Effect of Mg on the Structural, Optical and Thermoluminescence Properties of Li3Al3(BO3)4: Shift in Main Glow Peak
Abstract
1. Introduction
2. Experimental Route
3. Results and Discussions
3.1. Structural Analysis
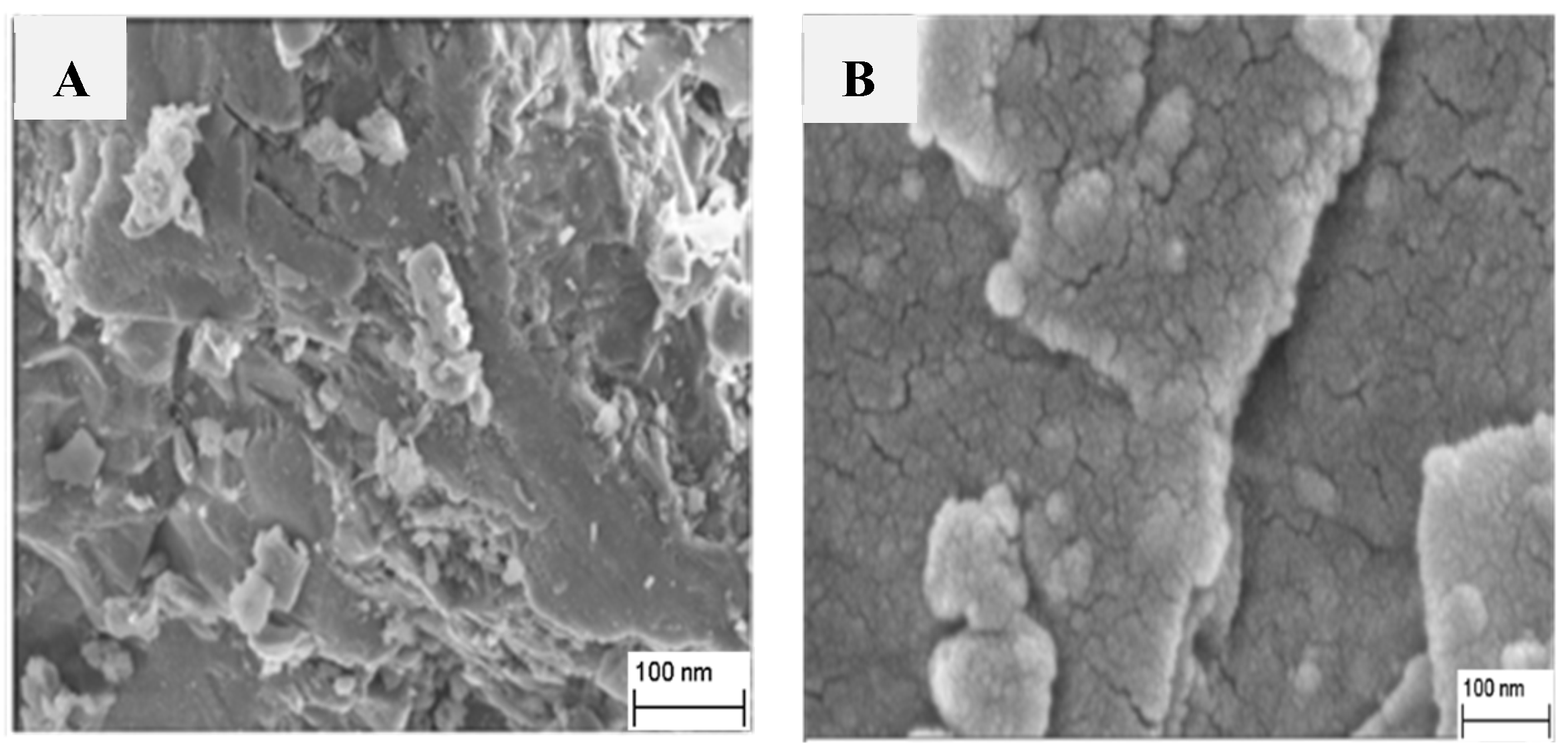
3.2. Morphology Survey
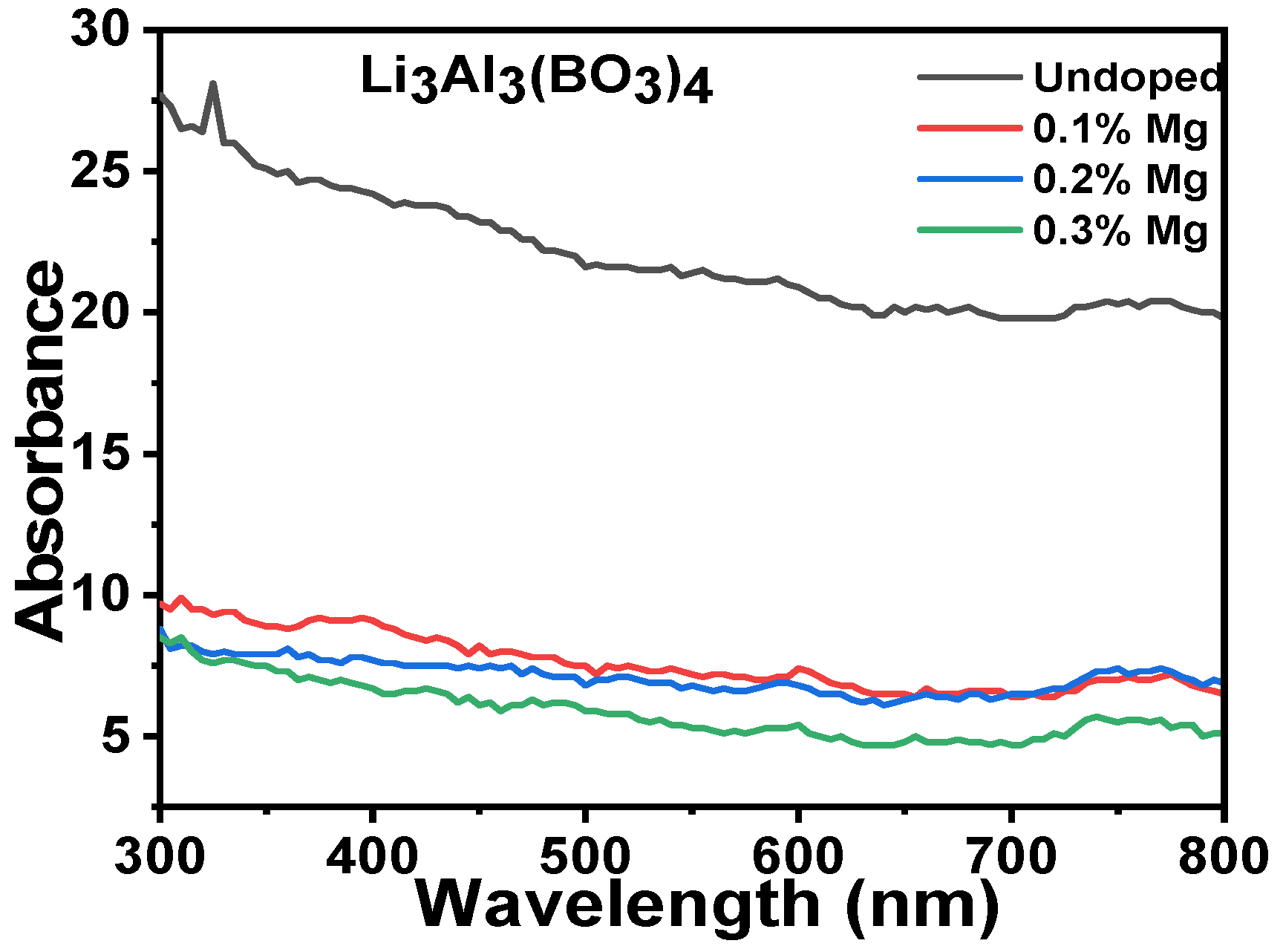
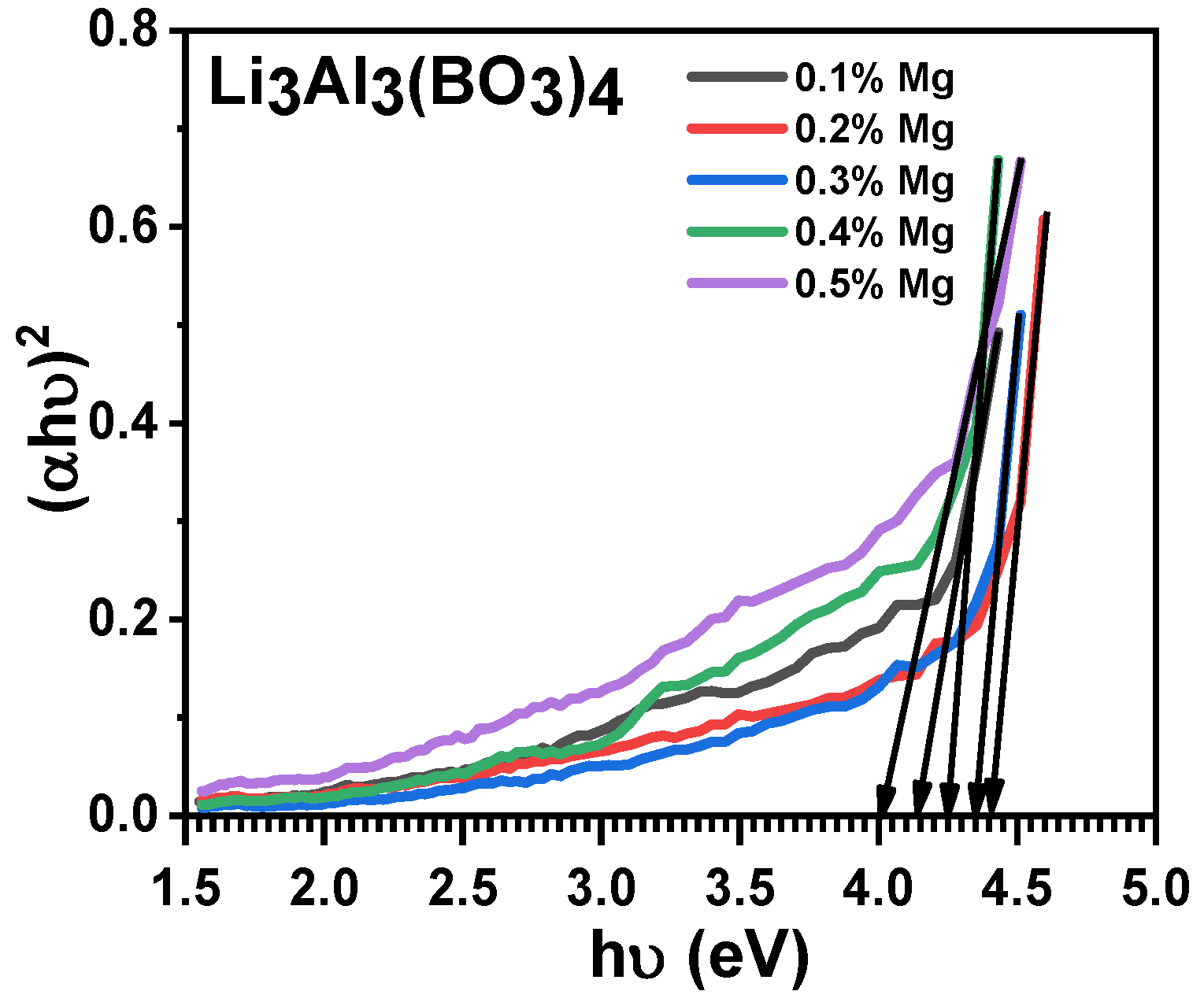
3.3. Optical Properties
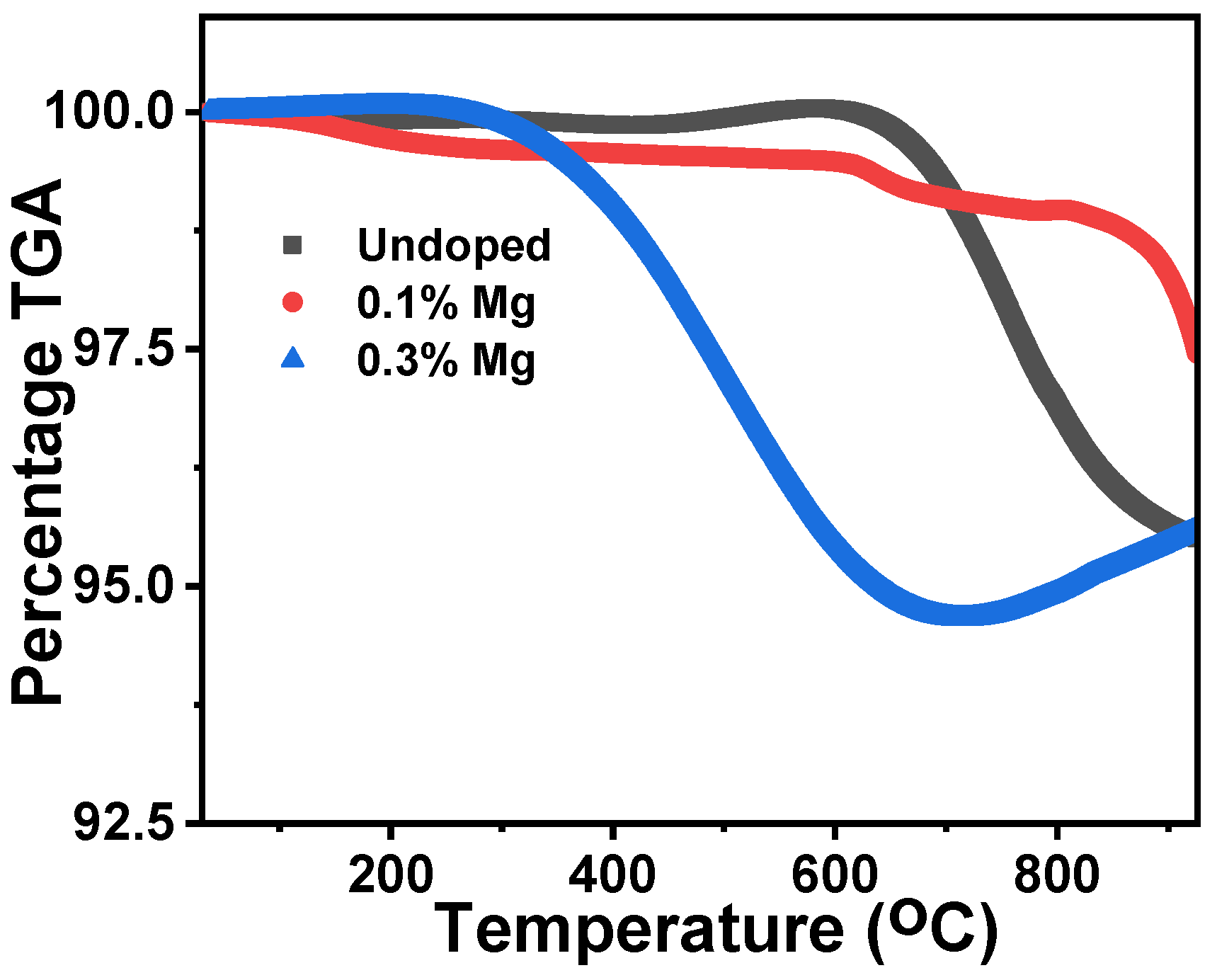
3.4. Thermal Gravimetric Analysis (TGA)
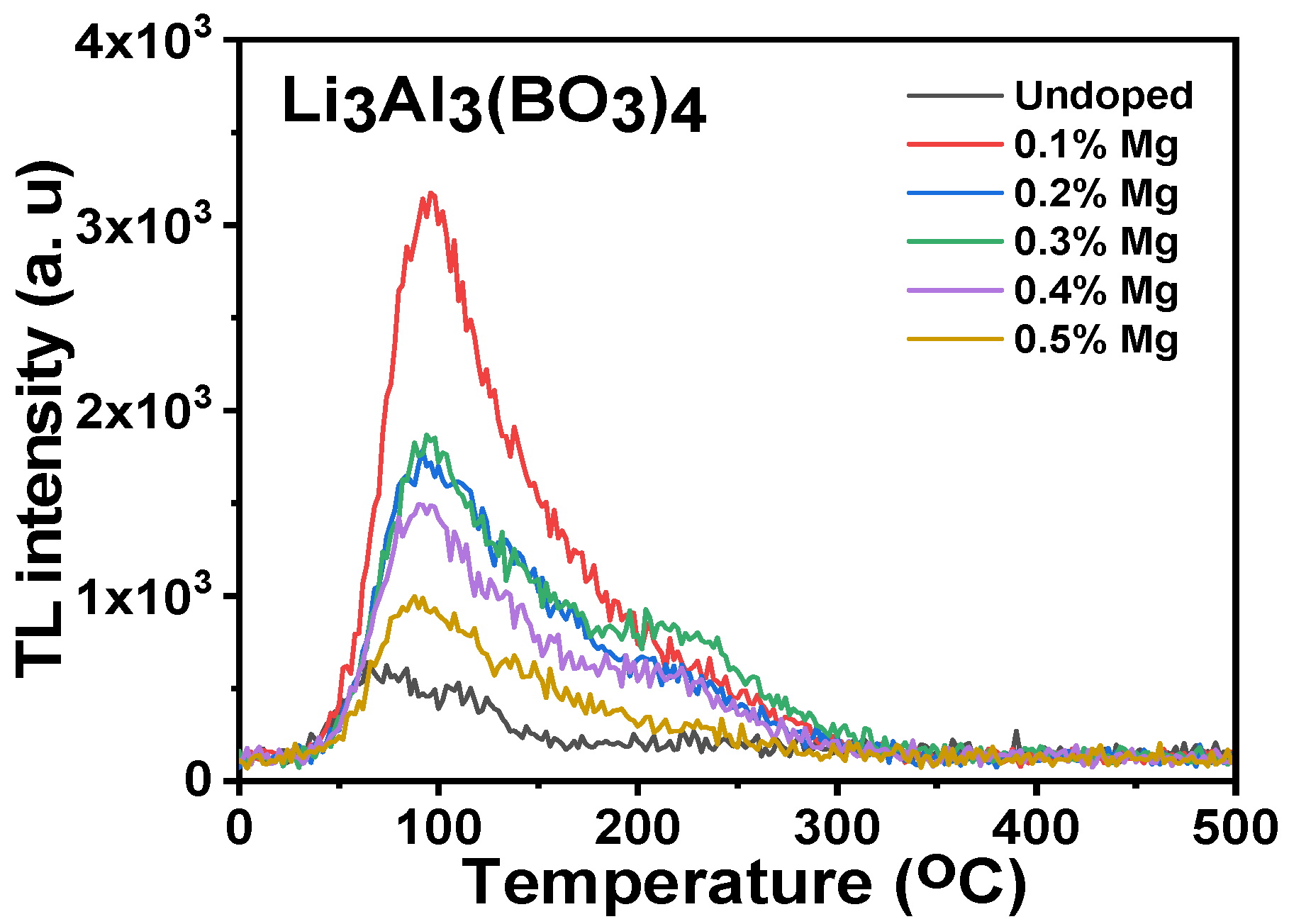
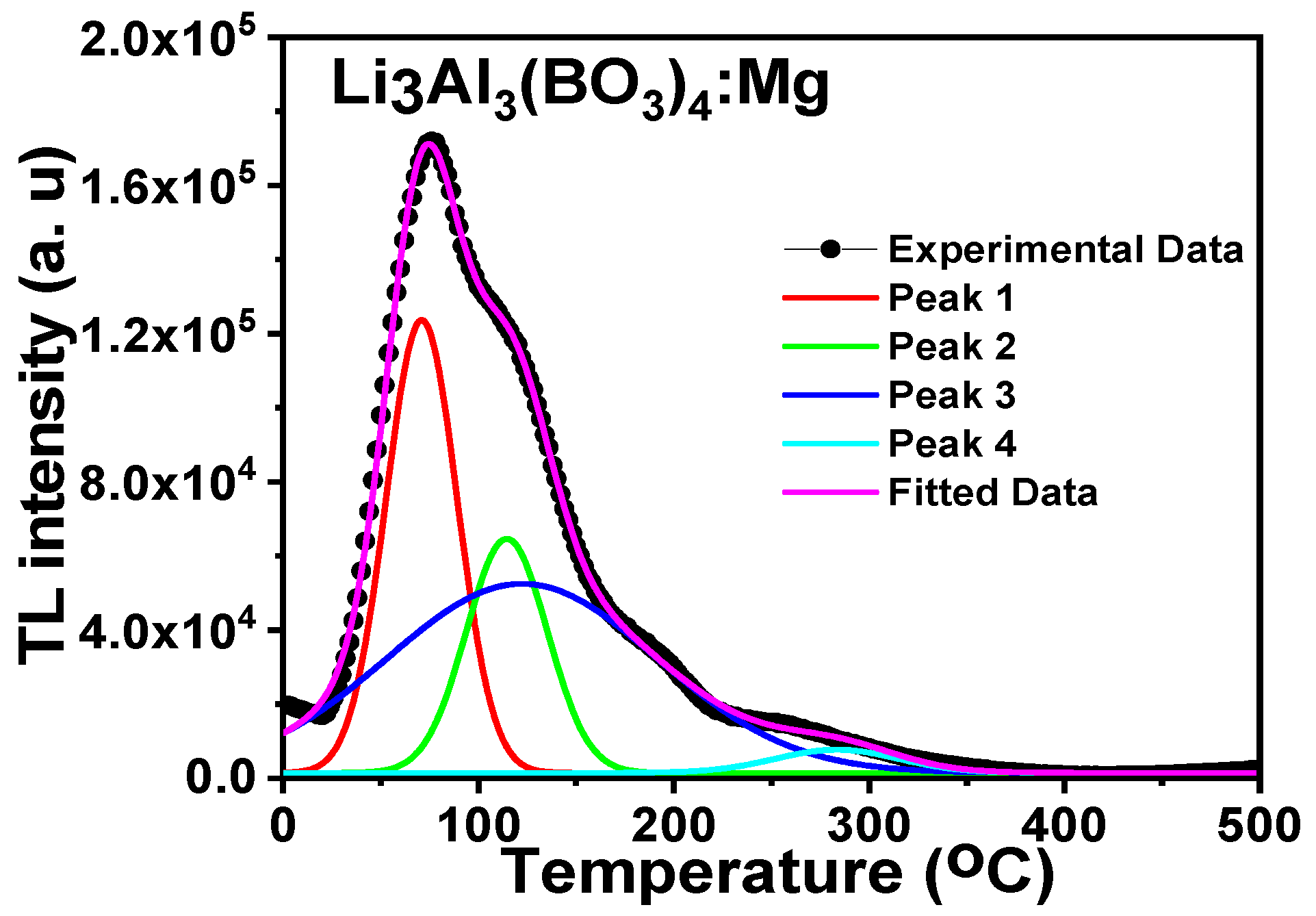
3.5. Thermoluminescence
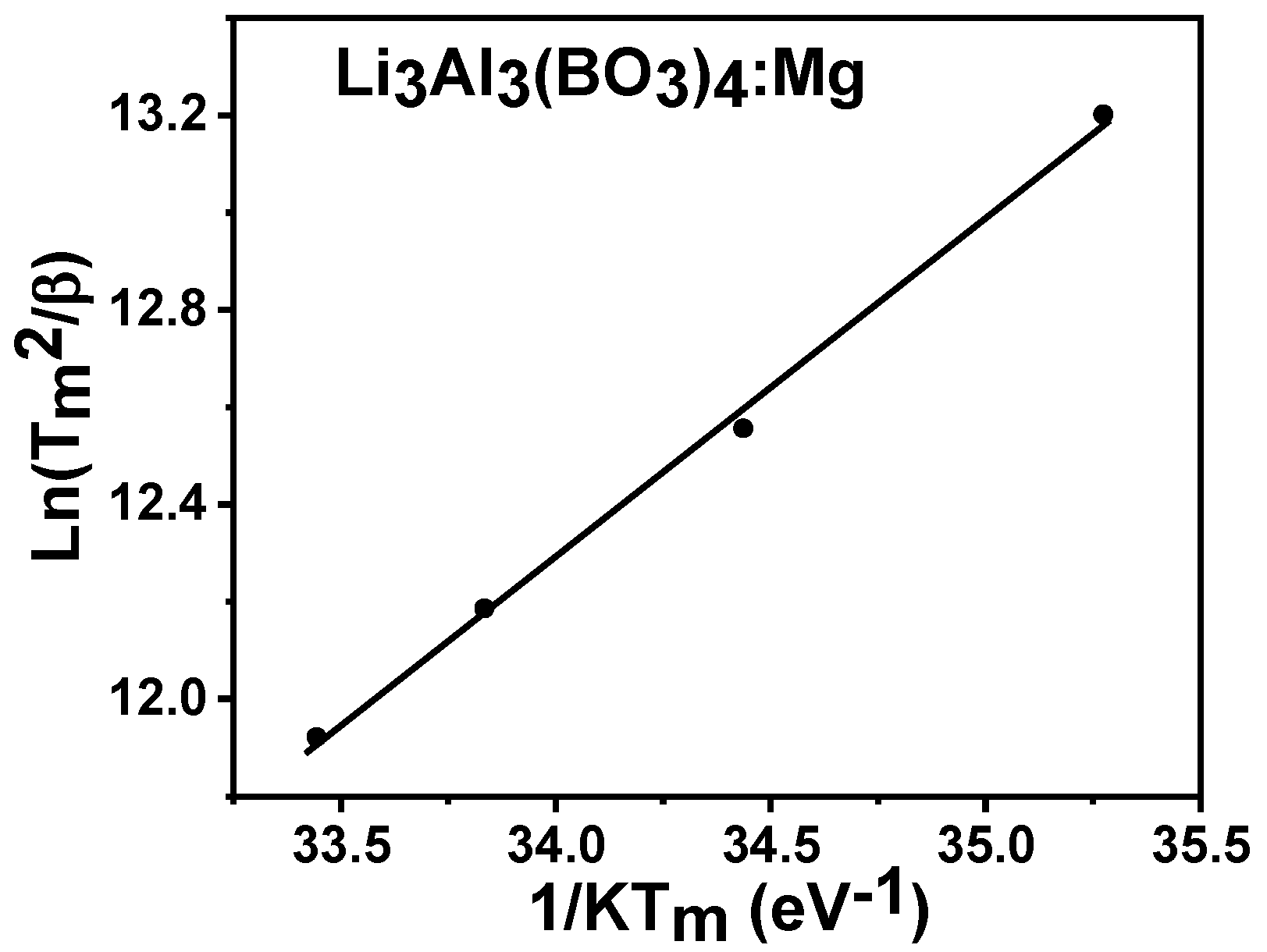
3.6. Kinetic Analysis
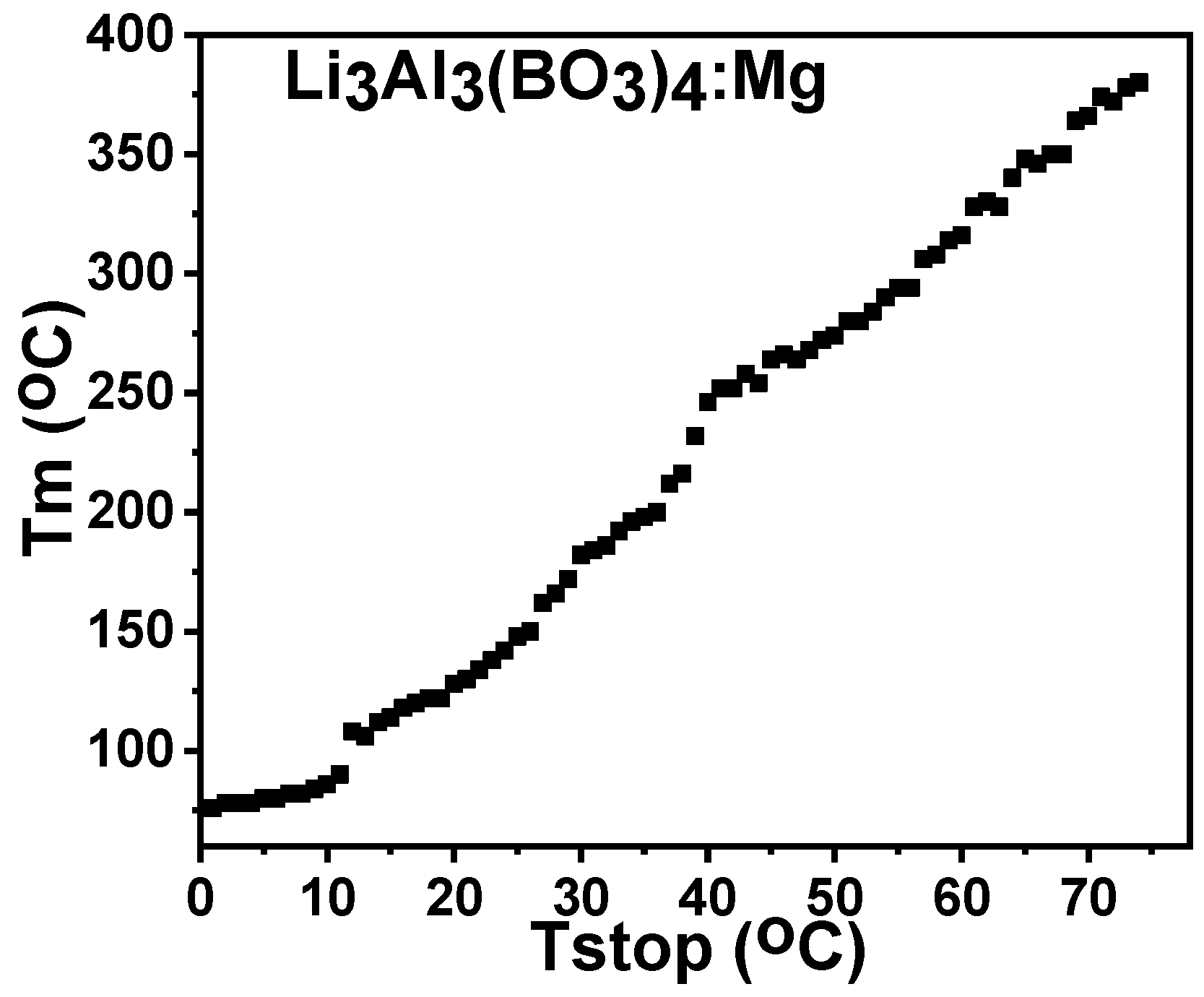
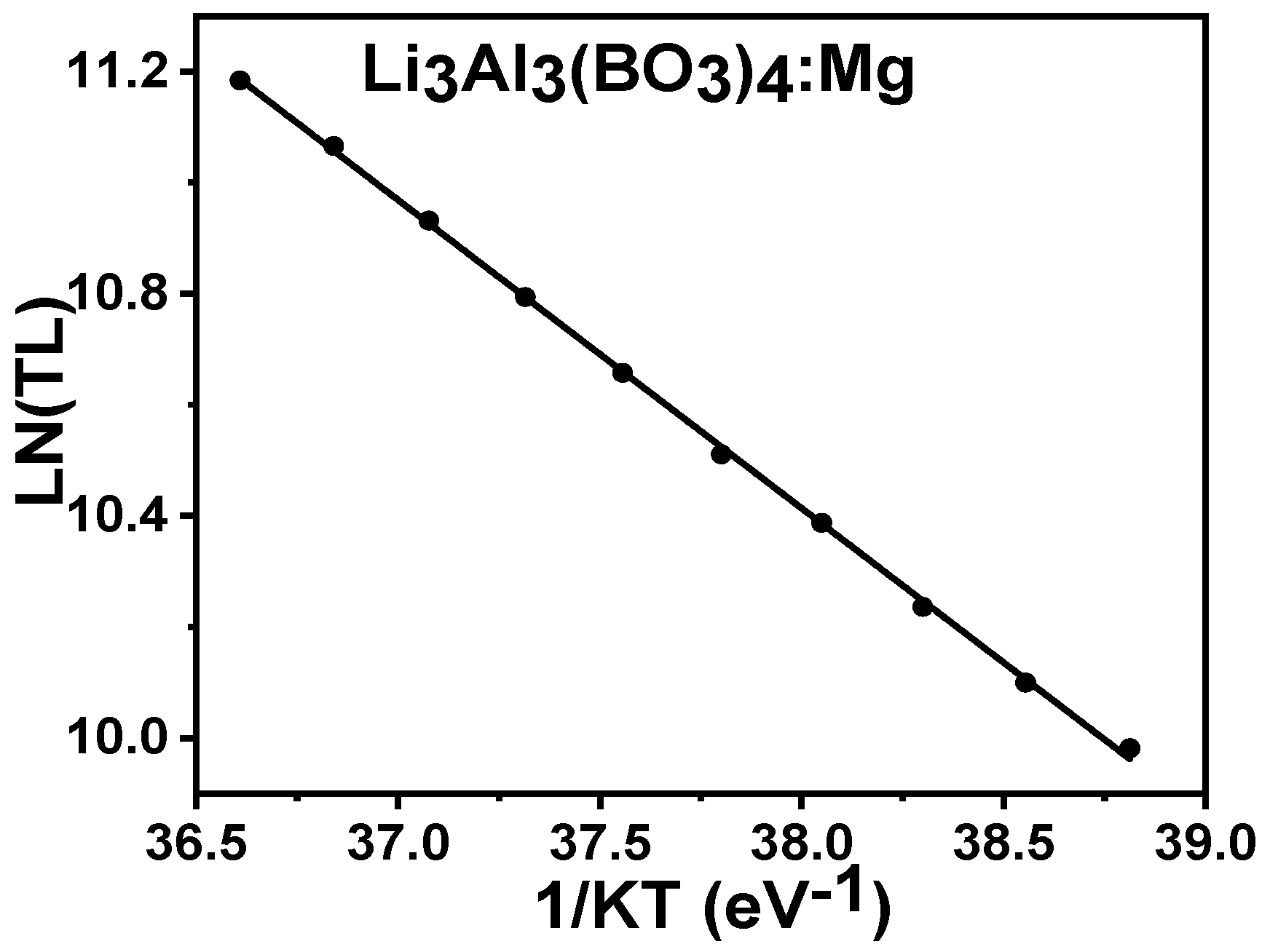
3.6.1. Initial Rise Method
3.6.2. Curve Deconvolution
3.6.3. Variable Heating Rate (VHR) Method
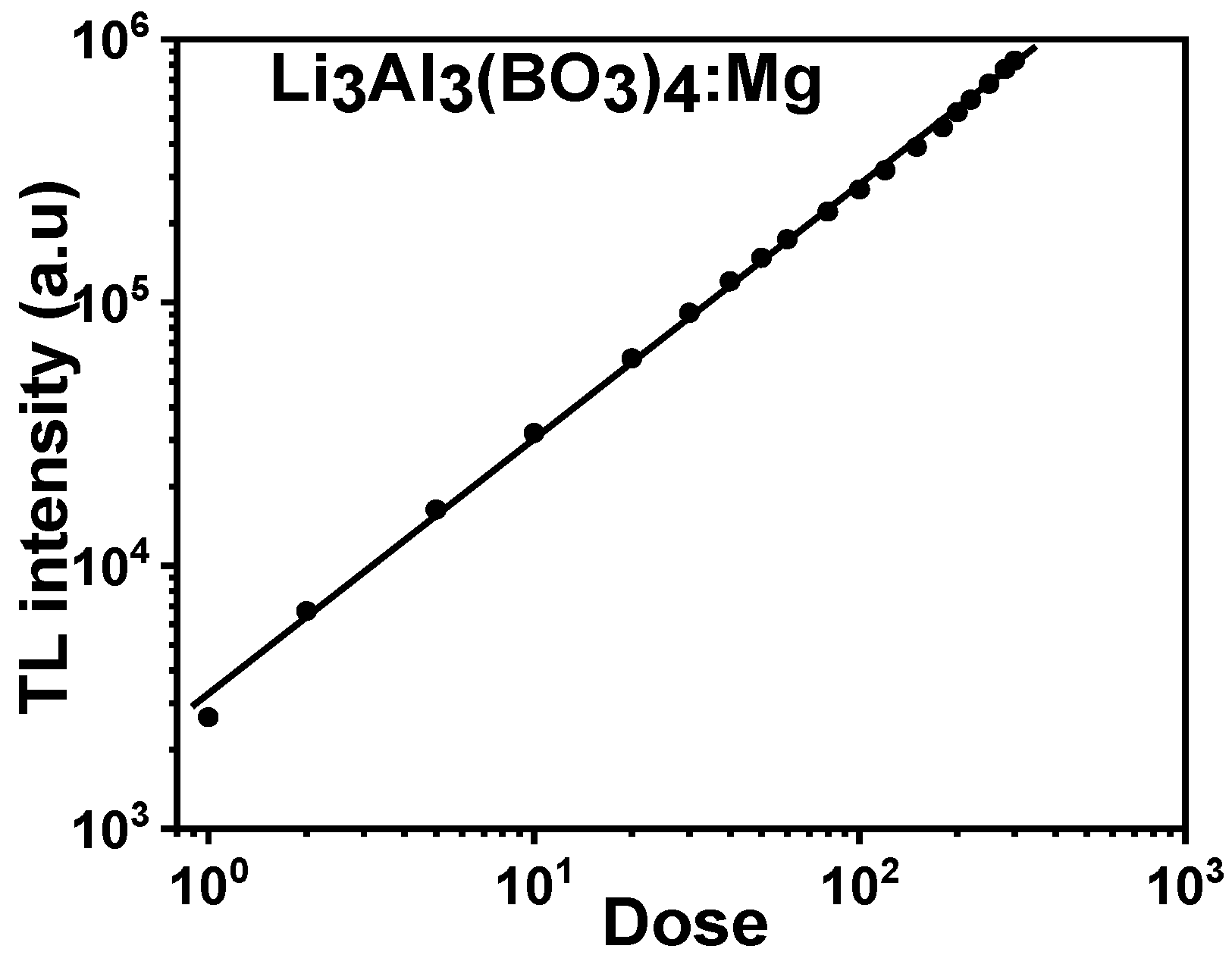
3.7. Dose–Response
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ishii, Y.; Arai, K.; Namikawa, H.; Tanaka, M.; Negishi, A.; Handa, T. Preparation of Cerium-Activated Silica Glasses: Phosphorus and Aluminum Codoping Effects on Absorption and Fluorescence Properties. J. Am. Ceram. Soc. 1987, 70, 72. [Google Scholar] [CrossRef]
