Design, Synthesis, Fungicidal and Insecticidal Activities of Novel Diamide Compounds Combining Pyrazolyl and Polyfluoro-Substituted Phenyl into Alanine or 2-Aminobutyric Acid Skeletons
Abstract
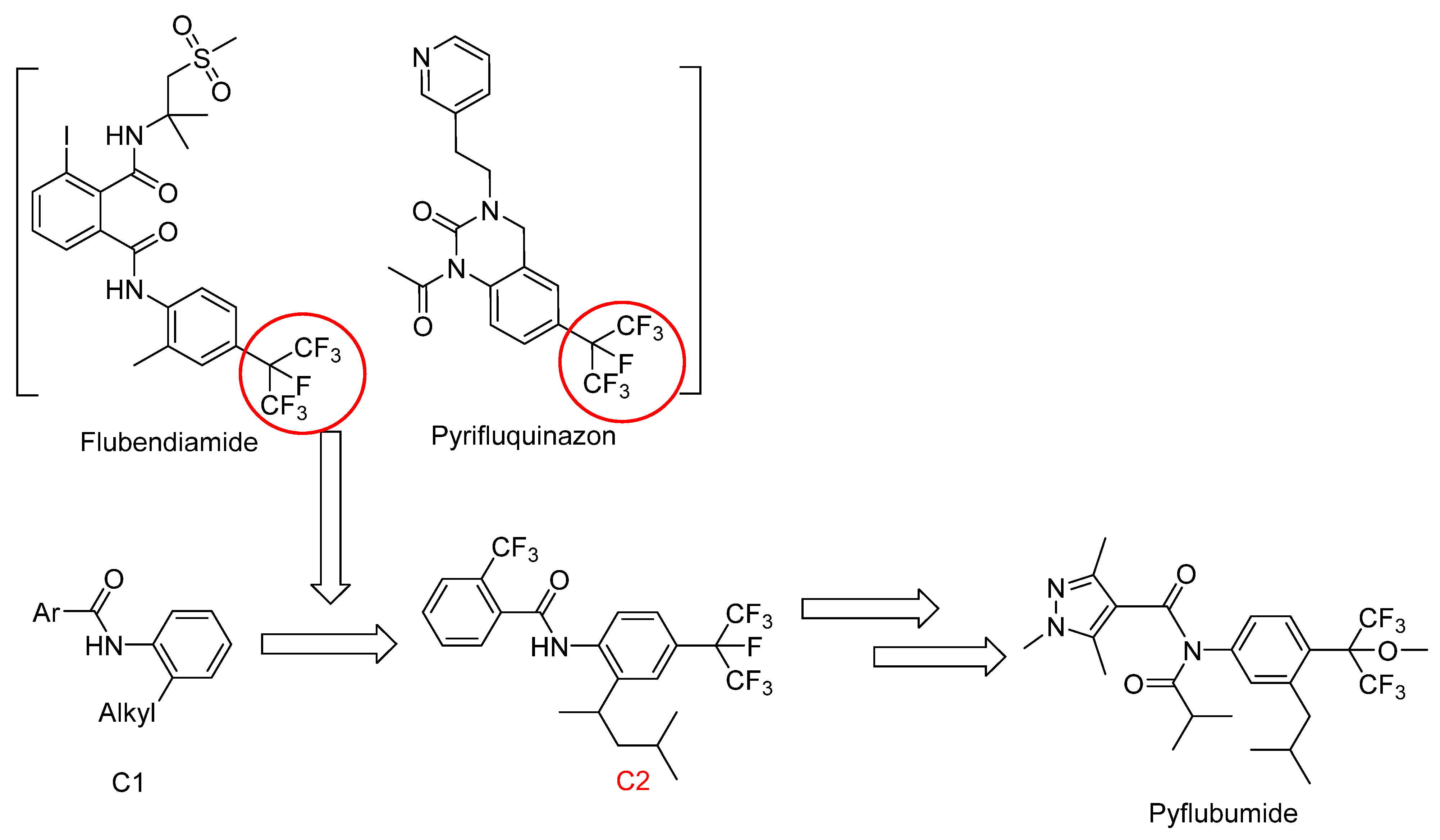
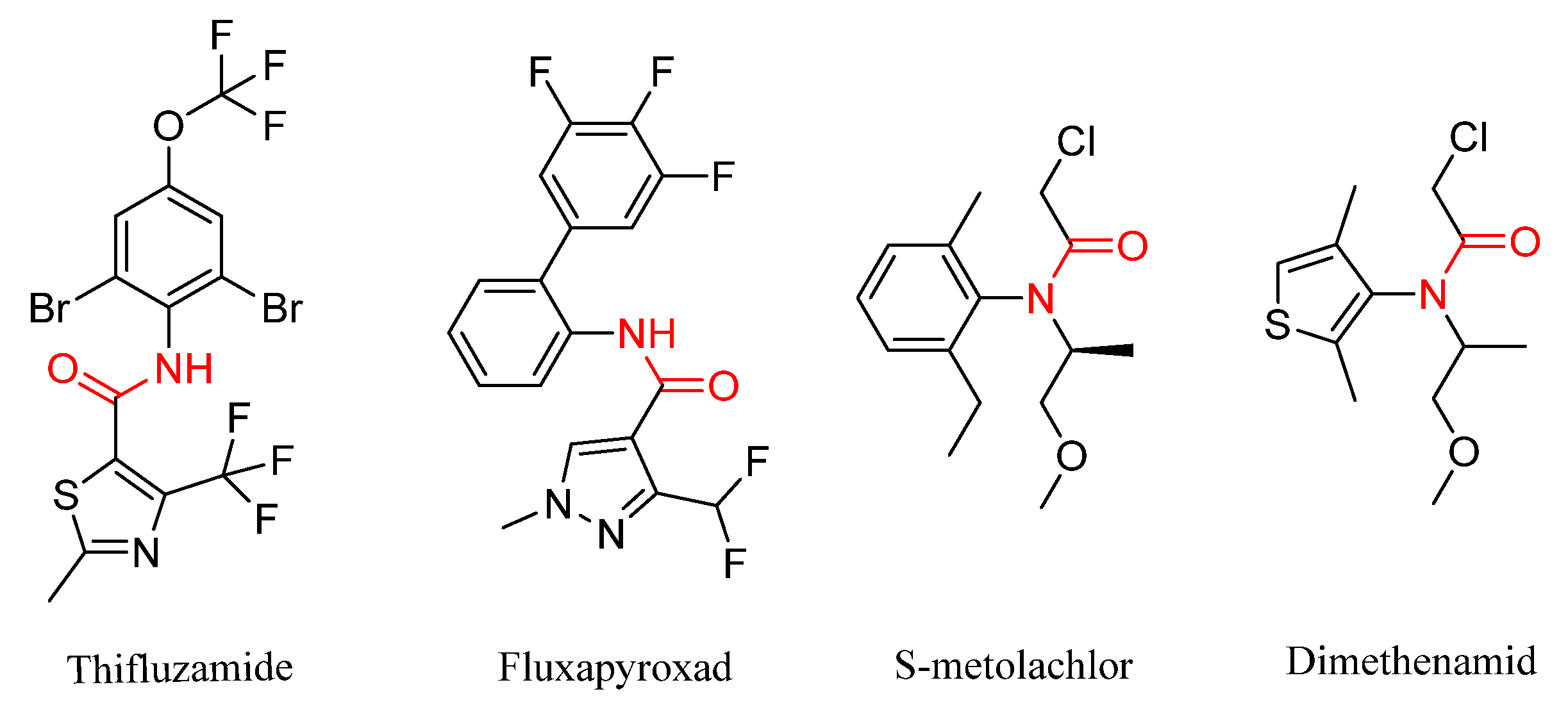
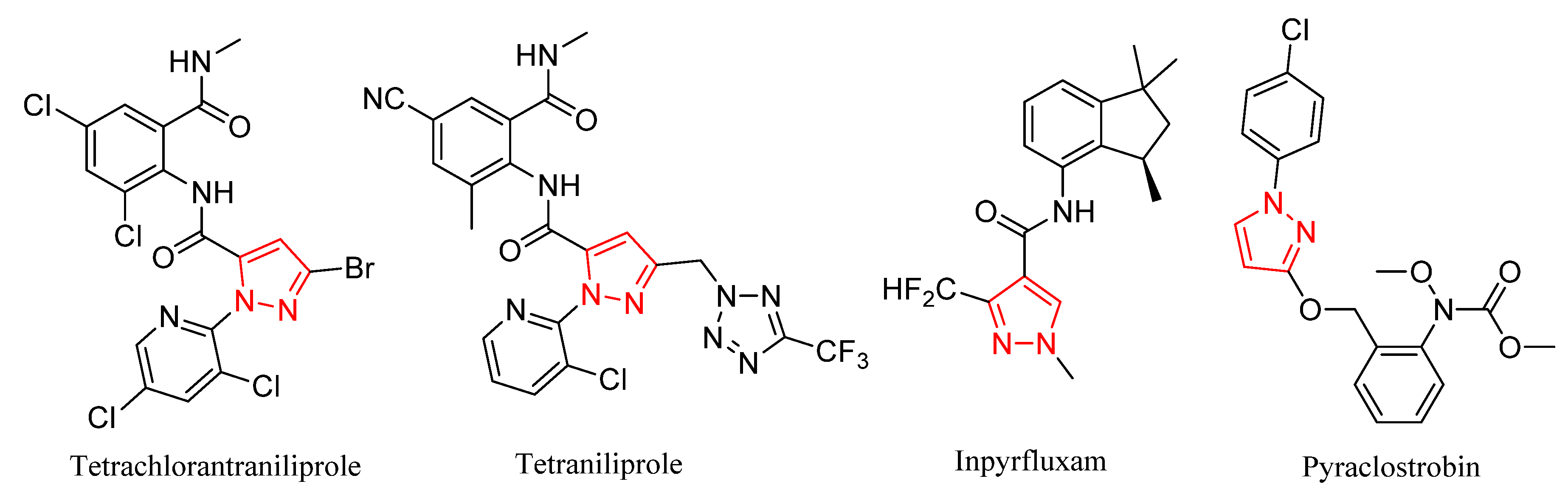
