Osthole Antagonizes Microglial Activation in an NRF2-Dependent Manner
Abstract
1. Introduction
2. Results
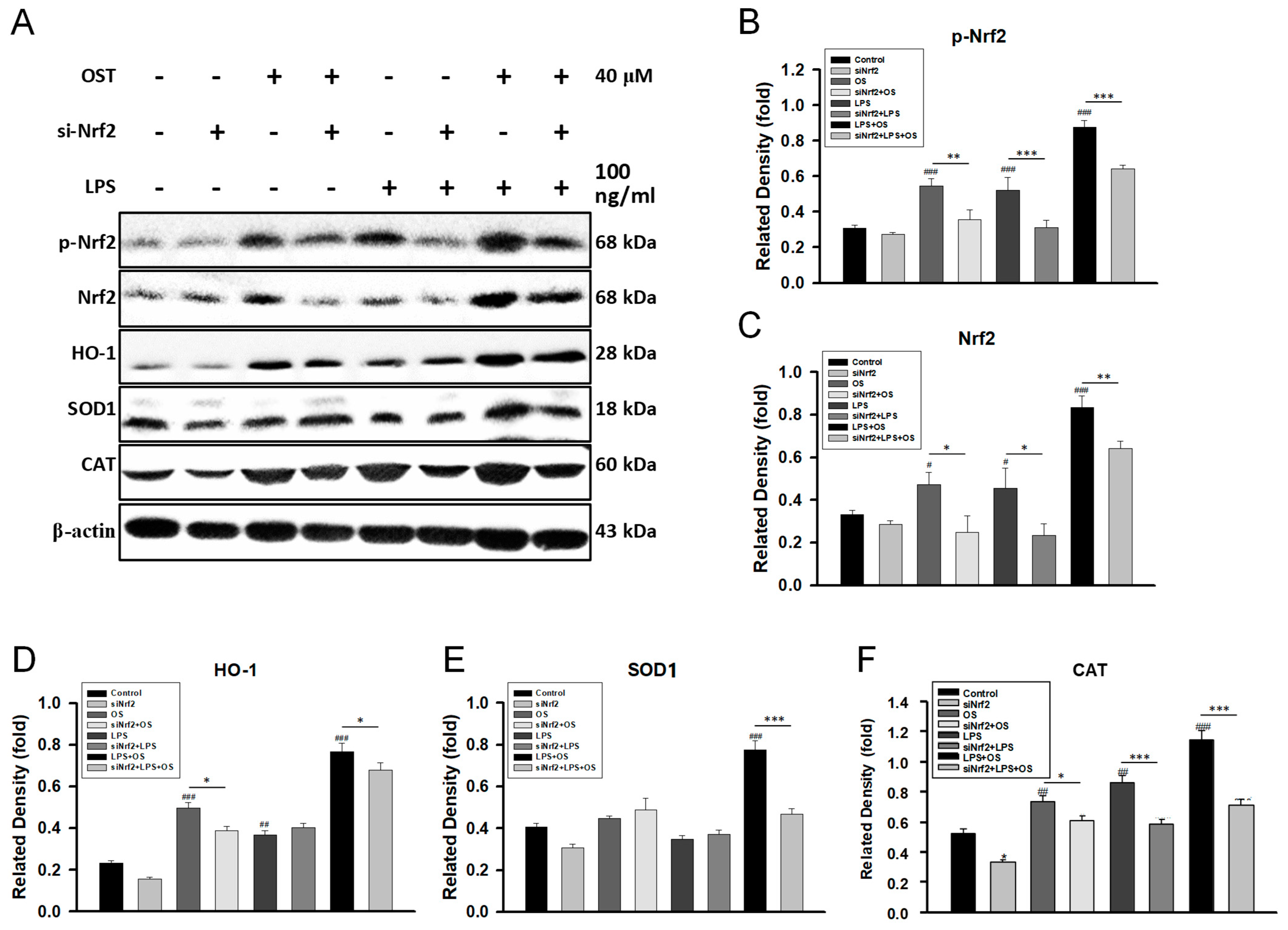
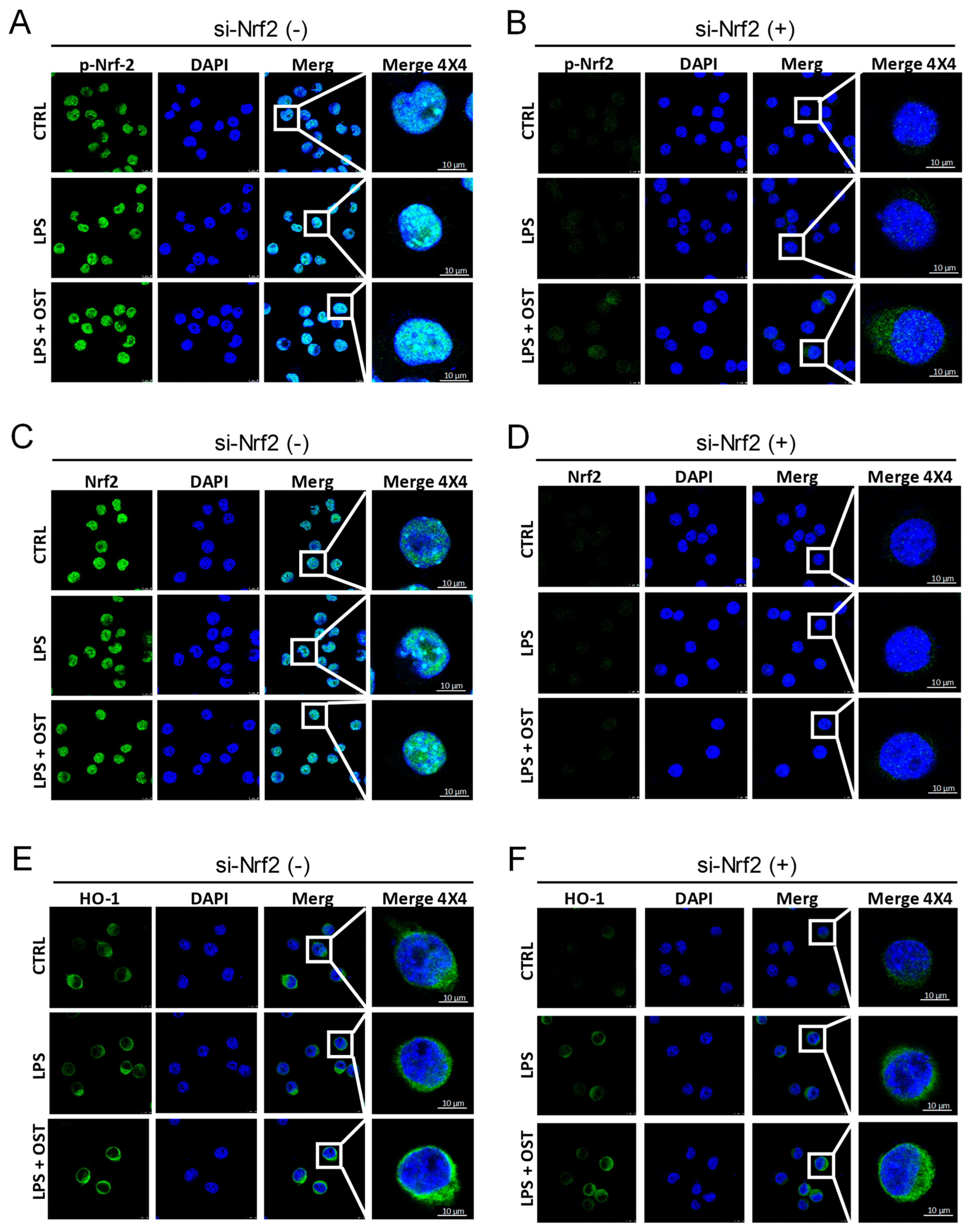
2.1. OST Activated the Nrf2/HO-1 Signaling Pathway in LPS-Stimulated BV2 Cells
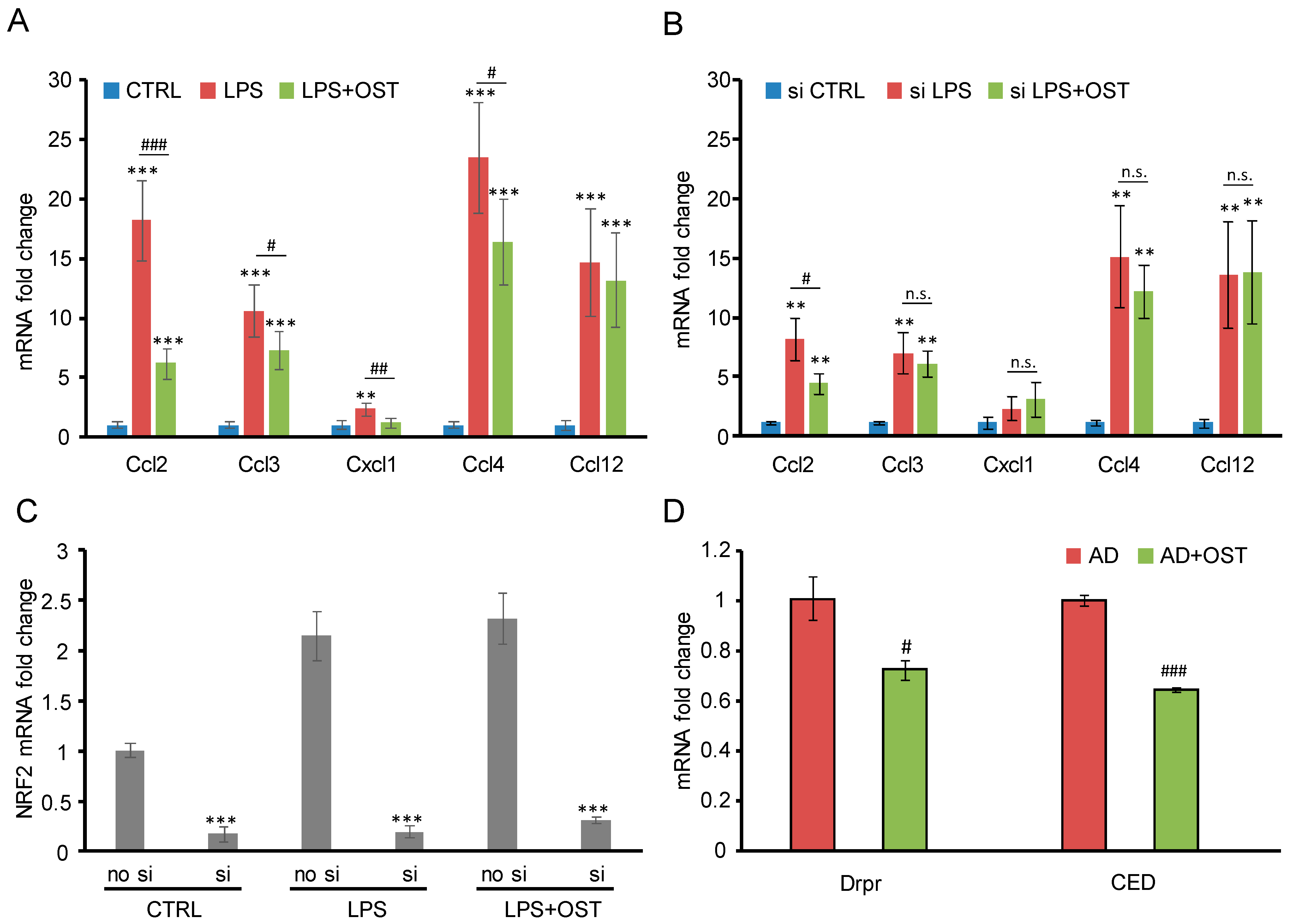
2.2. Nrf2 siRNA Suppressed OST-Induced Antagonism toward Microglial Activation
2.3. OST Ameliorated Alzheimer’s Disease Symptoms in Drosophila Animal Model
3. Discussion
4. Materials and Methods
4.1. Osthole (OST)
4.2. Western Blots
4.3. Immunofluorescence (IF) Assay
4.4. Nrf2 siRNA Transfection
4.5. SOD/Catalase Activity
4.6. Lipid Hydroperoxide (LPO) Assay
4.7. Quantitative PCR
4.8. Fly Stock Maintenance, Lifespan and Antigeotaxis Assays
4.9. Drosophila Learning and Memory Platform (T-Maze)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, J.; Matsumoto, Y.; Kreutzberg, G.W. Microglia: Intrinsic immuneffector cell of the brain. Brain Res. Rev. 1995, 20, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.; Ramalho, T.C. Future therapeutic perspectives into the Alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional regulation by Nrf2. Antioxid. Redox. Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a therapeutic target in neurodegenerative diseases. ASN Neuro 2020, 12, 1759091419899782. [Google Scholar] [CrossRef]
- Li, Y.M.; Jia, M.; Li, H.Q.; Zhang, N.D.; Wen, X.; Rahman, K.; Zhang, Q.Y.; Qin, L.P. Cnidium monnieri: A Review of Traditional Uses, Phytochemical and Ethnopharmacological Properties. Am. J. Chin. Med. 2015, 43, 835–877. [Google Scholar] [CrossRef]