- Chiodni, N.; Fasoli, M.; Martini, M.; Rosetta, E.; Spinolo, G.; Vedda, A. High-efficiency SiO2: Ce3+ glass scintillators. Appl. Phys. Lett. 2002, 81, 4374. [Google Scholar] [CrossRef]
- Friebele, E.J.; Nikl, M.; Solovieva, N.; Baraldi, A.; Capelletti, R. Radiation protection of fiber optic materials: Effect of cerium doping on the radiation-induced absorption. Appl. Phys. Lett. 1975, 27, 210. [Google Scholar] [CrossRef]
- Korzhik, M.V.; Trower, W.P. Origin of scintillation in cerium-doped oxide crystals. Appl. Phys. Lett. 1995, 66, 2327. [Google Scholar] [CrossRef]
- Ike, P.O.; Folley, D.E.; Agwu, K.K.; Chithambo, M.L.; Chikwembani, S.; Fabian, I. EzemaInfluence of dysprosium doping on the structural, thermoluminescence and optical properties of lithium aluminium borate. J. Luminescence 2021, 233, 117932. [Google Scholar] [CrossRef]
- Hawthorne, F. The structure hierarchy hypothesis. Mineral. Mag. 2014, 78, 957–1027. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Filatov, S.K. High-temperature borate crystal chemistry. Z. Für Krist. Cryst. Mater. 2013, 228, 395–428. [Google Scholar] [CrossRef]
- Wright, A. Borate structures: Crystalline and vitreous. Phys. Chem. Glass Eur. J. Glass Sci. Technol. B 2010, 51, 1–39. [Google Scholar]
