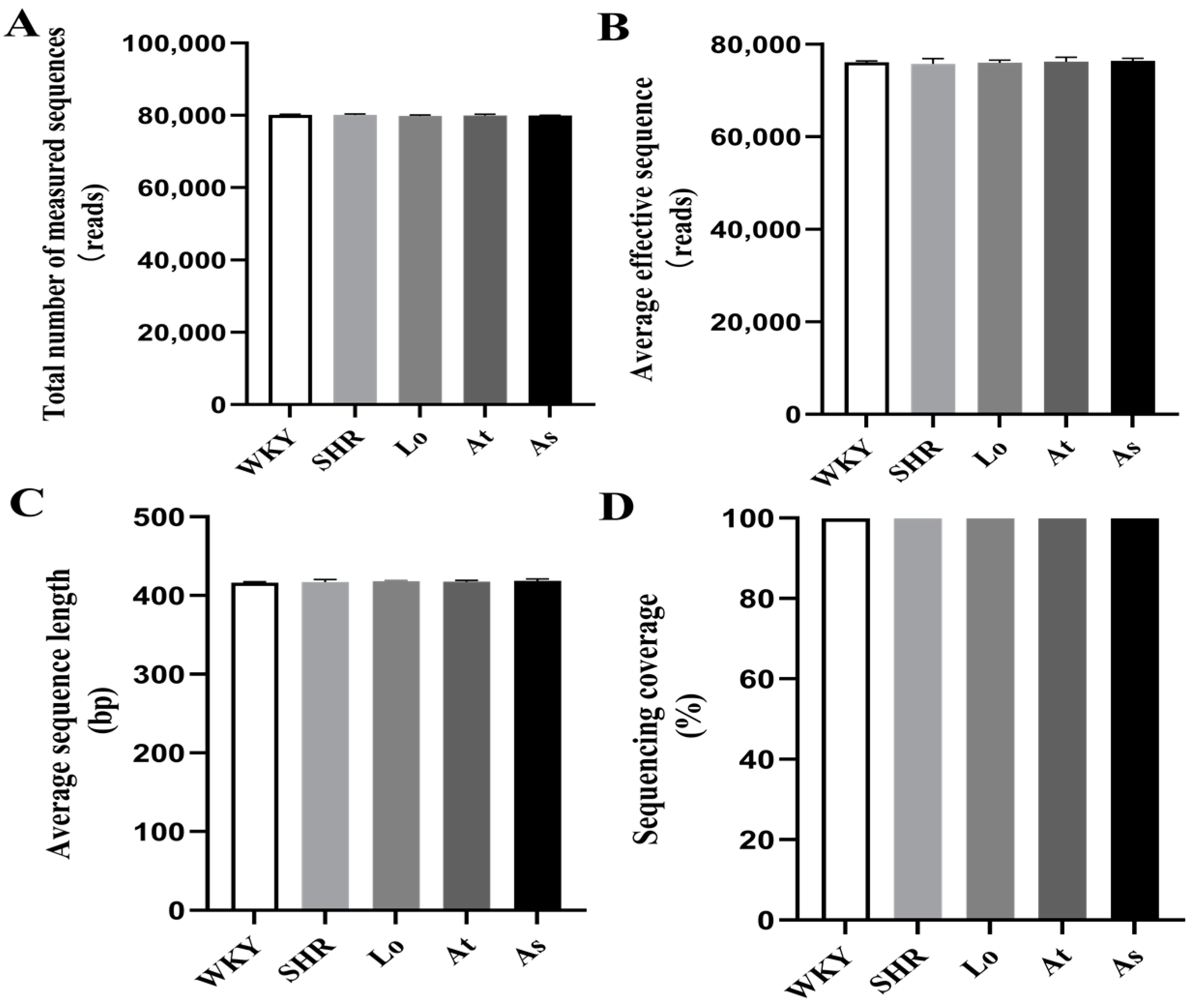
2.2. Quality Control of Sequencing Data
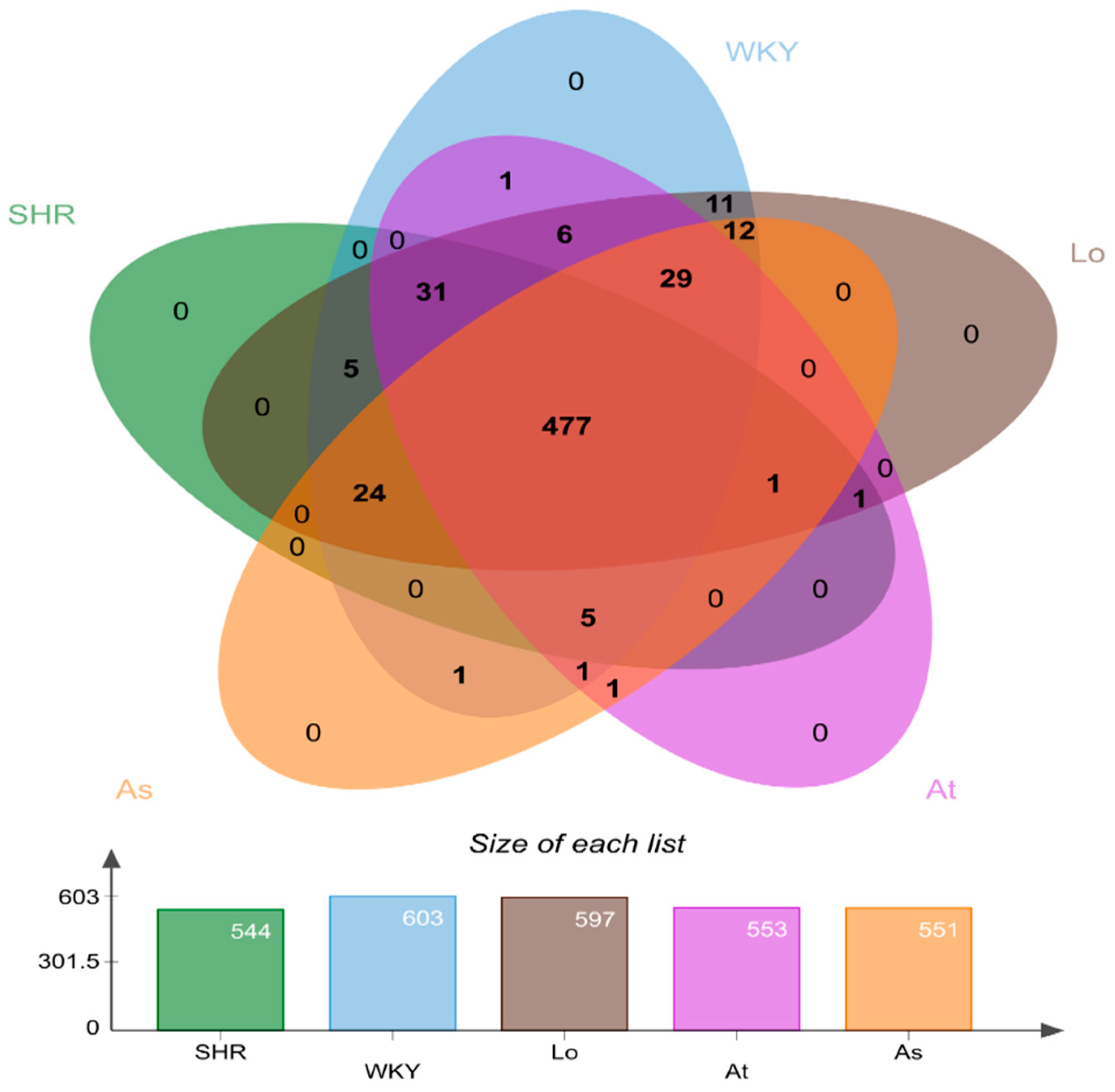
2.3. Operational Taxonomic Units (OTUs) Analysis
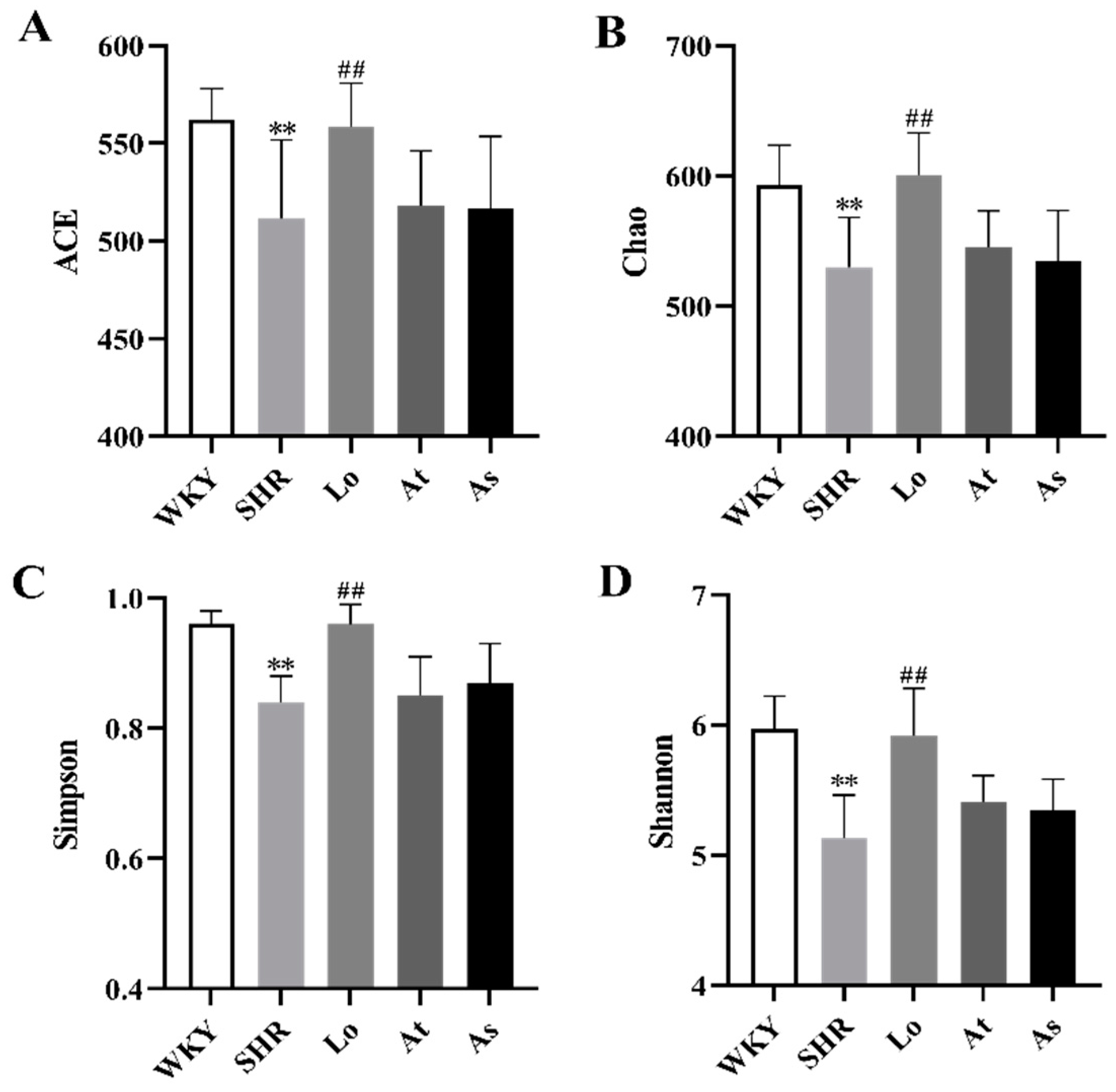
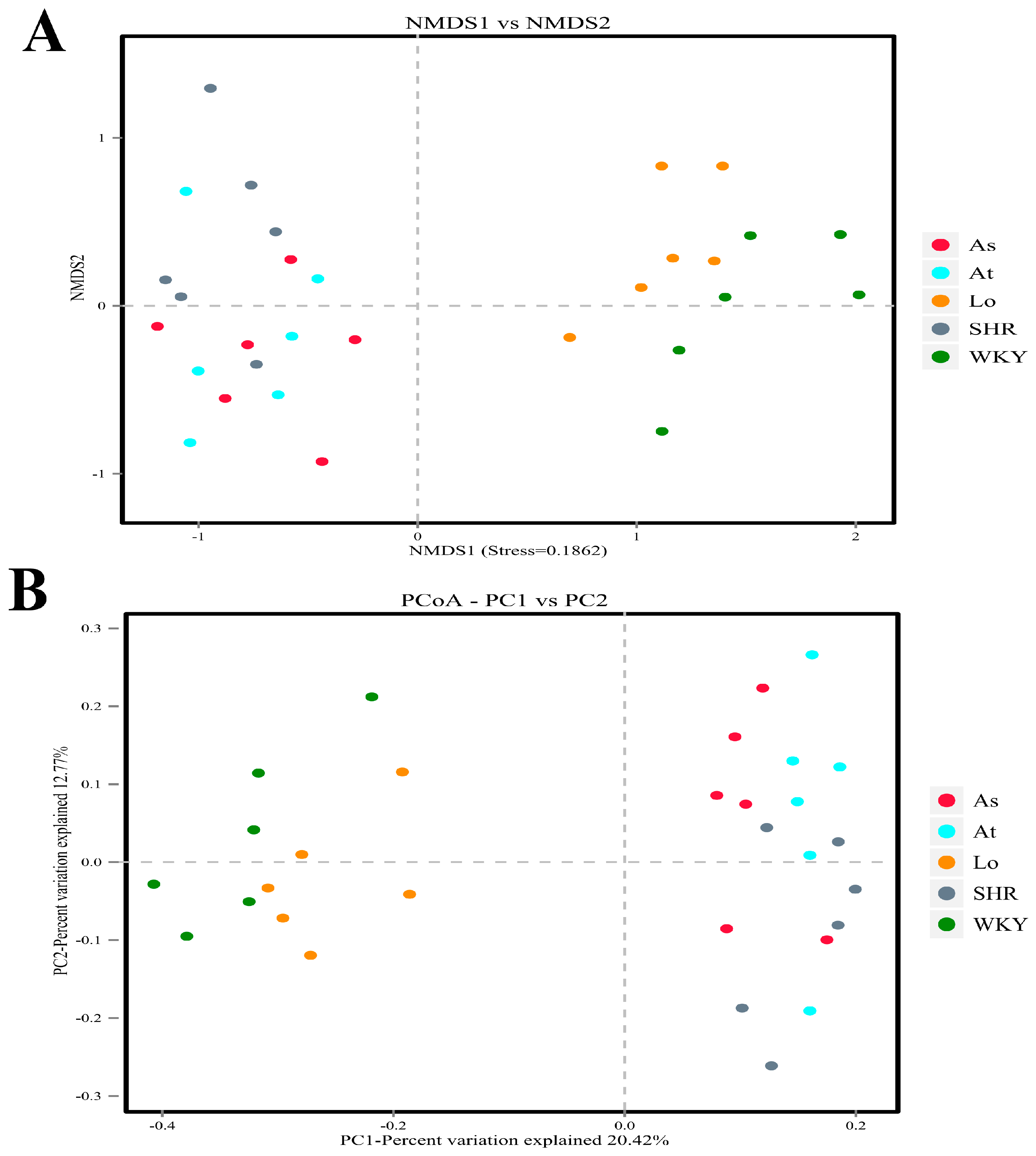
2.4. Alpha and Beta Diversity of Gut Microbiota
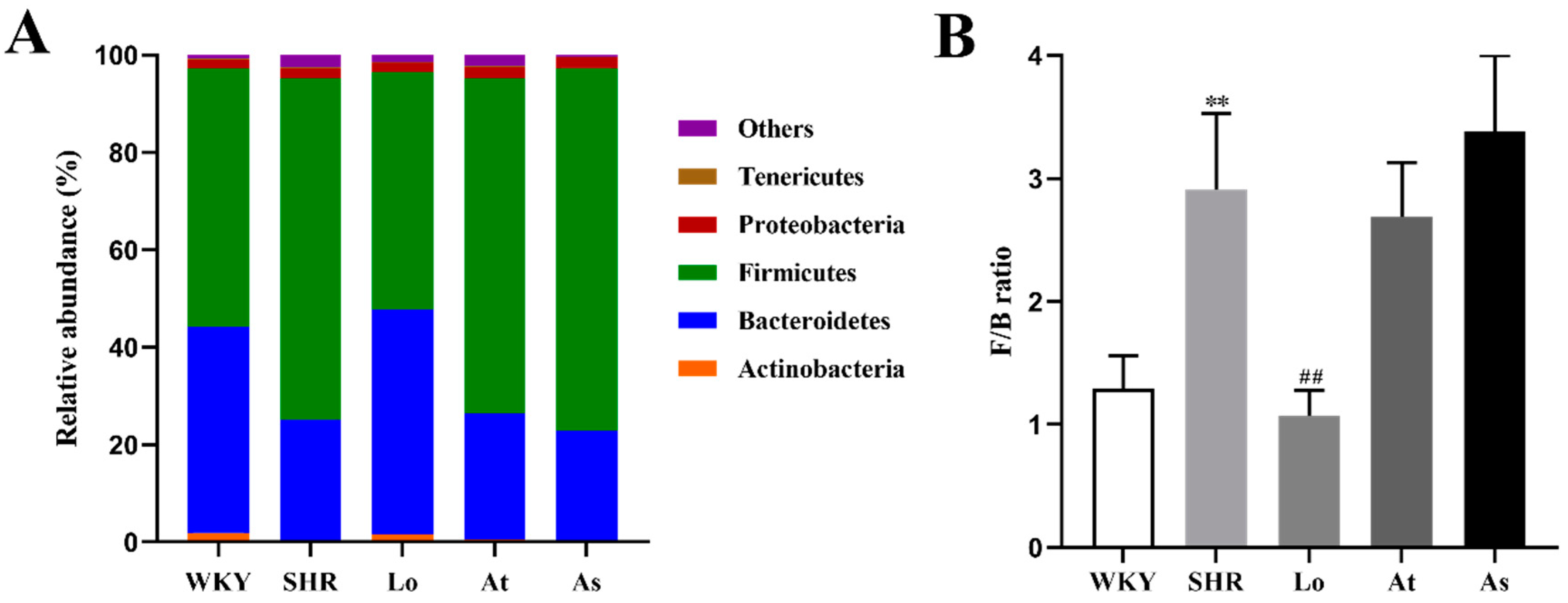
2.5. Structural Analysis of Gut Microbiota
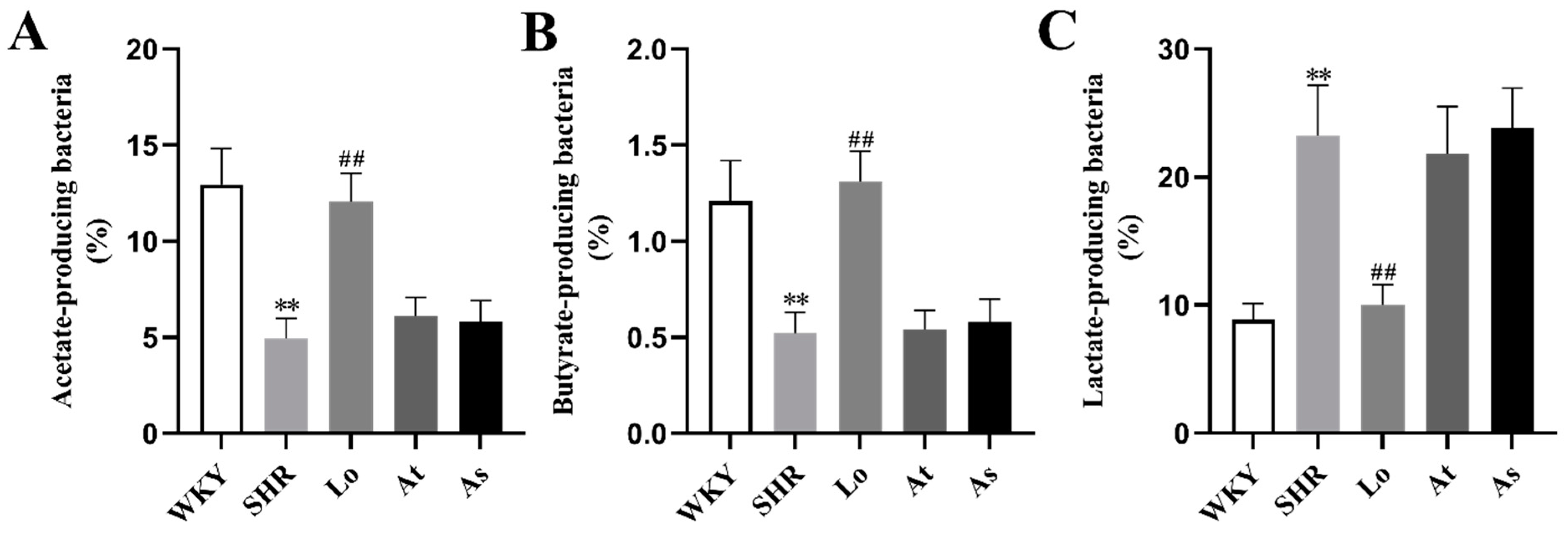
2.6. The Abundance of Acetate-, Butyrate-, and Lactate-Producing Bacteria
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
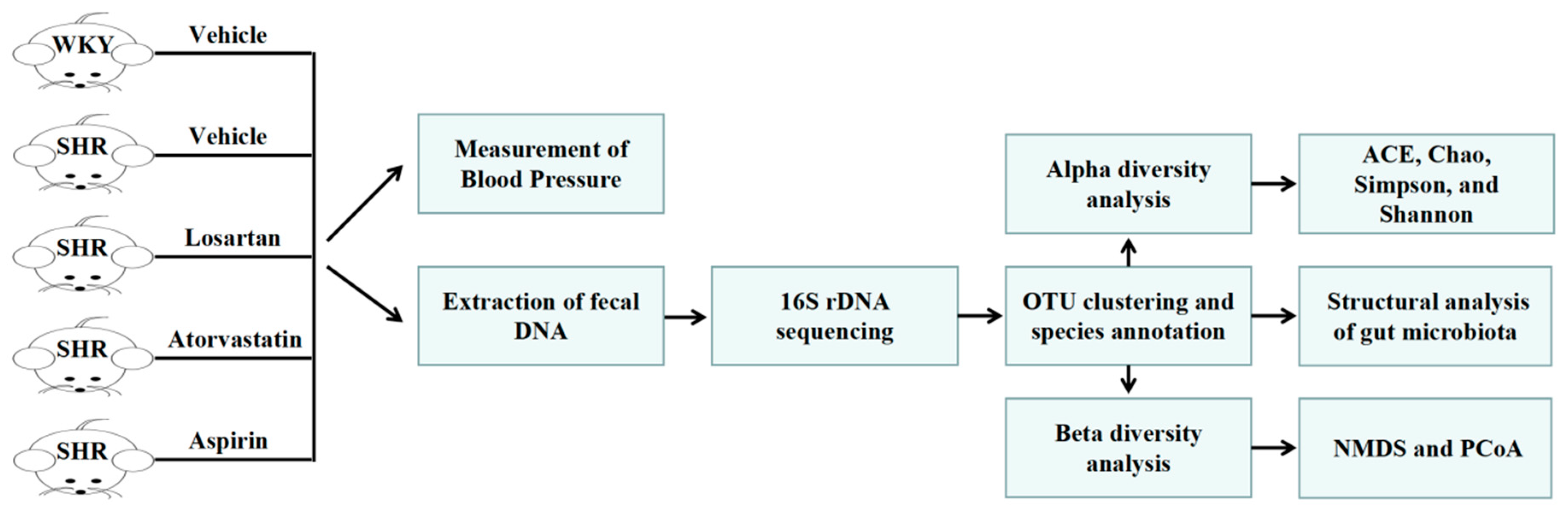
4.2. Animal Experiments and Design
4.3. Measurement of Blood Pressure
4.4. Collection of Fecal Samples
4.5. 16S rDNA High-Throughput Sequencing
4.6. Bioinformatics Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yue, Q.; Cai, M.; Xiao, B.; Zhan, Q.; Zeng, C. The Microbiota-Gut-Brain Axis and Epilepsy. Cell. Mol. Neurobiol. 2022, 42, 439–453. [Google Scholar] [CrossRef]
- Miele, L.; Giorgio, V.; Alberelli, M.A.; De Candia, E.; Gasbarrini, A.; Grieco, A. Impact of Gut Microbiota on Obesity, Diabetes, and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2015, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Moya-Perez, A. Microbiota, inflammation