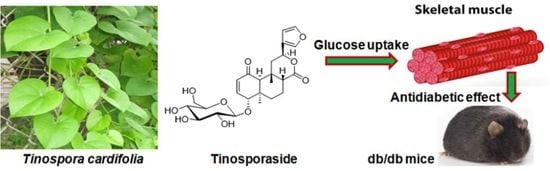
Tinosporaside from Tinospora cordifolia Encourages Skeletal Muscle Glucose Transport through Both PI-3-Kinase- and AMPK-Dependent Mechanisms
Abstract
1. Introduction
2. Results
2.1. Tinosporaside Augments Glucose Uptake in L6 Cells
2.2. Effect of Tinosporaside on GLUT4 Translocation in L6-GLUT4myc Myotubes
2.3. Effect of Wortmannin on Tinosporaside-Stimulated Glucose Uptake in L6 Myotubes
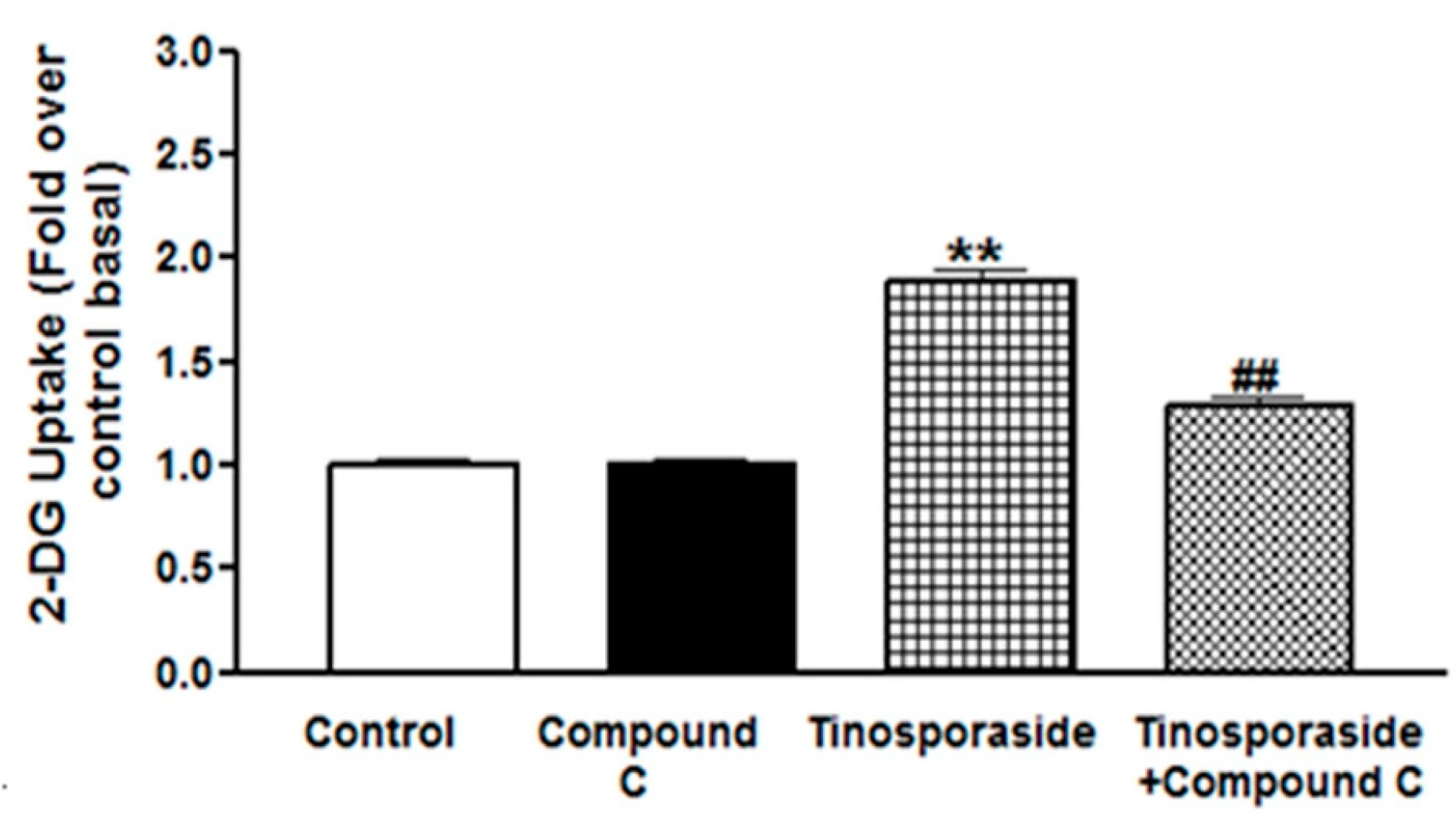
2.4. Effect of Compound C on Tinosporaside-Stimulated Glucose Uptake in L6 Myotubes
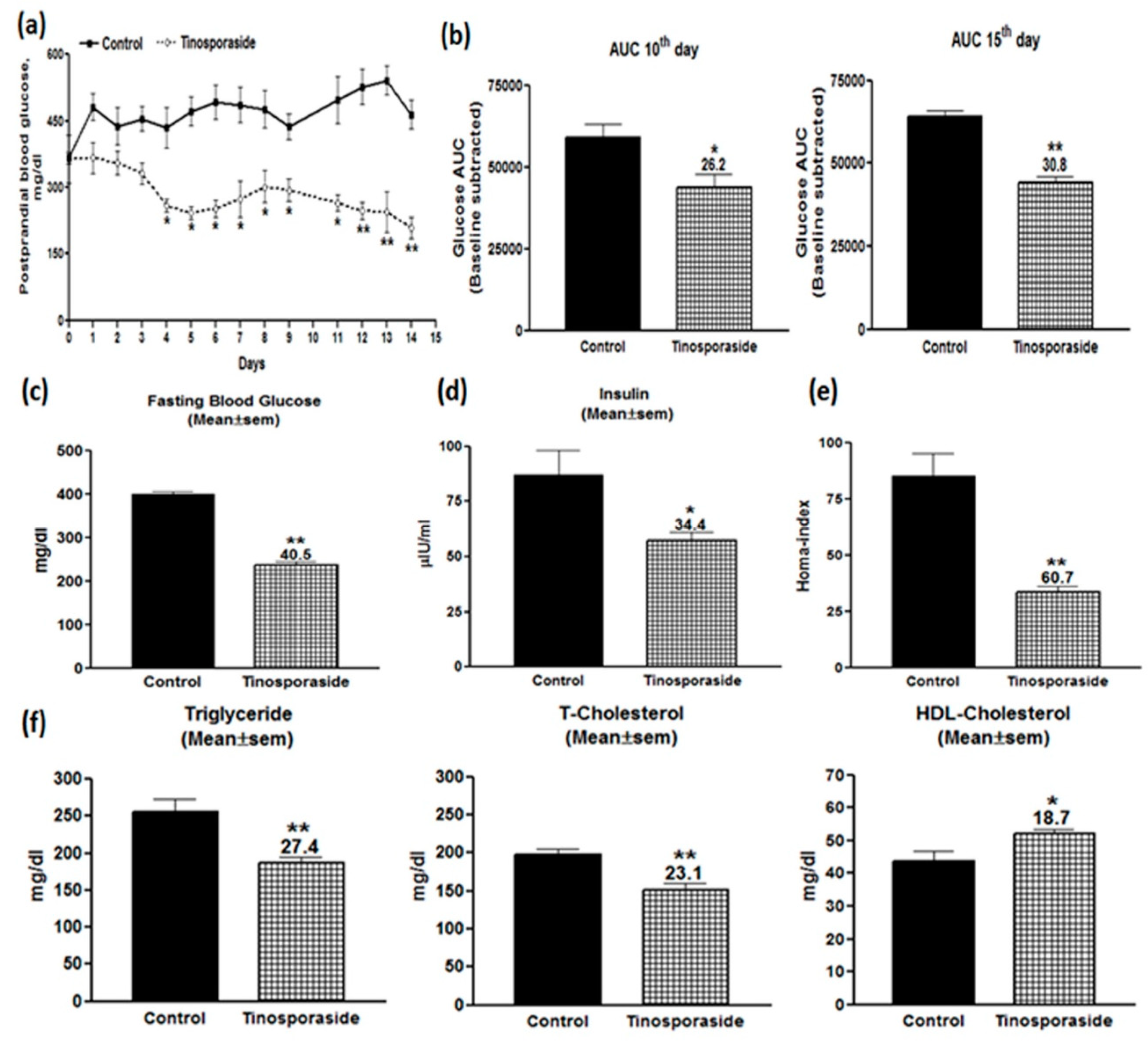
2.5. Antidiabetic Effect of Tinosporaside on C57BL-Ks db/db Mice
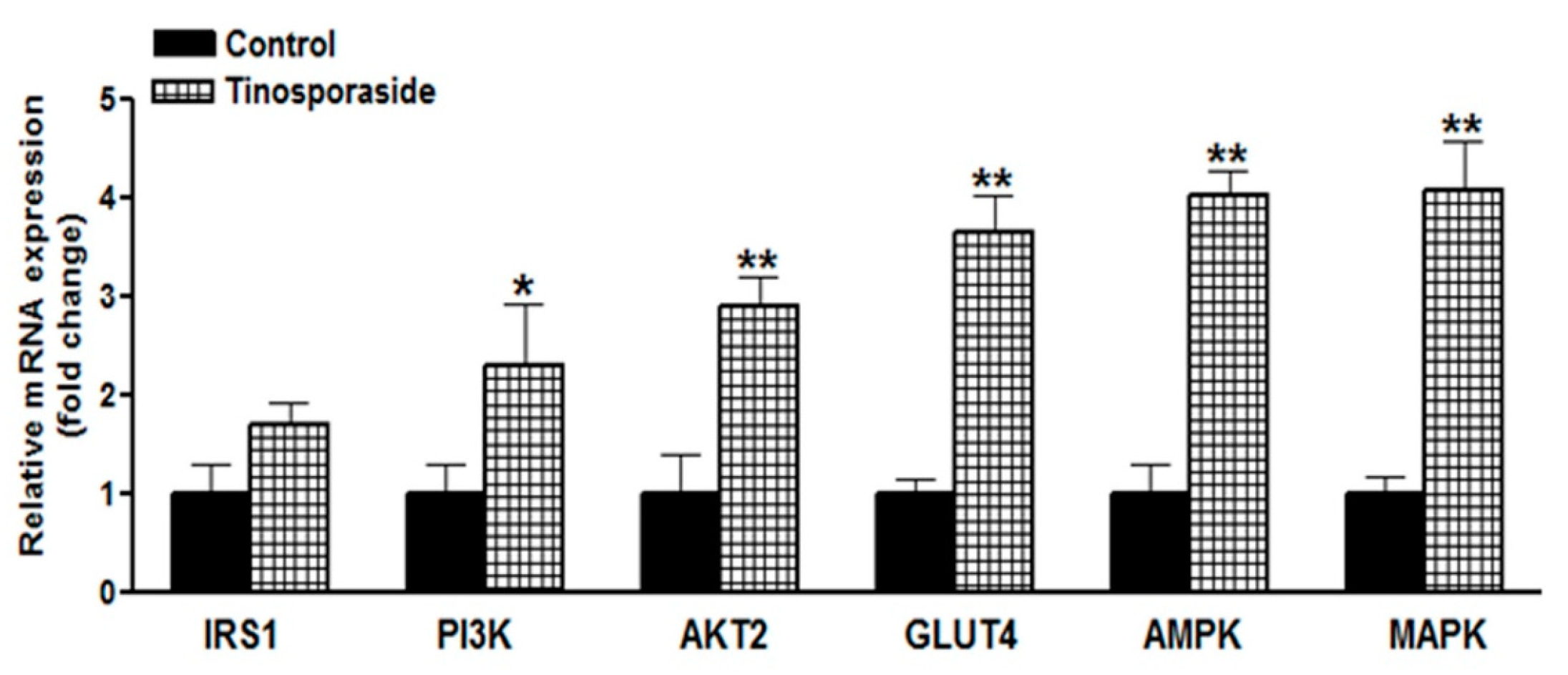
2.6. Effect of Tinosporaside on Gene Expression in the Skeletal Muscle of db/db Mice
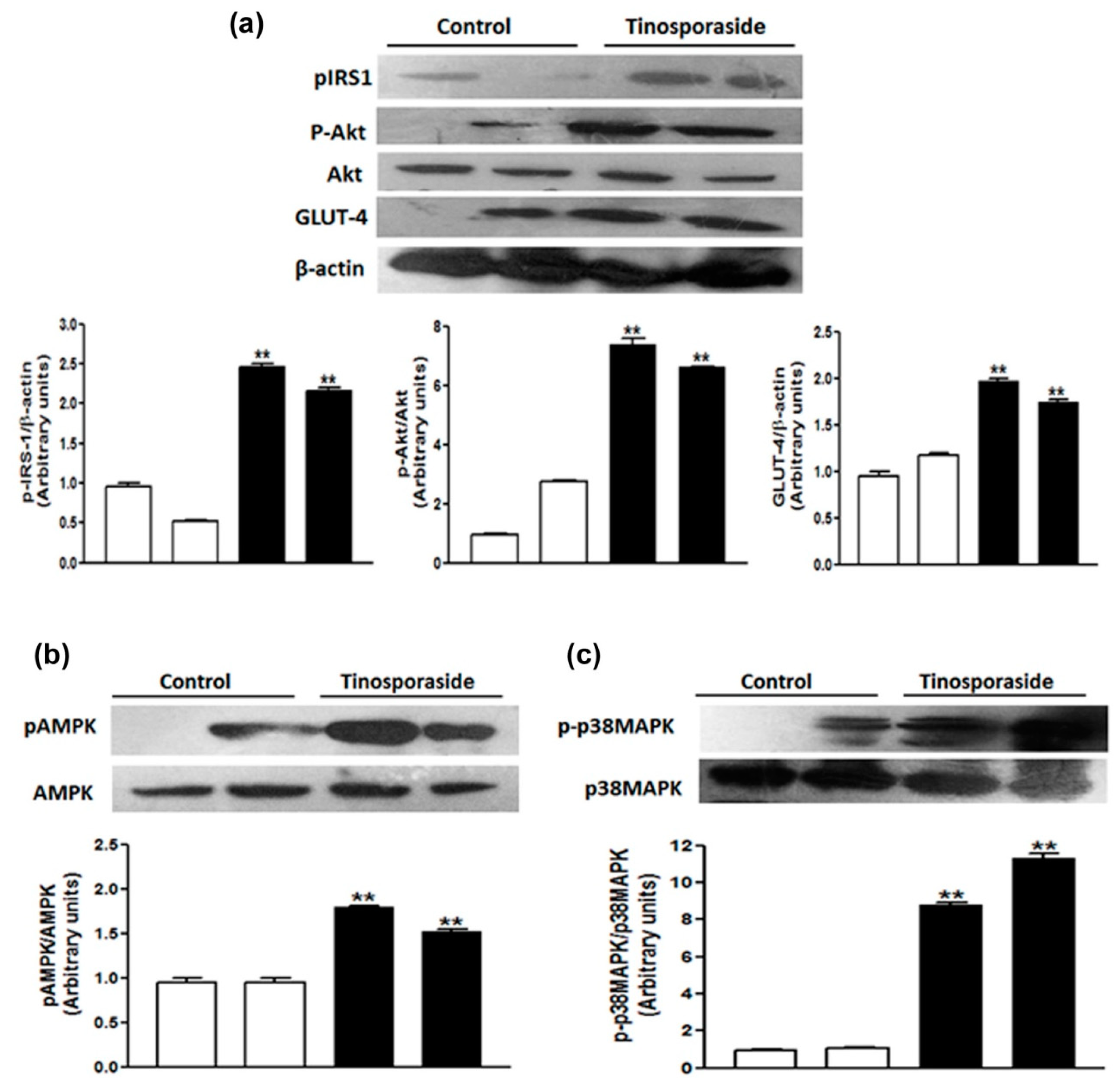
2.7. Effect of Tinosporaside on the Protein Levels of the PI3K and AMPK Signaling Pathways in Skeletal Muscle of db/db Mice
3. Discussion
4. Material and Method
4.1. Materials
4.2. Plant Material
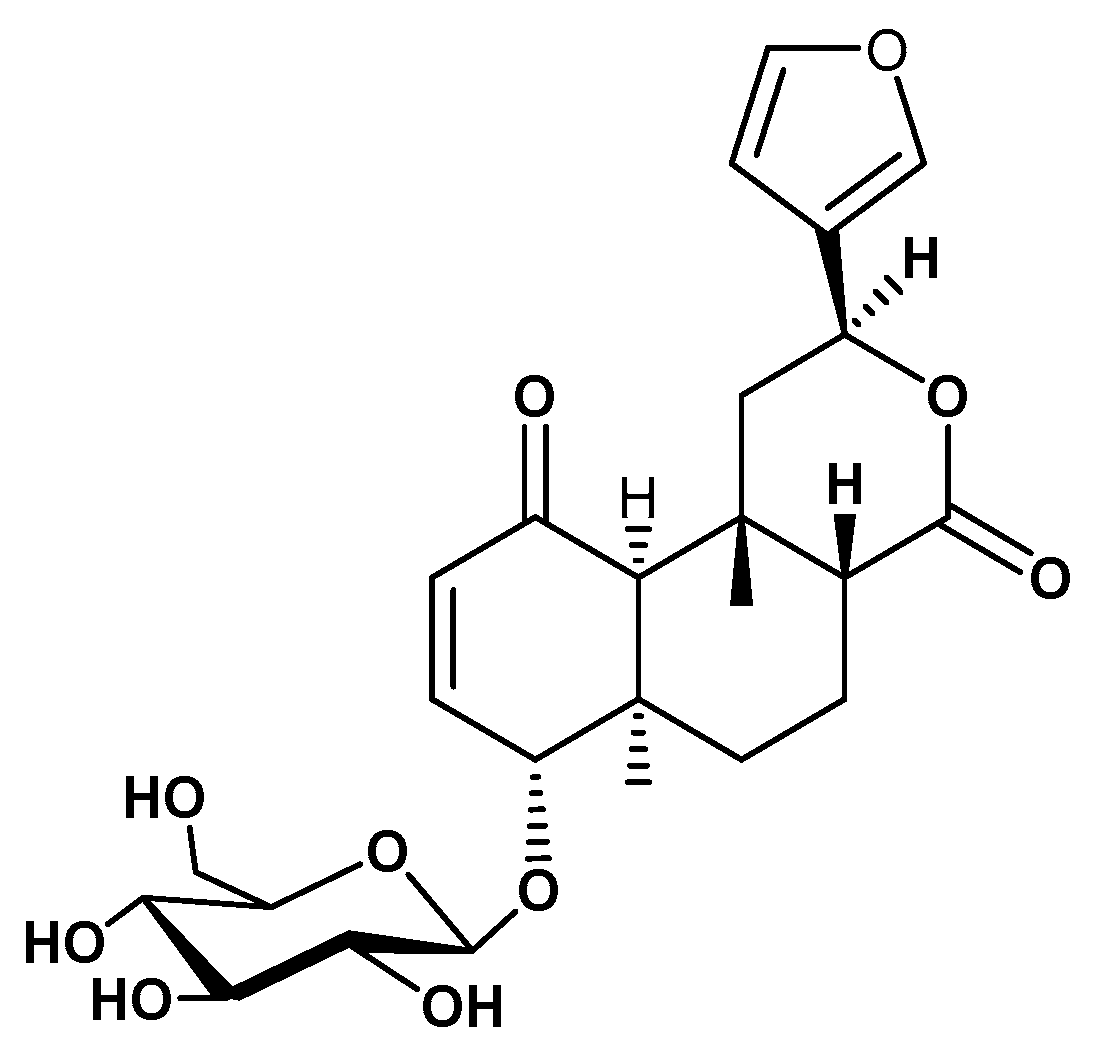
4.3. Extraction and Isolation of Tinosporaside
4.4. Cell Culture
4.5. Glucose Uptake Assay
4.6. GLUT4 Translocation Measurement
4.7. Cell Viability Assay
4.8. Animals
4.9. Antihyperglycemic Activity Evaluation in db/db Mice
4.10. Biochemical Estimation
4.11. Gene expression Analysis