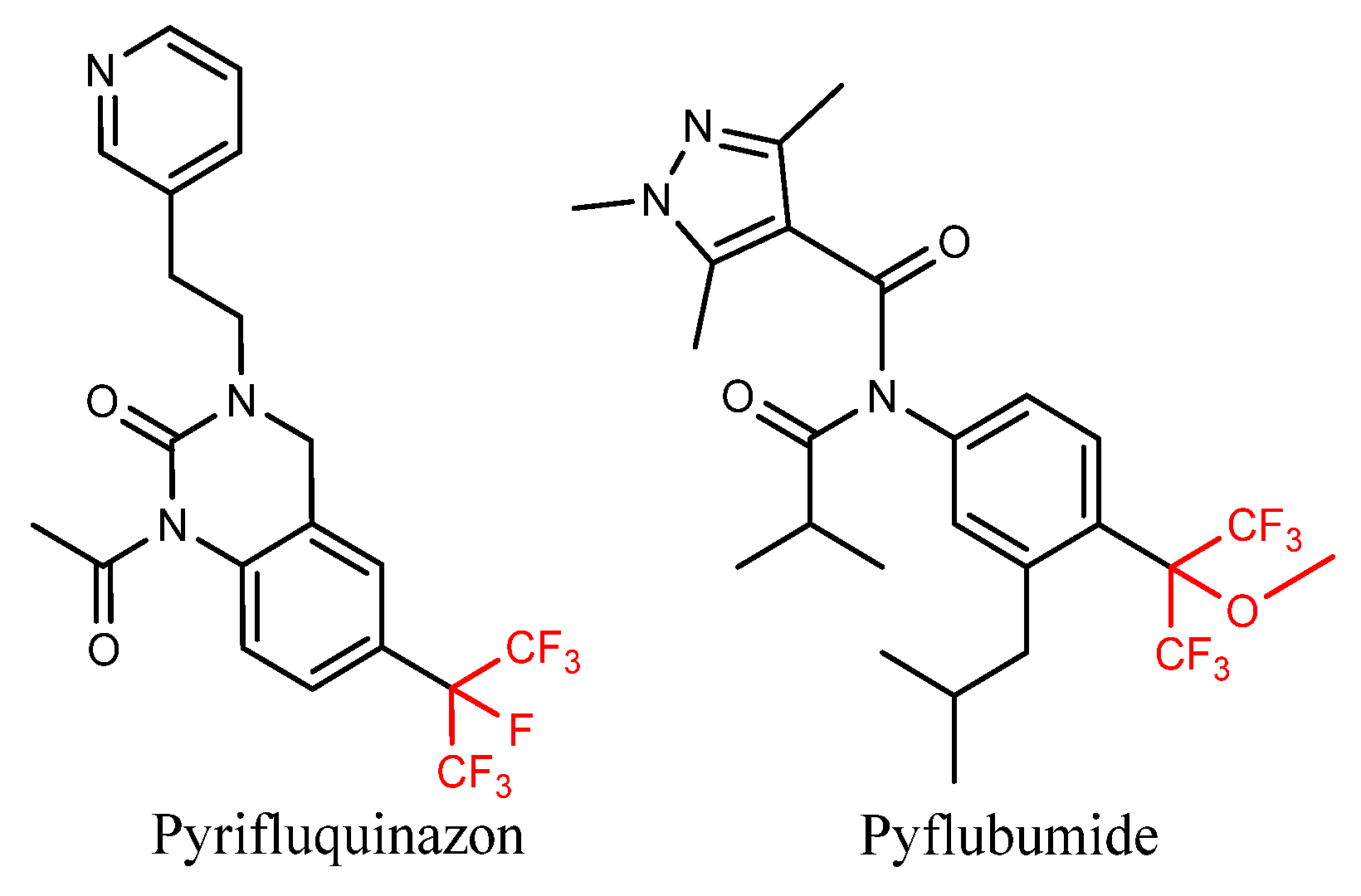
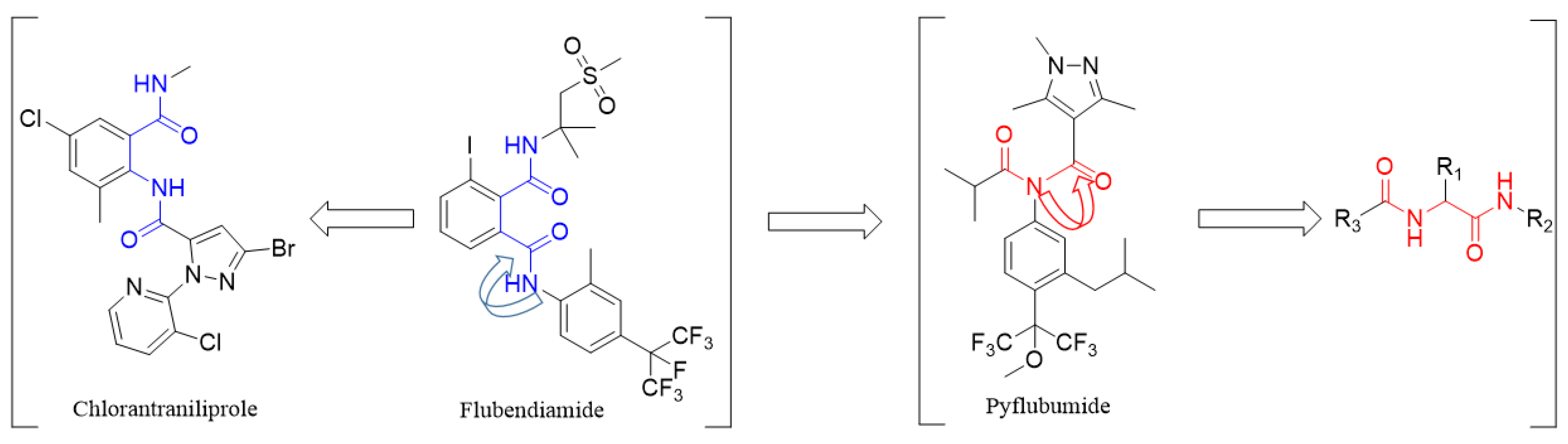
1. Introduction
2. Results and Discussion
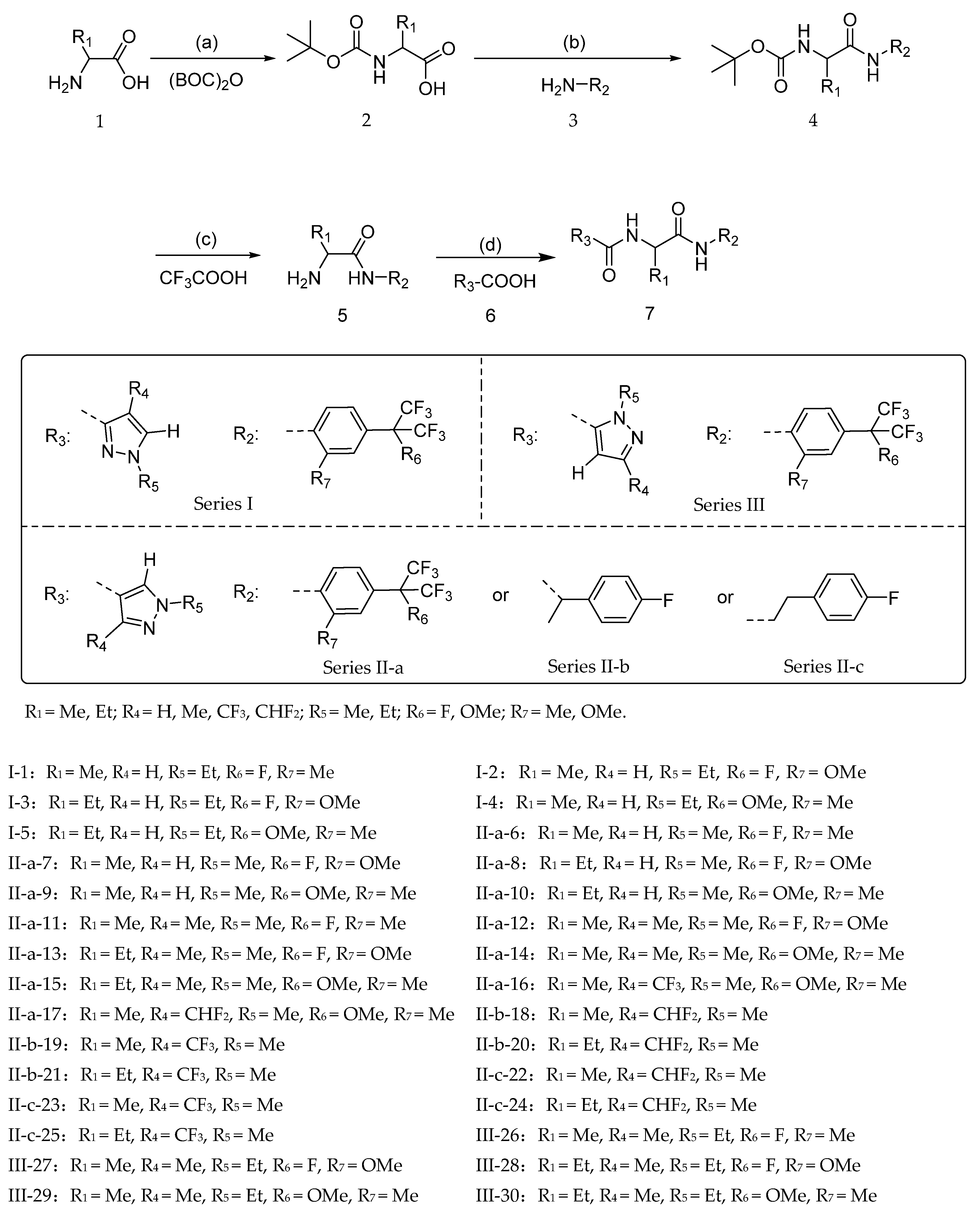
2.1. Chemistry
2.2. Structure–Activity Relationship
2.2.1. Insecticidal and Acaricidal Activity
2.2.2. Fungicidal Activity