- Belokoneva, E. Systematic, properties, and structure predictions of new borate materials. Cryst. Res. Technol. 2008, 43, 1173–1182. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Filatov, S.K. Strong anisotropic thermal expansion in borates. Phys. Stat. Solid. 2008, 245, 2469–2476. [Google Scholar] [CrossRef]
- Bubnova, R.; Filatov, S. High-Temperature Crystal Chemistry Borates and Borosilicates. Science 2008, 228, 395–428. [Google Scholar]
- Yuan, G.; Xue, D. Crystal chemistry of borates: The classification and algebraic description by topological type of fundamental building blocks. Acta Crystallogr. 2007, 63, 353–362. [Google Scholar] [CrossRef]
- Yu, D.; Xue, D. Bond analyses of borates from the Inorganic Crystal Structure Database. Acta Crystallogr. 2006, 62, 702–709. [Google Scholar] [CrossRef]
- Belokoneva, E. Borate crystal chemistry in terms of the extended OD theory: Topology and symmetry analysis. Crystallogr. Rev. 2005, 11, 151–198. [Google Scholar] [CrossRef]
- Parthé, E. New examples of crystallized and amorphous borates where the ratio of BO3 triangles to BO4 tetrahedra can be calculated from the chemical formula. J. Alloys Compd. 2004, 367, 126–131. [Google Scholar] [CrossRef]