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The growing epidemic of diabetes mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M. Highlighting diabetes mellitus: The epidemic continues. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Jaldin-Fincati, J.R.; Pavarotti, M.; Frendo-Cumbo, S.; Bilan, P.J.; Klip, A. Update on GLUT4 vesicle traffic: Cornerstone of insulin action. Trends Endocrinol. Metab. 2017, 28, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 2008, 413, 201–215. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- He, L.; Zhang, F.J.; Li, H.Y.; Li, L.; Song, L.G.; Mao, Y.; Li, J.; Liu, H.M.; Li, F.L.; Xu, L.Y.; et al. Anti-diabetic role of adropin in streptozotocin induced diabetic rats via alteration of PI3K/Akt and insulin signaling pathway. J. Oleo Sci. 2021, 70, 657–664. [Google Scholar] [CrossRef]
- de Wendt, C.; Espelage, L.; Eickelschulte, S.; Springer, C.; Toska, L.; Scheel, A.; Bedou, A.D.; Benninghoff, T.; Cames, S.; Stermann, T.; et al. Contraction-mediated glucose transport in skeletal muscle is regulated by a framework of AMPK, TBC1D1/4, and Rac1. Diabetes 2021, 70, 2796–2809. [Google Scholar] [CrossRef]
- Kumar, P.; Kamle, M.; Mahato, D.K.; Bora, H.; Sharma, B.; Rasane, P.; Bajpai, V.K. Tinospora cordifolia (Giloy): Phytochemistry, ethnopharmacology, clinical application and conservation strategies. Curr. Pharm. Biotechnol. 2020, 21, 1165–1175. [Google Scholar] [CrossRef]