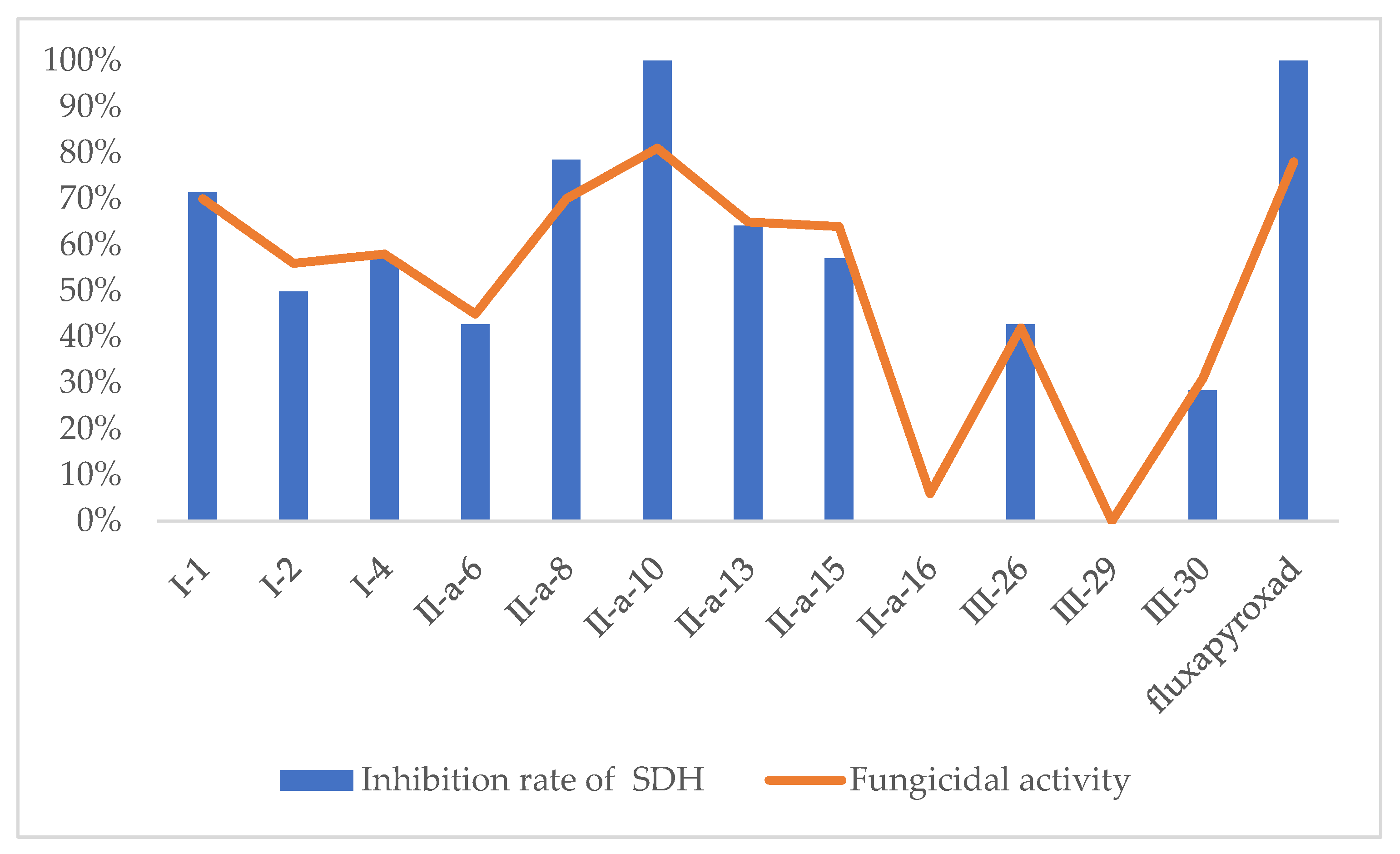
2.3. Enzyme Activity Assay
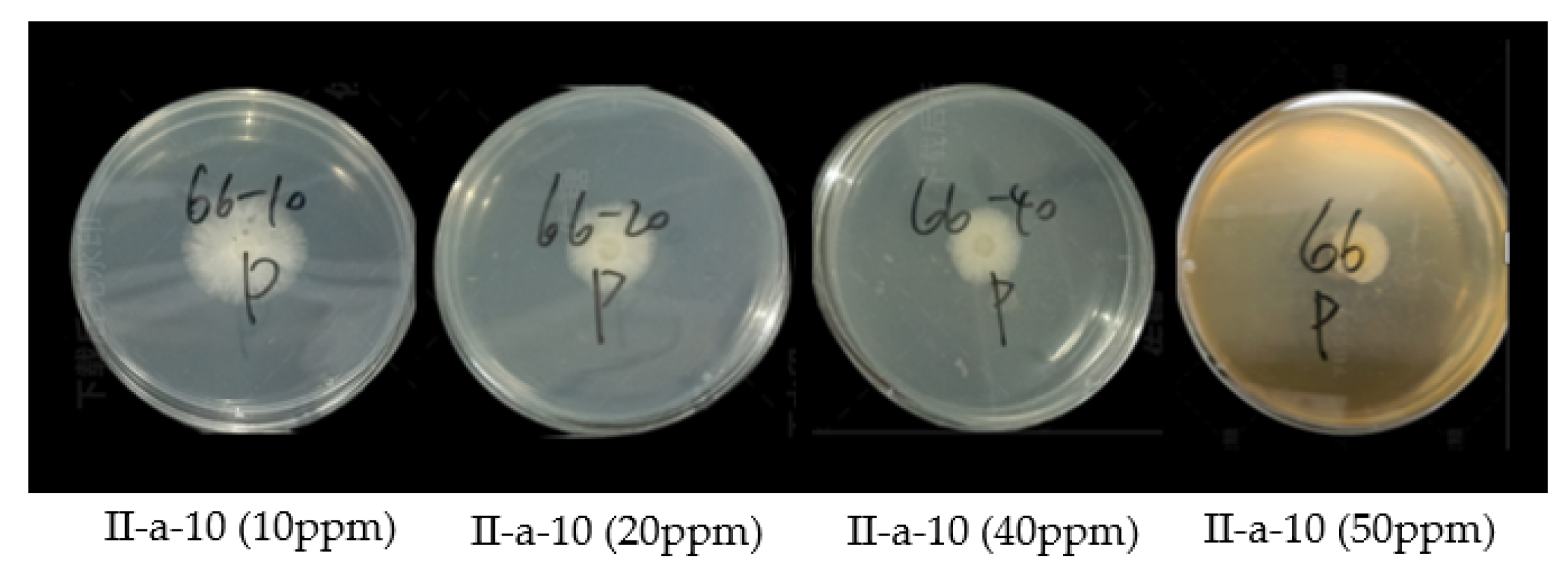
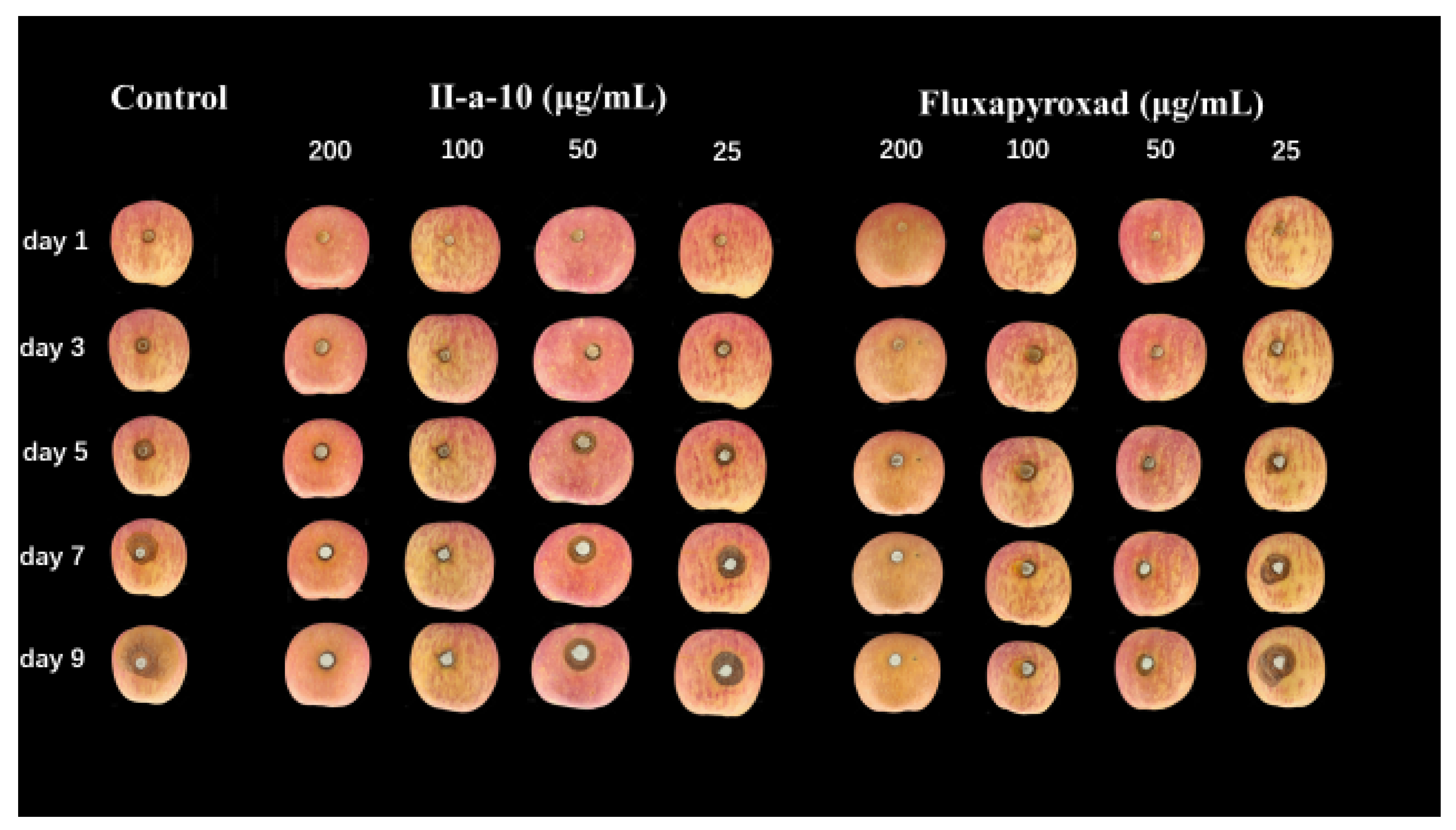
2.4. In Vivo Curative Effect of Compound II-a-10 against Cytospora sp.
3. Materials and Methods
3.1. Instruments and Materials
3.2. Synthesis
3.3. Biological Assay
3.3.1. Insecticidal and Acaricidal Activity
3.3.2. Fungicidal Activity
3.4. Enzymatic Activity
3.5. In Vivo Efficacy of Compound II-a-10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Chen, Y.; Li, B.; Chen, T.; Tian, S. Reactive oxygen species: A generalist in regulating development and pathogenicity of phytopathogenic fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3344–3349. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS One 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- FAO Crop and Livestock Statistics. Available online: https://www.fao.org/faostat/zh/#data/QCL (accessed on 1 November 2022).