- Touboul, M.; Penin, N.; Nowogrocki, G. Borates: A survey of main trends concerning crystal-chemistry, polymorphism and dehydration process of alkaline and pseudo-alkaline borates. Solid State Sci. 2003, 5, 1327–1342. [Google Scholar] [CrossRef]
- Parthé, E. Calculation of the BO3 triangle to BO4 tetrahedron ratio in borates. Z. Für Krist. 2002, 217, 179–200. [Google Scholar] [CrossRef]
- Becker, P. A contribution to borate crystal chemistry: Rules for the occurrence of polyborate anion types. Z. Für Krist. 2001, 216, 523–533. [Google Scholar] [CrossRef]
- Filatov, S.; Bubnova, R. Borate Crystal Chemistry. Phys. Chem. Glasses 2000, 41, 216–224. [Google Scholar]
- Grice, J.; Burns, P.; Hawthorne, F. Borate minerals. II. A hierarchy of structures based upon the borate fundamental building block. Can. Mineral. 1999, 37, 731–762. [Google Scholar]
- Strunz, H. Classification of borate minerals. Eur. J. Mineral. 1997, 9, 225–232. [Google Scholar] [CrossRef]
- Hawthorne, F.; Burns, P.; Grice, J. The crystal chemistry of boron. Rev. Mineral. 1996, 33, 41–116. [Google Scholar]
- Burns, P.; Grice, J.; Hawthorne, F. Borate minerals. I. Polyhedral clasters and fundamental building blocks. Can. Mineral. 1995, 33, 1131–1151. [Google Scholar]