- Choudhry, N.; Singh, S.; Siddiqui, M.B.; Khatoon, S. Impact of seasons and dioecy on therapeutic phytoconstituents of Tinospora cordifolia, a Rasayana drug. Biomed Res. Int. 2014, 2014, 902138. [Google Scholar] [CrossRef]
- Yates, C.R.; Bruno, E.J.; Yates, M.E.D. Tinospora Cordifolia: A review of its immunomodulatory properties. J. Diet. Suppl. 2022, 19, 271–285. [Google Scholar] [CrossRef]
- Arunachalam, K.; Yang, X.; San, T.T. Tinospora cordifolia (Willd.) Miers: Protection mechanisms and strategies against oxidative stress-related diseases. J. Ethnopharmacol. 2022, 283, 114540. [Google Scholar] [CrossRef]
- Jena, S.; Munusami, P.; Mm, B.; Chanda, K. Computationally approached inhibition potential of Tinospora cordifolia towards COVID-19 targets. Virusdisease 2021, 32, 65–77. [Google Scholar] [CrossRef]
- Birla, H.; Rai, S.N.; Singh, S.S.; Zahra, W.; Rawat, A.; Tiwari, N.; Singh, R.K.; Pathak, A.; Singh, S.P. Tinospora cordifolia suppresses neuroinflammation in parkinsonian mouse model. Neuromol. Med. 2019, 21, 42–53. [Google Scholar] [CrossRef]
- Patial, V.; Katoch, S.; Chhimwal, J.; Singh, P.P.; Suresh, P.S.; Padwad, Y. Tinospora cordifolia activates PPARγ pathway and mitigates glomerular and tubular cell injury in diabetic kidney disease. Phytomedicine 2021, 91, 153663. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedee, B.P.; Bisht, D.; Dash, A.K.; Kumar, D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019, 5, e02437. [Google Scholar] [CrossRef]