- He, X.K. Research and development of crop protection machinery and chemical application technology in China. Chin. J. Pestic. Sci. 2019, 21, 921–930. [Google Scholar]
- Wang, Z.C.; Kang, Z.J.; Shi, X.Y.; Gao, X.W. Research progresses on the metabolic mechanisms of organophosphate insecticides. Chin. J. Pestic. Sci. 2015, 17, 1–14. [Google Scholar]
- Yosri, N.; Khalifa, S.A.; Guo, Z.; Xu, B.; Zou, X.; El-Seedi, H.R. Marine organisms: Pioneer natural sources of polysaccharides/proteins for green synthesis of nanoparticles and their potential applications. Int. J. Biol. Macromol. 2021, 193, 1767–1798. [Google Scholar]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and how to kill a plant cell: Infection strategies of plant pathogenic fungi. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, J.; Longo, S.B.; York, R. Agriculture, pesticide use, and economic development: A global examination (1990–2014). Rural Sociol. 2020, 85, 519–544. [Google Scholar] [CrossRef]
- Leadbeater, A. Recent developments and challenges in chemical disease control. Plant Prot. Sci. 2016, 51, 163–169. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.; Tohnishi, M.; Nakao, H. Flubendiamide, a new insecticide characterizedby its novel chemistry and biology. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2007; pp. 127–135. [Google Scholar]
- Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J. Pestic. Sci. 2005, 30, 354–360. [Google Scholar] [CrossRef]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef]
- George, P.L.; Thomas, P.S.; Thomas, M.S. Arthropodicidal Anthranilamides. WO Patent 2003015519, 27 February 2003. [Google Scholar]
- Nakao, T.; Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Machiya, K.; Fujioka, S.; Nakano, M.; Inagaki, K. Development of a novel acaricide, pyflubumide. Pestic. Sci. 2017, 42, 132–136. [Google Scholar] [CrossRef]
- Nakano, M.; Yasokawa, N.; Suwa, A.; Fujioka, S.; Furuya, T.; Sakata, K. Mode of action of novel acaricide pyflubumide: Effects on the mitochondrial respiratory chain. J. Pestic. Sci. 2015, 40, 19–24. [Google Scholar] [CrossRef]
- Furuya, T.; Suwa, A.; Nakano, M.; Fujioka, S.; Yasokawa, N.; Machiya, K. Synthesis and biological activity of a novel acaricide, pyflubumide. J. Pestic. Sci. 2015, 40, 38–43. [Google Scholar] [CrossRef]
- Sharma, A.K.; Singh, D.P.; Kumar, J.; Singh, A.; Tewari, A.N.; Singh, K.P.; Karwasra, S.S.; Grewal, A.S. Efficacy of thifluzamide in the control of loose smut of wheat (Triticum aestivum) caused by Ustilago segetum. Indian J. Agric. Sci. 2001, 71, 648–649. [Google Scholar]
- Ebbinghaus, D.; Huser-Hahn, I.; Dittgen, J. Use of Succinate Dehydrogenase Inhibitors for Increasing the Resistance of Plants or Parts of Plants to Abiotic Stress. U.S. Patent 20100324101A1, 23 December 2010. [Google Scholar]
- O’Connell, P.J.; Harms, C.T.; Allen, J.R.F. Metolachlor, S-metolachlor and their role within sustainable weed-management. Crop Prot. 1998, 17, 207–212. [Google Scholar] [CrossRef]
- Rahman, A.; James, T.K.; Popay, A.J. Weed control and soil persistence studies with dimethenamid in maize. N. Z. Plant Prot. 1992, 45, 84–88. [Google Scholar] [CrossRef]
- Deepak, S.A.; Oros, G.; Raj, S.N. Iprovalicarb has potential for the control of downy mildew of pearl millet. Acta Phytopathol. Et Entomol. Hung. 2004, 39, 55–69. [Google Scholar] [CrossRef]
- Lamberth, C. Carboxylic Acid Amide Fungicides for the Control of Downy Mildew Diseases; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2016. [Google Scholar]
- Zhu, L.; Liu, W.; Jiang, J.; Wang, S.; Zhao, P.; Shi, B. Fungicide Composition Containing Valifenalate and Application in Prevention and Control of Downy Mildew. CN Patent 109673655, 26 April 2019. [Google Scholar]
- Nossier, E.S.; Fahmy, H.H.; Khalifa, N.M.; El-Eraky, W.I.; Baset, M.A. Design and synthesis of novel pyrazole-substituted different nitrogenous heterocyclic ring systems as potential anti-inflammatory agents. Molecules 2017, 22, 512. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.H.; Khalifa, N.M.; Ismail, M.M.F.; El-Sahrawy, H.M.; Nossier, E.S. Biological validation of novel polysubstituted pyrazole candidates with in vitro anticancer activities. Molecules 2016, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Tetrachlorantraniliprole Containing Insecticidal Composition. CN Patent 103931643, 23 July 2014. [Google Scholar]
- Fischer, R.; Grondal, C.; Gesing, E.R.; Wroblowsky, H.-J.; Hence, A.; Franken, E.-M.; Voerste, A.; Görgens, U. Triazole-Substituted Anthranilamides as Pesticides. U.S. Patent 20110275676, 20 October 2011. [Google Scholar]
- Ayer, K.M.; Villani, S.M.; Choi, M.-W.; Cox, K.D. Characterization of the VisdhC and VisdhD genes in Venturia inaequalis, and sensitivity to fluxapyroxad, pydiflumetofen, inpyrfluxam, and benzovindiflupyr. Plant Dis. 2019, 103, 1092–1100. [Google Scholar] [CrossRef]