- Dong, W.P.; Zhang, Z.; Liu, Z.J.; Liu, H.; Wang, X.Y.; Bi, S.H.; Wang, X.W.; Ma, T.; Zhang, W.D. Protective effects of osthole, a natural derivative of coumarin, against intestinal ischemia-reperfusion injury in mice. Int. J. Mol. Med. 2013, 31, 1367–1374. [Google Scholar] [CrossRef]
- Tang, D.Z.; Hou, W.; Zhou, Q.A.; Zhang, M.J.; Holz, J.; Sheu, T.J.; Li, T.F.; Cheng, S.D.; Shi, Q.; Harris, S.E.; et al. Osthole Stimulates Osteoblast Differentiation and Bone Formation by Activation of beta-Catenin-BMP Signaling. J. Bone Min. Res. 2010, 25, 1234–1245. [Google Scholar] [CrossRef]
- Liang, H.J.; Suk, F.M.; Wang, C.K.; Hung, L.F.; Liu, D.Z.; Chen, N.Q.; Chen, Y.C.; Chang, C.C.; Liang, Y.C. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem. Biol. Interact. 2009, 181, 309–315. [Google Scholar] [CrossRef]
- Huang, R.L.; Chen, C.C.; Huang, Y.L.; Hsieh, D.J.; Hu, C.P.; Chen, C.F.; Chang, C.M. Osthole increases glycosylation of hepatitis B surface antigen and suppresses the secretion of hepatitis B virus in vitro. Hepatology 1996, 24, 508–515. [Google Scholar] [CrossRef]
- Zhang, L.R.; Jiang, G.R.; Yao, F.; He, Y.; Liang, G.Q.; Zhang, Y.S.; Hu, B.; Wu, Y.; Li, Y.S.; Liu, H.Y. Growth Inhibition and Apoptosis Induced by Osthole, A Natural Coumarin, in Hepatocellular Carcinoma. PLoS ONE 2012, 7, e37865. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 1. [Google Scholar] [CrossRef]