- Pandey, M.; Chikara, S.K.; Vyas, M.K.; Shrama, R.; Thakur, G.S.; Bisen, P.S. Tinospora cordifolia: A climbing shrug in health care management. Int. J. Pharm. Bio. Sci. 2012, 3, 612–628. [Google Scholar]
- Sangeetha, M.K.; Priya, C.D.M.; Vasanthi, H.R. Anti-diabetic property of Tinospora cordifolia and its active compound is mediated through the expression of Glut-4 in L6 myotubes. Phytomedicine 2013, 20, 246–248. [Google Scholar] [CrossRef]
- Joladarashi, D.; Chilkunda, N.D.; Salimath, P.V. Glucose uptake-stimulatory activity of Tinospora cordifolia stem extracts in Ehrlich ascites tumor cell model system. J. Food Sci. Technol. 2014, 51, 178–182. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT-4 glucose transporter. Cell Metab. 2007, 4, 237–252. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.T.; Song, Z.; Zhang, W.C.; Jiao, B.; Yu, Z.B. Impaired translocation of GLUT4 results in insulin resistance of atrophic soleus muscle. Biomed. Res. Int. 2015, 2015, 291987. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Koyabashi, K.; Forte, T.M.; Taniguchi, S.; Ishida, B.Y.; Oka, K.; Chan, L. The db/db mouse, a model for diabetic dyslipidemia: Molecular characterization and effect of western diet feeding. Metabolism 2000, 49, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Scalzo, R.L.; Reusch, J.E.B. Glucose uptake by skeletal muscle within the contexts of type 2 diabetes and exercise: An integrated approach. Nutrients 