and obesity. Adv. Exp. Med. Biol. 2014, 817, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Lee, S.M.; Shen, Y.; Khosravi, A.; Mazmanian, S.K. Host-bacterial symbiosis in health and disease. Adv. Immunol. 2010, 107, 243–274. [Google Scholar] [CrossRef]
- McDermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Cianci, R.; Franza, L.; Schinzari, G.; Rossi, E.; Ianiro, G.; Tortora, G.; Gasbarrini, A.; Gambassi, G.; Cammarota, G. The Interplay between Immunity and Microbiota at Intestinal Immunological Niche: The Case of Cancer. Int. J. Mol. Sci. 2019, 20, 501. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, C.; Xing, Y.; Xue, G.; Zhang, Q.; Pan, F.; Wu, G.; Hu, Y.; Guo, Q.; Lu, A.; et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat. Commun. 2017, 8, 1896. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Drapkina, O.M.; Yafarova, A.A.; Kaburova, A.N.; Kiselev, A.R. Targeting Gut Microbiota as a Novel Strategy for Prevention and Treatment of Hypertension, Atrial Fibrillation and Heart Failure: Current Knowledge and Future Perspectives. Biomedicines 2022, 10, 2019. [Google Scholar] [CrossRef]
- Shen, X.; Li, L.; Sun, Z.; Zang, G.; Zhang, L.; Shao, C.; Wang, Z. Gut Microbiota and Atherosclerosis-Focusing on the Plaque Stability. Front. Cardiovasc. Med. 2021, 8, 668532. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, J.; Tang, P.; Li, J.; Xiong, X.; Deng, H. The Role of the Gut Microbiota in Coronary Heart Disease. Curr. Atheroscler. Rep. 2020, 22, 77. [Google Scholar] [CrossRef]
- Chacon, M.R.; Lozano-Bartolome, J.; Portero-Otin, M.; Rodriguez, M.M.; Xifra, G.; Puig, J.; Blasco, G.; Ricart, W.; Chaves, F.J.; Fernandez-Real, J.M. The gut mycobiome composition is linked to carotid atherosclerosis. Benef. Microbes 2018, 9, 185–198. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Nijiati, Y.; Maimaitiyiming, D.; Yang, T.; Li, H.; Aikemu, A.J.E. Research on the improvement of oxidative stress in rats with high-altitude pulmonary hypertension through the participation of irbesartan in regulating intestinal flora. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4540–4553. [Google Scholar] [CrossRef]
- Wu, W.K.; Ivanova, E.A.; Orekhov, A.N. Gut microbiome: A possible common therapeutic target for treatment of atherosclerosis and cancer. Semin. Cancer Biol. 2021, 70, 85–97. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Grigorova, E.V.; Belkova, N.L.; Nemchenko, U.M.; Klimenko, E.S.; Pogodina, A.V.; Romanitsa, A.I.; Novikova, E.A.; Rychkova, L.V. Metasequencing of V3-V4 Variable Regions of 16S rRNA Gene in Opportunistic Microbiota and Gut Biocenosis in Obese Adolescents. Bull. Exp. Biol. Med. 2021, 170, 321–325. [Google Scholar] [CrossRef]
- Peterson, D.A.; Frank, D.N.; Pace, N.R.; Gordon, J.I. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 2008, 3, 417–427. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, V.R.; Petrosino, J.F.; Bryan, R.M., Jr.; Durgan, D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Jin, H.; Wu, Z.; Yan, C.; Liu, Z.; Xu, X.; Liu, S.; Zhu, F. Zhengganxifeng Decoction Affects Gut Microbiota and Reduces Blood Pressure via Renin–Angiotensin System. Biol. Pharm. Bull. 2019, 42, 1482–1490. [Google Scholar] [CrossRef]
- Shi, C.W.; Cheng, M.Y.; Yang, X.; Lu, Y.Y.; Yin, H.D.; Zeng, Y.; Wang, R.Y.; Jiang, Y.L.; Yang, W.T.; Wang, J.Z.; et al. Probiotic Lactobacillus rhamnosus GG Promotes Mouse Gut Microbiota Diversity and T Cell Differentiation. Front. Microbiol. 2020, 11, 607735. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; de la Visitacion, N.; Toral, M.; Sanchez, M.; Gomez-Guzman, M.; Jimenez, R.; Romero, M.; Duarte, J. Mycophenolate mediated remodeling of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rats. Biomed. Pharmacother. 2021, 135, 111189. [Google Scholar] [CrossRef]