- Du, M.; Sun, Z.; Lu, Y.; Li, Y.-Z.; Xu, H.-R.; Zeng, C.-Q. Osthole inhibits proliferation and induces apoptosis in BV-2 microglia cells in kainic acid-induced epilepsy via modulating PI3K/AKt/mTOR signalling way. Pharm. Biol. 2019, 57, 238–244. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhou, L.; Wang, X.; Lian, Q. Anti-inflammatory effect and mechanism of osthole in rats. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2005, 28, 1002–1006. [Google Scholar]
- Wang, Y.; Zhou, Y.; Wang, X.; Zhen, F.; Chen, R.; Geng, D.; Yao, R. Osthole alleviates MPTP-induced Parkinson’s disease mice by suppressing Notch signaling pathway. Int. J. Neurosci. 2019, 129, 833–841. [Google Scholar] [CrossRef]
- Kong, L.; Yao, Y.; Xia, Y.; Liang, X.; Ni, Y.; Yang, J. Osthole alleviates inflammation by down-regulating NF-κB signaling pathway in traumatic brain injury. Immunopharmacol. Immunotoxicol. 2019, 41, 349–360. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gong, Q.; Shi, J.; Li, F. Osthole improves cognitive function of vascular dementia rats: Reducing Aβ deposition via inhibition NLRP3 inflammasome. Biol. Pharm. Bull. 2020, 43, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Meng, X.; Liu, F.; Wang, F.; Yang, J.; Wang, H.; Xie, G. Protective effects of osthole against inflammation induced by lipopolysaccharide in BV2 cells. Mol. Med. Rep. 2018, 17, 4561–4566. [Google Scholar] [CrossRef] [PubMed]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX: Altern. Anim. Exp. 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635. [Google Scholar] [CrossRef] [PubMed]
- Glezer, I.; Simard, A.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef]
- Ekdahl, C.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef]
- Finelli, A.; Kelkar, A.; Song, H.-J.; Yang, H.; Konsolaki, M. A model for studying Alzheimer’s Aβ42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. 2004, 26, 365–375. [Google Scholar] [CrossRef]