2022, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V.; Sajan, M.P.; Standaert, M.L. Insulin-sensitive protein kinases (a typical protein kinase C and protein kinase B/Akt): Actions and defects in obesity and type II diabetes. Exp. Biol. Med. 2005, 230, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Dey, C.S. AKT ISOFORMS-AS160-GLUT4: The defining axis of insulin resistance. Rev. Endocr. Metab. Disord. 2021, 22, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Pandeti, S.; Arha, D.; Mishra, A.; Reddy, S.S.; Srivastava, A.K.; Narender, T.; Tamrakar, A.K. Glucose uptake stimulatory potential and antidiabetic activity of the Arnebin-1 from Arnabia nobelis. Eur. J. Pharmacol. 2016, 789, 449–457. [Google Scholar] [CrossRef]
- Treebak, J.T.; Glund, S.; Deshmukh, A.; Klein, D.K.; Long, Y.C.; Jensen, T.E.; Jorgensen, S.B.; Viollet, B.; Andersson, L.; Neumann, D.; et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 2006, 55, 2051–2058. [Google Scholar] [CrossRef]
- Shanak, S.; Bassalat, N.; Barghash, A.; Kadan, S.; Ardah, M.; Zaid, H. Drug discovery of plausible lead natural compounds that target the insulin signaling pathway: Bioinformatics approaches. Evid. Based Complement. Alternat. Med. 2022, 2022, 2832889. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.R.; Oh, G.T.; Park, H.S.; Lee, K.U.; Lane, M.D.; et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Šmejkal, K.; Malaník, M.; et al. Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Kim, M.S.; Hur, H.J.; Kwon, D.Y.; Hwang, J.T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell. Endocrinol. 2012, 358, 127–134. [Google Scholar] [CrossRef]