- Bubnova, R.; Volkov, S.; Albert, B.; Filatov, S. Borates—Crystal Structures of Prospective Nonlinear Optical Materials: High Anisotropy of the Thermal Expansion Caused by Anharmonic Atomic Vibrations. Crystals 2017, 7, 93. [Google Scholar] [CrossRef]
- Kim, K.H.; Hummel, F.A. Studies in lithium oxide systems: XII, Li2O-B2O3-Al2O3. J. Am. Ceram. Soc. 1962, 45, 487–489. [Google Scholar] [CrossRef]
- He, M.; Chen, X.L.; Hu, B.Q.; Zhou, T.; Xu, Y.P.; Xu, T. The ternary system Li2O–Al2O3–B2O3: Compounds and phase relations. J. Solid State Chem. 2002, 165, 187–192. [Google Scholar] [CrossRef]
- Ohashi, M.; Ogawa, H.; Kan, A.; Tanaka, E. Microwave dielectric properties of low temperature sintered Li3AlB2O6 ceramic. J. Eur. Ceram. Soc. 2005, 25, 2877–2881. [Google Scholar] [CrossRef]
- Abdullaev, G.; Rza-zade, P.; Mamedov, K. The system Na2O-Al2O3-B2O3. Russ. J. Inorg. Chem. 1983, 28, 208–211. [Google Scholar]
- Abdullaev, G.; Mamedov, K. Refinement of the crystal-structure of lithium alumoborate LI6. Kristallografiya 1982, 27, 381–383. [Google Scholar]
- Abdullaev, G.; Mamedov, K. Crystal structure of lithium alumoborate Li6 ) 7Al2(BO3)4{. )]. Kristallografiya 1974, 19, 165–168. [Google Scholar]