- MacDonald, J.M.; Beach, M.G.; Porpiglia, E.; Sheehan, A.E.; Watts, R.J.; Freeman, M.R. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 2006, 50, 869–881. [Google Scholar] [CrossRef]
- Hu, Y.; Wen, Q.P.; Liang, W.B.; Kang, T.G.; Ren, L.; Zhang, N.; Zhao, D.; Sun, D.; Yang, J.X. Osthole Reverses Beta-Amyloid Peptide Cytotoxicity on Neural Cells by Enhancing Cyclic AMP Response Element-Binding Protein Phosphorylation. Biol. Pharm. Bull. 2013, 36, 1950–1958. [Google Scholar] [CrossRef]
- Yao, Y.J.; Gao, Z.; Liang, W.B.; Kong, L.; Jiao, Y.A.; Li, S.H.; Tao, Z.Y.; Yan, Y.H.; Yang, J.X. Osthole promotes neuronal differentiation and inhibits apoptosis via Wnt/beta-catenin signaling in an Alzheimer’s disease model. Toxicol. Appl. Pharm. 2015, 289, 474–481. [Google Scholar] [CrossRef]
- Dong, X.H.; Zhang, D.S.; Zhang, L.; Li, W.; Meng, X.Y. Osthole improves synaptic plasticity in the hippocampus and cognitive function of Alzheimer’s disease rats via regulating glutamate. Neural. Regen. Res. 2012, 7, 2325–2332. [Google Scholar] [CrossRef]
- Liu, H.; Xue, X.H.; Shi, H.J.; Qi, L.F.; Gong, D.R. Osthole Upregulates BDNF to Enhance Adult Hippocampal Neurogenesis in APP/PS1 Transgenic Mice. Biol. Pharm. Bull. 2015, 38, 1439–1449. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef]
- Mohler, J.; Mahaffey, J.W.; Deutsch, E.; Vani, K. Control of Drosophila head segment identity by the bZIP homeotic gene cnc. Development 1995, 121, 237–247. [Google Scholar] [CrossRef]