- Um, J.H.; Park, S.J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kühne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W.; et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef]
- Khan, M.A.; Gray, A.I.; Waterman, P.G. Tinosporaside, an 18-norclerodane glucoside from Tinospora cordifolia. Phytochemistry 1989, 28, 273–275. [Google Scholar] [CrossRef]
- Maurya, R.; Wazir, V.; Tyagi, A.; Kapil, R.S. Clerodane diterpenoids from Tinospora cordifolia. Phytochemistry 1995, 38, 659–661. [Google Scholar] [CrossRef]
- Prasad, J.; Maurya, C.K.; Pandey, J.; Jaiswal, N.; Madhur, G.; Srivastava, A.K.; Narender, T.; Tamrakar, A.K. Diastereomeric mixture of calophyllic acid and isocalophyllic acid stimulates glucose uptake in skeletal muscle cells: Involvement of PI-3-Kinase- and ERK1/2-dependent pathways. Mol. Cell. Endocrinol. 2013, 370, 11–19. [Google Scholar] [CrossRef]
- Pandey, J.; Dev, K.; Chattopadhyay, S.; Kadan, S.; Sharma, T.; Maurya, R.; Sanyal, S.; Siddiqi, M.I.; Zaid, H.; Tamrakar, A.K. β-Sitosterol-D-glucopyranoside mimics estrogenic properties and stimulates glucose utilization in skeletal muscle cells. Molecules 2021, 26, 3129. [Google Scholar] [CrossRef]