- Bibiloni, R.; Membrez, M.; Chou, C.J. Gut Microbiota, Obesity and Diabetes. Ann. Nestlé Engl. Ed. 2009, 67, 39–47. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Fan, X.; Richel, A.; Everaert, N.; Yang, X.; Ren, G. Administration with Quinoa Protein Reduces the Blood Pressure in Spontaneously Hypertensive Rats and Modifies the Fecal Microbiota. Nutrients 2021, 13, 2446. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Tian, C.; Chen, X.; Li, H.; Wei, X.; Lin, W.; Zheng, N.; Jiang, A.; Feng, R.; et al. Targeting the Gut Microbiota to Investigate the Mechanism of Lactulose in Negating the Effects of a High-Salt Diet on Hypertension. Mol. Nutr. Food Res. 2019, 63, e1800941. [Google Scholar] [CrossRef]
- Gronlund, M.M.; Gueimonde, M.; Laitinen, K.; Kociubinski, G.; Gronroos, T.; Salminen, S.; Isolauri, E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin. Exp. Allergy 2007, 37, 1764–1772. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Lamas, A.; del Carmen Mondragón, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic Effects against Virus Infections: New Weapons for an Old War. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Grangette, C. Bifidobacteria and subsets of dendritic cells: Friendly players in immune regulation! Gut 2012, 61, 331–332. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Natarajan, N.; Pluznick, J.L. From microbe to man: The role of microbial short chain fatty acid metabolites in host cell biology. Am. J. Physiol. Cell Physiol. 2014, 307, C979–C985. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; An, J.; Song, Y.; Lee, C.K.; Kim, K.; Kong, H. Alterations in Gut Microbiota by Statin Therapy and Possible Intermediate Effects on Hyperglycemia and Hyperlipidemia. Front. Microbiol. 2019, 10, 1947. [Google Scholar] [CrossRef] [PubMed]
- Prizment, A.E.; Staley, C.; Onyeaghala, G.C.; Vivek, S.; Thyagarajan, B.; Straka, R.J.; Demmer, R.T.; Knights, D.; Meyer, K.A.; Shaukat, A.; et al. Randomised clinical study: Oral aspirin 325 mg daily vs placebo alters gut microbial composition and bacterial taxa associated with colorectal cancer risk. Aliment. Pharmacol. Ther. 2020, 52, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Zhang, M.; Zhang, Y.; Bai, X.; Zhang, Y.; Guo, X. Effects of Oat Complex High-Fiber Formula Powder on the Composition of Intestinal Microbiota and Enzyme Activities in Mice Induced by a High-Fat Diet. Front. Nutr. 2022, 9, 871556. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, W.; Zhang, S.; Chen, L.; Tang, F.; Sheng, Y.; Ao, H.; Peng, C. Gut microbial modulation in the treatment of chemotherapy-induced diarrhea with Shenzhu Capsule. BMC Complement Altern. Med. 2019, 19, 126. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, B.; Zheng, W.; Chen, X.; Zhang, J.; Yan, R.; Zhang, T.; Yu, L.; Dong, Y.; Ma, B. Liupao tea extract alleviates diabetes mellitus and modulates gut microbiota in rats induced by streptozotocin and high-fat, high-sugar diet. Biomed. Pharmacother. 2019, 118, 109262. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Antharam, V.C.; Li, E.C.; Ishmael, A.; Sharma, A.; Mai, V.; Rand, K.H.; Wang, G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013, 51, 2884–2892. [Google Scholar] [CrossRef]


| Phylum | WKY | SHR | Lo | At | As |
|---|---|---|---|---|---|
| Actinobacteria (%) | 1.88 ± 0.43 | 0.29 ± 0.04 ** | 1.50 ± 0.23 ## | 0.43 ± 0.10 | 0.34 ± 0.06 |
| Bacteroidetes (%) | 42.22 ± 5.37 | 24.79 ± 3.99 ** | 46.22 ± 4.72 ## | 25.95 ± 3.00 | 22.53 ± 3.20 |
| Firmicutes (%) | 53.10 ± 5.18 | 70.14 ± 3.83 ** | 48.75 ± 5.13 ## | 68.80 ± 3.06 | 74.43 ± 3.33 |