- Jiang, L.H.; Zhang, Y.L.; Li, C.Y.; Hao, J.Q.; Su, Q. Thermoluminescence studies of LiSrBO3: RE3+ (RE= Dy, Tb, Tm and Ce). Appl. Radiat. Isot. 2010, 68, 196–200. [Google Scholar] [CrossRef]
- Vinila, V.S.; Jacob, R.; Mony, A.; Nair, H.G.; Issac, S.; Rajan, S.; Nair, A.S.; Isac, J. XRD studies on nano crystalline ceramic superconductor PbSrCaCuO at different treating temperatures. Cryst. Struct. Theory Appl. 2014, 3, 43963. [Google Scholar] [CrossRef][Green Version]
- Bos, A.J. On the energy conversion in thermoluminescence dosimetry materials. Radiat. Meas. 2001, 33, 737–744. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum Press: New York, NY, USA, 1974; p. 159. [Google Scholar]
- Parvinder, K.; Gurinder, P.S.; Simranpreet, K.; Singh, D.P. Modifier role of cerium in lithium aluminium borate glasses. J. Mol. Struct. 2012, 1020, 83–87. [Google Scholar]
- Yukihara, E.G.; Milliken, E.D.; Oliveira, L.C.; Orante-Barron, V.R.; Jacobsohn, L.G.; Blair, W.M. Systematic development of new thermoluminescence and optically stimulated luminescence materials. J. Lumin. 2013, 133, 203–210. [Google Scholar] [CrossRef]