- Tamrakar, A.K.; Jaiswal, N.; Yadav, P.P.; Maurya, R.; Srivastava, A.K. Pongamol from Pongamia pinnata stimulates glucose uptake by increasing surface GLUT-4 level in skeletal muscle cells. Mol. Cell. Endocrinol. 2011, 339, 98–104. [Google Scholar] [CrossRef]
- Kadan, S.; Melamed, S.; Benvalid, S.; Tietel, Z.; Sasson, Y.; Zaid, H. Gundelia tournefortii: Fractionation, chemical composition and anti-diabetic efficacy. Molecules 2021, 3785, 1–22. [Google Scholar]
- Mishra, A.; Srivastava, R.; Srivastava, S.P.; Gautam, S.; Tamrakar, A.K.; Maurya, R.; Srivastava, A.K. Antidiabetic activity of heartwood of Pterocarpus marsupium Roxb. and analysis of phytoconstituents. Indian J. Exp. Biol. 2013, 51, 363–374. [Google Scholar] [PubMed]
- Pandey, J.; Maurya, R.; Raykhera, R.; Srivastava, M.N.; Yadav, P.P.; Tamrakar, A.K. Murraya koenigii (L.) Spreng. ameliorates insulin resistance in dexamethasone-treated mice by enhancing peripheral insulin sensitivity. J. Sci. Food Agric. 2014, 94, 2282–2288. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| IRS1 | AGGAGGAGGGAGGAGAAGG | GAAGAGATCGGGGAAGACG |
| PI3KCG | CCATGAGGAAACCCAGTGAG | GCGGAGGTTGTCCTCTCTTA |
| AKT2 | GGGCCTGACTCCGAGAAG | CCGCTCCTTATTTATGAACTGG |
| GLUT4 | GACGGACACTCCATCTGTTG | CATAGCTCATGGCTGGAACC |
| AMPK | CCTTCGGGAAAGTGAAGGT | GAATCTTCTGCCGGTTGAGT |
| MAPK | TGAAGTTGAACAGGCTCTGG | AATGGCGCTTCAGCAATG |