| Proteobacteria (%) | 1.80 ± 0.31 | 2.00 ± 1.19 | 1.92 ± 0.69 | 2.39 ± 1.08 | 2.16 ± 0.27 |
| Tenericutes (%) | 0.21 ± 0.06 | 0.25 ± 0.14 | 0.22 ± 0.09 | 0.20 ± 0.09 | 0.18 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Liu, Q.; Zhou, X.; Zhao, Y.; Yang, K.; Li, L.; Zhu, D. Effects of Losartan, Atorvastatin, and Aspirin on Blood Pressure and Gut Microbiota in Spontaneously Hypertensive Rats. Molecules 2023, 28, 612. https://doi.org/10.3390/molecules28020612
Dong S, Liu Q, Zhou X, Zhao Y, Yang K, Li L, Zhu D. Effects of Losartan, Atorvastatin, and Aspirin on Blood Pressure and Gut Microbiota in Spontaneously Hypertensive Rats. Molecules. 2023; 28(2):612. https://doi.org/10.3390/molecules28020612
Chicago/Turabian StyleDong, Shuai, Qi Liu, Xue Zhou, Yubo Zhao, Kang Yang, Linsen Li, and Dan Zhu. 2023. "Effects of Losartan, Atorvastatin, and Aspirin on Blood Pressure and Gut Microbiota in Spontaneously Hypertensive Rats" Molecules 28, no. 2: 612. https://doi.org/10.3390/molecules28020612
APA StyleDong, S., Liu, Q., Zhou, X., Zhao, Y., Yang, K., Li, L., & Zhu, D. (2023). Effects of Losartan, Atorvastatin, and Aspirin on Blood Pressure and Gut Microbiota in Spontaneously Hypertensive Rats. Molecules, 28(2), 612. https://doi.org/10.3390/molecules28020612