- Chang, C.-I.; Hu, W.-C.; Shen, C.-P.; Hsu, B.-D.; Lin, W.-Y.; Sung, P.-J.; Wang, W.-H.; Wu, J.-B.; Kuo, Y.-H. 8-Alkylcoumarins from the fruits of Cnidium monnieri protect against hydrogen peroxide induced oxidative stress damage. Int. J. Mol. Sci. 2014, 15, 4608–4618. [Google Scholar] [CrossRef]
- Liao, P.-C.; Chien, S.-C.; Ho, C.-L.; Wang, E.I.-C.; Lee, S.-C.; Kuo, Y.-H.; Jeyashoke, N.; Chen, J.; Dong, W.-C.; Chao, L.K. Osthole regulates inflammatory mediator expression through modulating NF-κB, mitogen-activated protein kinases, protein kinase C, and reactive oxygen species. J. Agric. Food Chem. 2010, 58, 10445–10451. [Google Scholar] [CrossRef]
- Leung, Y.-M.; Kuo, Y.-H.; Chao, C.-C.; Tsou, Y.-H.; Chou, C.-H.; Lin, C.-H.; Wong, K.-L. Osthol is a use-dependent blocker of voltage-gated Na+ channels in mouse neuroblastoma N2A cells. Planta Med. 2010, 76, 34–40. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Chen, D.-R.; Lin, W.-J.; Lin, Y.-H.; Chen, J.-Y.; Kuo, Y.-H.; Chung, J.-G.; Hsia, T.-C.; Hsieh, W.-T. Ergosta-7, 9 (11), 22-trien-3β-ol Attenuates Inflammatory Responses via Inhibiting MAPK/AP-1 Induced IL-6/JAK/STAT Pathways and Activating Nrf2/HO-1 Signaling in LPS-Stimulated Macrophage-like Cells. Antioxidants 2021, 10, 1430. [Google Scholar] [CrossRef]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of Nrf2 pathway contributes to neuroprotection by the dietary flavonoid tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar] [CrossRef]
- Bae, J.H.; Yang, M.J.; Jeong, S.-h.; Kim, J.; Hong, S.P.; Kim, J.W.; Kim, Y.H.; Koh, G.Y. Gatekeeping role of Nf2/Merlin in vascular tip EC induction through suppression of VEGFR2 internalization. Sci. Adv. 2022, 8, eabn2611. [Google Scholar] [CrossRef] [PubMed]