- Hernández, M.F.; Suárez, G.; Cipollone, M.; Conconi, M.S.; Aglietti, E.F.; Rendtorff, N.M. Formation, microstructure and properties of aluminum borate ceramics obtained from alumina and boric acid. Ceram. Int. 2017, 43, 2188–2195. [Google Scholar] [CrossRef]
- Ike, P.O.; Nwanya, A.C.; Agwu, K.K.; Ezema, F.I. Structural, thermal and thermoluminescence response of transition and alkaline earth metals activated on aluminium borate [Al2(B4O7)3:Mn, Mg]. Ceram. Int. 2022, 48, 15533–15540. [Google Scholar] [CrossRef]
- Pagonis, V.; Kitis, G.; Furetta, C. Numerical and Practical Exercises Thermoluminescence; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Kitis, G. TL glow-curve deconvolution functions for various kinetics orders and continuous trap distribution: Acceptance criteria for E and s values. J. Radioanal. Nucl. Chem. 2001, 3, 697–703. [Google Scholar] [CrossRef]
- Gartia, R.K.; Singh, L.L. Evaluation of trapping parameter of quartz by deconvolutionof the glow curves. Radiat. Meas. 2011, 46, 664–668. [Google Scholar] [CrossRef]
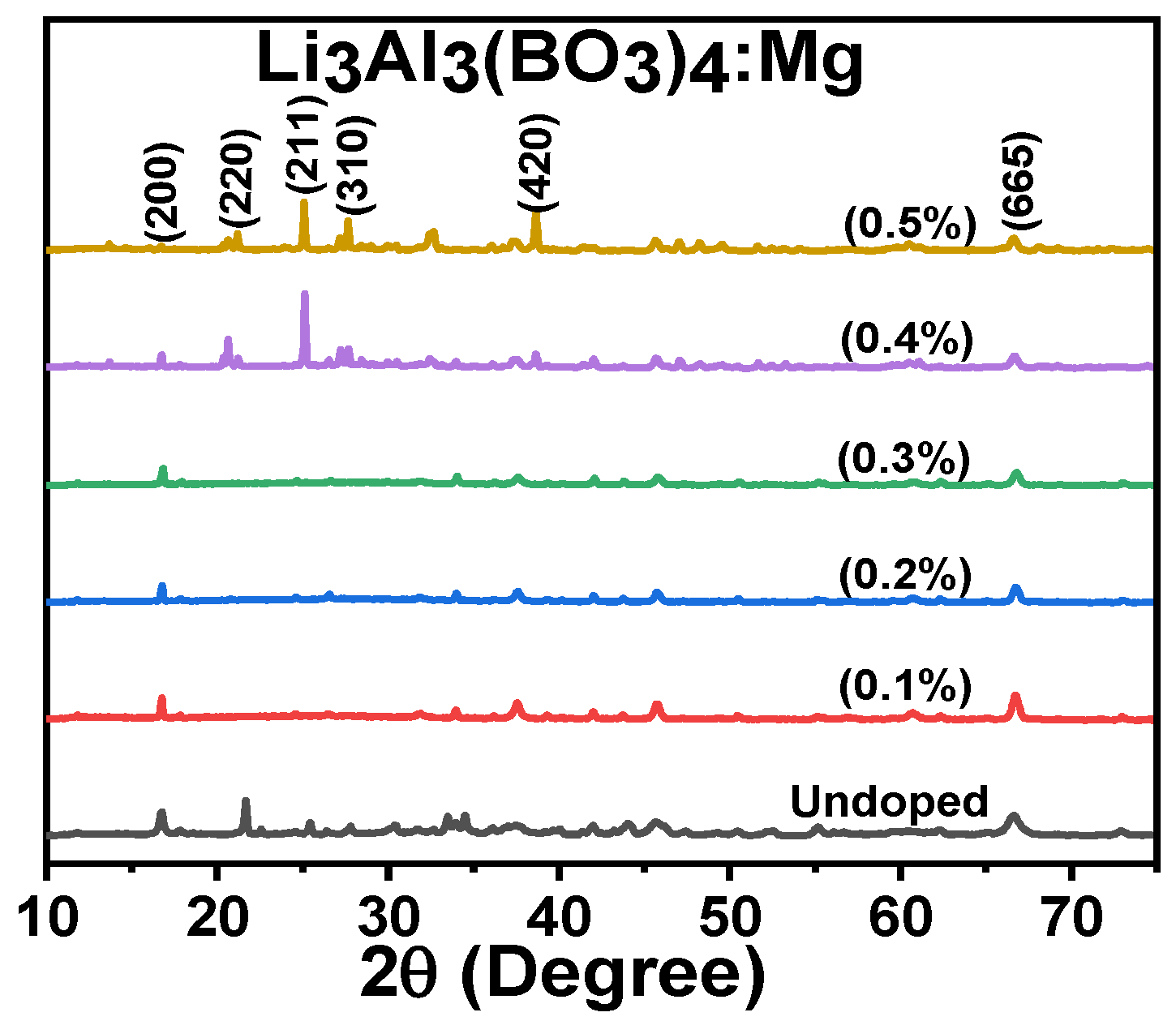



| Samples of Li3Al3(BO3)4 | FWHM (°) | S (nm) |
|---|---|---|
| Undoped | 0.15096 | 53.95 |
| Mg (0.1%) | 0.37878 | 34.85 |
| Mg (0.2%) | 0.19061 | 42.82 |
| Mg (0.3%) | 0.18364 | 45.24 |