- Turechek, W.W.; Peres, N.A.; Werner, N.A. Pre- and Post-Infection Activity of Pyraclostrobin for Control of Anthracnose Fruit Rot of Strawberry Caused by Colletotrichum acutatum. Plant Dis. 2006, 90, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Angst, M.; Pedroni, D.; Senn, R. Methods of Controlling Insects in the Family Nitidulidae Using Pyrifluquinazon. WO Patent 2009000470 A1, 31 December 2008. [Google Scholar]
- Katti, S.; Srinivasarao, K.; Soni, A. Synthesis and antimalarial activity of new 4-aminoquinolines active against drug resistantstrains. RSC Adv. 2016, 6, 105676–105689. [Google Scholar]
- Masanobu, O.; Oona, Y.A.; Eiji, K.; Kenji, T. A Process for Producing Perfluoroalkylaniline Derivatives. EP Patent 1006102 A2, 7 June 2000. [Google Scholar]
- Noboru, A.; Osamu, S. Preparation of Aniline Derivatives. JP Patent 2012067060A, 5 April 2012. [Google Scholar]
- Ross, J.E.; Knipe, P.C.; Thompson, S. Hybrid Diphenylalkyne-Dipeptide Oligomers Induce Multistrand β-Sheet Formation. Chemistry 2015, 21, 13518–13521. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-J.; Zhou, C.; Dong, L.-F.; Feng, T.-T.; Wang, G.-A.; Wang, J.-J.; Gu, Y.-C.; Xu, Z.-P.; Cheng, J.-G.; Shao, X.-S.; et al. Diamides conformationally restricted with central amino acid: Design, synthesis, and biological activities. Int. J. Heterocycl. Chem. 2022, 59, 1045–1053. [Google Scholar] [CrossRef]
- Wei, Q.C.; Zheng, Z.C.; Wang, S.H.; Zheng, X.M. Pyrazole Amide Derivative and Preparation Method and Application Thereof. CN Patent 109721539, 7 May 2019. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, Y.Q.; Shao, X.S.; Xu, Z.P.; Xu, X.Y.; Li, Z. Synthesis and insecticidal evaluation of tetrahydroimidazo 1,2-a pyridin-5(1H)-one derivatives. Chin. Chem. Lett. 2016, 27, 7–10. [Google Scholar] [CrossRef]
- Luo, J.X.; Lai, T.; Guo, T.; Chen, F.; Zhang, L.L.; Ding, W.; Zhang, Y.Q. Synthesis and acaricidal activities of scopoletin phenolic ether derivatives: QSAR, molecular docking study and in silico ADME predictions. Molecules 2018, 23, 995. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kang, S.H.; Yuan, Q.K.; Luo, L.J.; Ma, J.; Shi, Q.C.; Yang, S. N-substituted 5-chloro-6-phenylpyridazin-3(2H)-ones: Synthesis, insecticidal activity against Plutella xylostella (L.) and SAR study. Molecules 2012, 17, 9413–9420. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhao, Z.; Li, J.; Yang, G.; Liu, Y.; Dai, L.; Zhang, Z.; Yang, Z.; Miao, X.; Yang, C.; et al. Insecticidal and antifungal activities of Rheum palmatum L. anthraquinones and structurally related compounds. Ind. Crops Prod. 2019, 137, 508–520. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, B.; Fan, Z.J.; Liu, X.M.; Wu, Q.F.; Li, H.P.; Wang, H.X. Synthesis of novel 3,4-chloroisothiazole-based imidazoles as fungicides and evaluation of their mode of action. J. Agric. Food Chem. 2018, 66, 7319–7327. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, B.; Fan, Z.; Yu, B.; Zhang, N.; Li, Z.; Zhu, Y.; Zhou, J.; Kalinina, T.A.; Glukhareva, T.V. Synthesis and bio logical activity of novel succinate dehydrogenase inhibitor deriva tives as potent fungicide candidates. J. Agric. Food Chem. 2019, 67, 13185–13194. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, B.; Fan, Z.; Yang, D.; Zhang, N.; Wu, Q.; Yu, B.; Zhou, S.; Kalinina, T.A.; Belskaya, N.P. Discovery of novel thiazole carboxamides as antifungal succinate dehydroge nase inhibitors. J. Agric. Food Chem. 2019, 67, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Zeun, R.; Scalliet, G.; Oostendorp, M. Biological activity of sedaxane—A novel broad-spectrum fungicide for seed treatment. Pest Manag. Sci. 2013, 69, 527. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Ma, K.Y.; Du, S.S.; Zhang, Z.J.; Wu, T.L.; Sun, Y.; Liu, Y.Q.; Yin, X.D.; Zhou, R.; Yan, Y.F.; et al. Antifungal exploration of quinoline derivatives against phytopathogenic fungi inspired by quinine alkaloids. J. Agric. Food Chem. 2021, 69, 12156–12170. [Google Scholar] [CrossRef]

| Against Aphis craccivora | Against Tetranychus cinnabarinus | Against Plutella xylostella | |||||
|---|---|---|---|---|---|---|---|
| Compd | 400 μg/mL | 200 μg/mL | 100 μg/mL | 400 μg/mL | 200 μg/mL | 400 μg/mL | 200 μg/mL |
| I-1 | 85.0% | 71.7% | 60.0% | 65.6% | 47.8% | 86.7% | 76.7% |
| I-2 | 81.7% | 61.7% | 41.7% | 53.3% | 33.3% | 65.6% | 47.8% |
| I-3 | 76.7% | 53.3% | 30.0% | 52.2% | 33.3% | 66.7% | 40.0% |
| I-4 | 93.3% | 78.3% | 60.0% | 53.3% | 26.7% | 65.6% | 46.7% |
| I-5 | 80.0% | 68.3% | 53.3% | 70.0% | 50.0% | 66.7% | 33.3% |
| II-a-6 | 85.0% | 71.7% | 60.0% | 50.0% | 33.3% | 50.0% | 30.0% |
| II-a-7 | 88.3% | 73.3% | 58.3% | 66.7% | 51.1% | 33.3% | 16.7% |
| II-a-8 | 68.3% | 56.7% | 41.7% | 63.3% | 46.7% | 46.7% | 23.3% |
| II-a-9 | 80.0% | 63.3% | 50.0% | 60.0% | 38.9% | 70.0% | 50.0% |
| II-a-10 | 90.0% | 78.3% | 61.7% | 58.9% | 41.1% | 46.7% | 16.7% |
| II-a-11 | 76.7% | 61.7% | 51.7% | 72.2% | 57.8% | 76.7% | 46.7% |
| II-a-12 | 86.7% | 68.3% | 53.3% | 60.0% | 41.1% | 60.0% | 36.7% |