| GAPDH | AGCTTGTCATCAACGGGAAG | TTTGATGTTAGTGGGGTCTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; Sharma, K.; Pandey, J.; Dev, K.; Kadan, S.; Sahai, M.; Ahmad, I.; Srivastava, A.K.; Tamrakar, A.K.; Zaid, H.; et al. Tinosporaside from Tinospora cordifolia Encourages Skeletal Muscle Glucose Transport through Both PI-3-Kinase- and AMPK-Dependent Mechanisms. Molecules 2023, 28, 483. https://doi.org/10.3390/molecules28020483
Mishra A, Sharma K, Pandey J, Dev K, Kadan S, Sahai M, Ahmad I, Srivastava AK, Tamrakar AK, Zaid H, et al. Tinosporaside from Tinospora cordifolia Encourages Skeletal Muscle Glucose Transport through Both PI-3-Kinase- and AMPK-Dependent Mechanisms. Molecules. 2023; 28(2):483. https://doi.org/10.3390/molecules28020483
Chicago/Turabian StyleMishra, Akansha, Khushbu Sharma, Jyotsana Pandey, Kapil Dev, Sleman Kadan, Mahendra Sahai, Ishbal Ahmad, Arvind K. Srivastava, Akhilesh K. Tamrakar, Hilal Zaid, and et al. 2023. "Tinosporaside from Tinospora cordifolia Encourages Skeletal Muscle Glucose Transport through Both PI-3-Kinase- and AMPK-Dependent Mechanisms" Molecules 28, no. 2: 483. https://doi.org/10.3390/molecules28020483
APA StyleMishra, A., Sharma, K., Pandey, J., Dev, K., Kadan, S., Sahai, M., Ahmad, I., Srivastava, A. K., Tamrakar, A. K., Zaid, H., & Maurya, R. (2023). Tinosporaside from Tinospora cordifolia Encourages Skeletal Muscle Glucose Transport through Both PI-3-Kinase- and AMPK-Dependent Mechanisms. Molecules, 28(2), 483. https://doi.org/10.3390/molecules28020483