| Mg (0.4%) | 0.11169 | 62.87 |
| Mg (0.5%) | 0.12084 | 67.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshoaibi, A.; Ike, P.O.; Nwanya, A.C.; Awada, C.; Islam, S.; Ezema, F.I. Effect of Mg on the Structural, Optical and Thermoluminescence Properties of Li3Al3(BO3)4: Shift in Main Glow Peak. Molecules 2023, 28, 504. https://doi.org/10.3390/molecules28020504
Alshoaibi A, Ike PO, Nwanya AC, Awada C, Islam S, Ezema FI. Effect of Mg on the Structural, Optical and Thermoluminescence Properties of Li3Al3(BO3)4: Shift in Main Glow Peak. Molecules. 2023; 28(2):504. https://doi.org/10.3390/molecules28020504
Chicago/Turabian StyleAlshoaibi, Adil, Patrick O. Ike, Assumpta C. Nwanya, Chawki Awada, Shumila Islam, and Fabian I. Ezema. 2023. "Effect of Mg on the Structural, Optical and Thermoluminescence Properties of Li3Al3(BO3)4: Shift in Main Glow Peak" Molecules 28, no. 2: 504. https://doi.org/10.3390/molecules28020504
APA StyleAlshoaibi, A., Ike, P. O., Nwanya, A. C., Awada, C., Islam, S., & Ezema, F. I. (2023). Effect of Mg on the Structural, Optical and Thermoluminescence Properties of Li3Al3(BO3)4: Shift in Main Glow Peak. Molecules, 28(2), 504. https://doi.org/10.3390/molecules28020504