| II-a-13 | 86.7% | 73.3% | 55.0% | 40.0% | 26.7% | 65.6% | 51.1% |
| II-a-14 | 90.0% | 73.3% | 56.7% | 73.3% | 61.1% | 66.7% | 50.0% |
| II-a-15 | 73.3% | 63.3% | 50.0% | 50.0% | 33.3% | 86.7% | 70.0% |
| II-a-16 | 43.3% | 26.7% | 10.0% | 43.3% | 27.8% | 46.7% | 26.7% |
| II-a-17 | 48.3% | 36.7% | 20.0% | 36.7% | 20.0% | 56.7% | 40.0% |
| II-b-18 | 50.0% | 28.3% | 20.0% | 47.8% | 30.0% | 46.7% | 13.3% |
| II-b-19 | 43.3% | 25.0% | 10.0% | 36.7% | 18.9% | 30.0% | 10.0% |
| II-b-20 | 58.3% | 45.0% | 25.0% | 40.0% | 23.3% | 53.3% | 26.7% |
| II-b-21 | 46.7% | 26.7% | 16.7% | 44.4% | 32.2% | 60.0% | 40.0% |
| II-c-22 | 55.0% | 35.0% | 10.0% | 31.1% | 18.9% | 43.3% | 26.7% |
| II-c-23 | 45.0% | 25.0% | 11.7% | 36.7% | 20.0% | 40.0% | 13.3% |
| II-c-24 | 65.0% | 51.7% | 35.0% | 25.6% | 15.6% | 36.7% | 10.0% |
| II-c-25 | 50.0% | 28.3% | 16.7% | 33.3% | 13.3% | 40.0% | 20.0% |
| III-26 | 93.3% | 81.7% | 65.0% | 72.2% | 55.6% | 46.7% | 26.7% |
| III-27 | 75.0% | 53.3% | 26.7% | 60.0% | 41.1% | 43.3% | 23.3% |
| III-28 | 86.7% | 66.7% | 46.7% | 73.3% | 54.4% | 36.7% | 26.7% |
| III-29 | 76.7% | 61.7% | 45.0% | 64.4% | 48.9% | 56.7% | 40.0% |
| III-30 | 70.0% | 55.0% | 45.0% | 53.3% | 36.7% | 80.0% | 53.3% |
| imidacloprid | 100% | 100% | 100% | — | — | — | — |
| fenpyroximate | — | — | — | 100% | 100% | — | — |
| flubendiamide | — | — | — | — | — | 100% | 100% |
| Fungicidal Activity (%) at 50 μg/mL | ||||||
|---|---|---|---|---|---|---|
| Compd | R. sa | C. s | B. c | F. g | P. a | S. s |
| I-1 | 32% | 70% | 55% | 40% | 29% | 81% |
| I-2 | 52% | 56% | 0% | 41% | 35% | 55% |
| I-3 | 53% | 45% | 1% | 26% | 7% | 47% |
| I-4 | 24% | 58% | 18% | 24% | 18% | 60% |
| I-5 | 0% | 70% | 31% | 33% | 3% | 0% |
| II-a-6 | 22% | 45% | 70% | 40% | 13% | 78% |
| II-a-7 | 47% | 58% | 31% | 51% | 34% | 60% |
| II-a-8 | 58% | 77% | 29% | 45% | 27% | 75% |
| II-a-9 | 11% | 37% | 78% | 35% | 0% | 32% |
| II-a-10 | 32% | 81% | 75% | 49% | 19% | 81% |
| II-a-11 | 27% | 75% | 66% | 43% | 15% | 79% |
| II-a-12 | 61% | 71% | 36% | 58% | 35% | 68% |
| II-a-13 | 50% | 65% | 0% | 16% | 21% | 64% |
| II-a-14 | 23% | 45% | 11% | 16% | 15% | 47% |
| II-a-15 | 0% | 64% | 45% | 45% | 8% | 0% |
| II-a-16 | 2% | 6% | 16% | 15% | 0% | 39% |
| II-a-17 | 9% | 73% | 78% | 52% | 2% | 85% |
| II-b-18 | 0% | 0% | 8% | 35% | 0% | 0% |
| II-b-19 | 0% | 1% | 20% | 31% | 4% | 0% |
| II-b-20 | 5% | 0% | 7% | 8% | 0% | 0% |
| II-b-21 | 10% | 0% | 25% | 9% | 0% | 34% |
| II-c-22 | 13% | 1% | 19% | 44% | 0% | 18% |
| II-c-23 | 5% | 0% | 3% | 22% | 0% | 8% |
| II-c-24 | 10% | 0% | 10% | 1% | 0% | 0% |
| II-c-25 | 25% | 0% | 14% | 0% | 0% | 0% |
| III-26 | 22% | 42% | 85% | 40% | 5% | 76% |
| III-27 | 57% | 44% | 9% | 29% | 20% | 43% |
| III-28 | 48% | 55% | 12% | 42% | 33% | 52% |
| III-29 | 40% | 0% | 0% | 0% | 10% | 16% |
| III-30 | 27% | 31% | 54% | 38% | 1% | 55% |
| fluxapyroxad | 97% | 78% | 84% | 16% | 31% | 90% |
| Fungi | Compd | EC50 (μg/mL) |
|---|---|---|
| Cytospora sp. | II-a-10 | 36.169 |
| fluxapyroxad | 19.354 | |
| B. cinerea | III-26 | 22.531 |
| fluxapyroxad | 0.835 | |
| S. sclerotiorum | I-1 | 14.711 |
| II-a-10 | 17.683 | |
| II-a-17 | 8.114 | |
| fluxapyroxad | 0.334 |
| Compd | Conc. (μg/mL) | 5 Day Control Efficacy (%) | 9 Day Control Efficacy (%) |
|---|---|---|---|
| II-a-10 | 200 | 84.21% | 83.78% |
| 100 | 73.69% | 77.70% | |
| 50 | 71.06% | 73.65% | |
| 25 | 52.64% | 59.46% | |
| fluxapyroxad | 200 | 89.48% | 87.16% |
| 100 | 81.58% | 78.38% | |
| 50 | 76.32% | 76.35% | |
| 25 | 55.27% | 53.38% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.-Y.; Feng, T.; Liu, Q.; Li, H.-T.; Wei, W.; Shi, R.-C.; Cao, Y.-M.; Liu, S.-Z. Design, Synthesis, Fungicidal and Insecticidal Activities of Novel Diamide Compounds Combining Pyrazolyl and Polyfluoro-Substituted Phenyl into Alanine or 2-Aminobutyric Acid Skeletons. Molecules 2023, 28, 561. https://doi.org/10.3390/molecules28020561
Xu Z-Y, Feng T, Liu Q, Li H-T, Wei W, Shi R-C, Cao Y-M, Liu S-Z. Design, Synthesis, Fungicidal and Insecticidal Activities of Novel Diamide Compounds Combining Pyrazolyl and Polyfluoro-Substituted Phenyl into Alanine or 2-Aminobutyric Acid Skeletons. Molecules. 2023; 28(2):561. https://doi.org/10.3390/molecules28020561
Chicago/Turabian StyleXu, Zhi-Yuan, Tong Feng, Qing Liu, Hui-Ting Li, Wei Wei, Rong-Chuan Shi, Yi-Ming Cao, and Shang-Zhong Liu. 2023. "Design, Synthesis, Fungicidal and Insecticidal Activities of Novel Diamide Compounds Combining Pyrazolyl and Polyfluoro-Substituted Phenyl into Alanine or 2-Aminobutyric Acid Skeletons" Molecules 28, no. 2: 561. https://doi.org/10.3390/molecules28020561
APA StyleXu, Z.-Y., Feng, T., Liu, Q., Li, H.-T., Wei, W., Shi, R.-C., Cao, Y.-M., & Liu, S.-Z. (2023). Design, Synthesis, Fungicidal and Insecticidal Activities of Novel Diamide Compounds Combining Pyrazolyl and Polyfluoro-Substituted Phenyl into Alanine or 2-Aminobutyric Acid Skeletons. Molecules, 28(2), 561. https://doi.org/10.3390/molecules28020561