- Waddell, S.; Quinn, W.G. Flies, genes, and learning. Annu. Rev. Neurosci. 2001, 24, 1283–1309. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.; Shim, W.-S. Caffeic acid phenethyl ester inhibits pseudo-allergic reactions via inhibition of MRGPRX2/MrgprB2-dependent mast cell degranulation. Arch. Pharmacal. Res. 2022, 45, 644–657. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-H.; Chen, M.-Y.; Kuo, Y.-H.; Cheng, J.; Chang, L.-Z.; Chang, M.-S.; Chuang, T.-N.; Hsieh, W.-T.; Xiao, Y.-R.; Wu, B.-T.; et al. Osthole Antagonizes Microglial Activation in an NRF2-Dependent Manner. Molecules 2023, 28, 507. https://doi.org/10.3390/molecules28020507
Liu C-H, Chen M-Y, Kuo Y-H, Cheng J, Chang L-Z, Chang M-S, Chuang T-N, Hsieh W-T, Xiao Y-R, Wu B-T, et al. Osthole Antagonizes Microglial Activation in an NRF2-Dependent Manner. Molecules. 2023; 28(2):507. https://doi.org/10.3390/molecules28020507
Chicago/Turabian StyleLiu, Chuan-Hsiu, Mei-Ying Chen, Yueh-Hsiung Kuo, Jack Cheng, Li-Zhong Chang, Meng-Shiun Chang, Tsai-Ni Chuang, Wen-Tsong Hsieh, Yan-Ru Xiao, Bor-Tsang Wu, and et al. 2023. "Osthole Antagonizes Microglial Activation in an NRF2-Dependent Manner" Molecules 28, no. 2: 507. https://doi.org/10.3390/molecules28020507
APA StyleLiu, C.-H., Chen, M.-Y., Kuo, Y.-H., Cheng, J., Chang, L.-Z., Chang, M.-S., Chuang, T.-N., Hsieh, W.-T., Xiao, Y.-R., Wu, B.-T., Lin, W.-Y., & Liu, H.-P. (2023). Osthole Antagonizes Microglial Activation in an NRF2-Dependent Manner. Molecules, 28(2), 507. https://doi.org/10.3390/molecules28020507






