Production of Gypenoside XVII from Ginsenoside Rb1 by Enzymatic Transformation and Their Anti-Inflammatory Activity In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
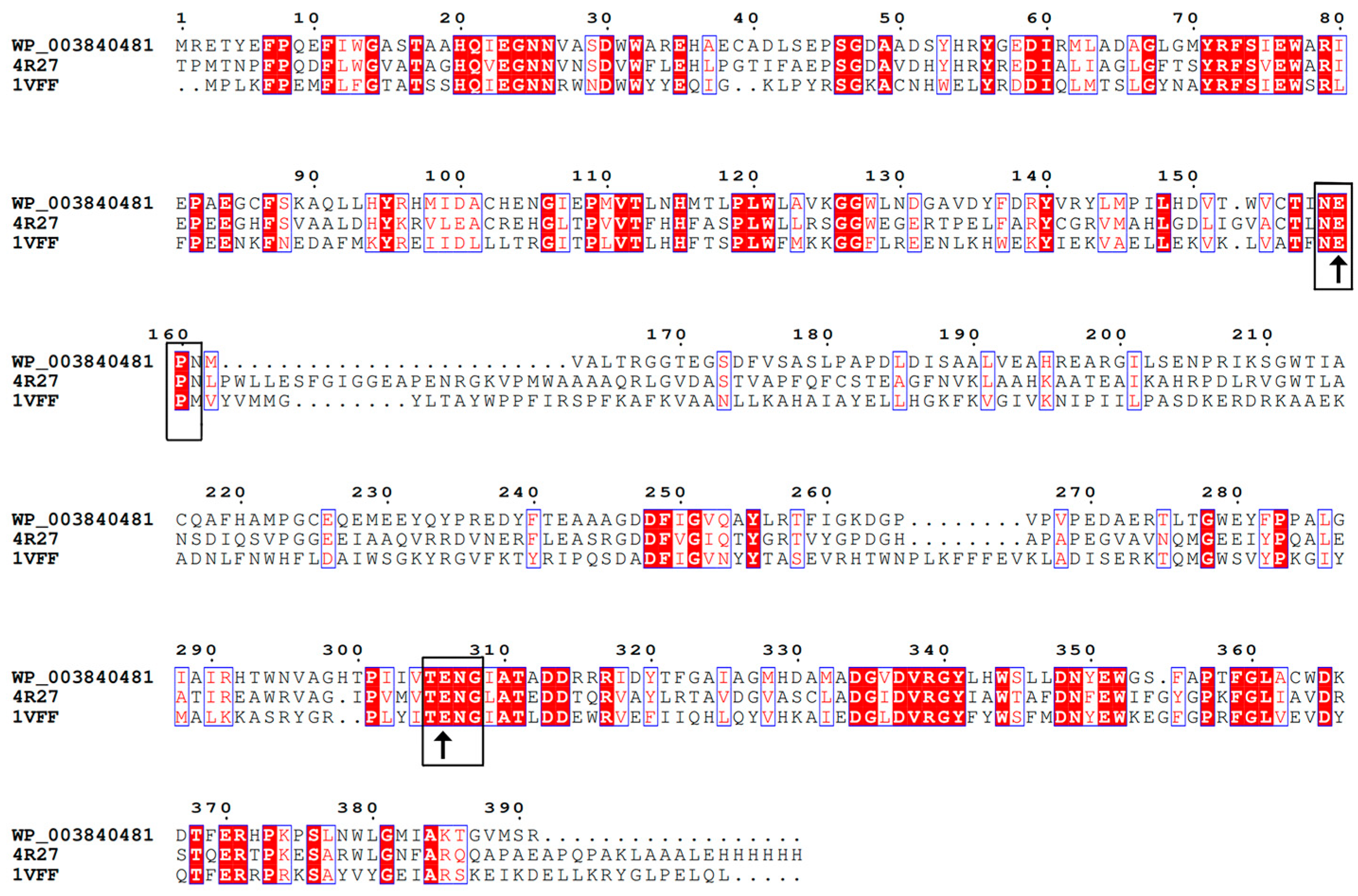
2.1. Sequence Analysis of BdbglB from B. dentium
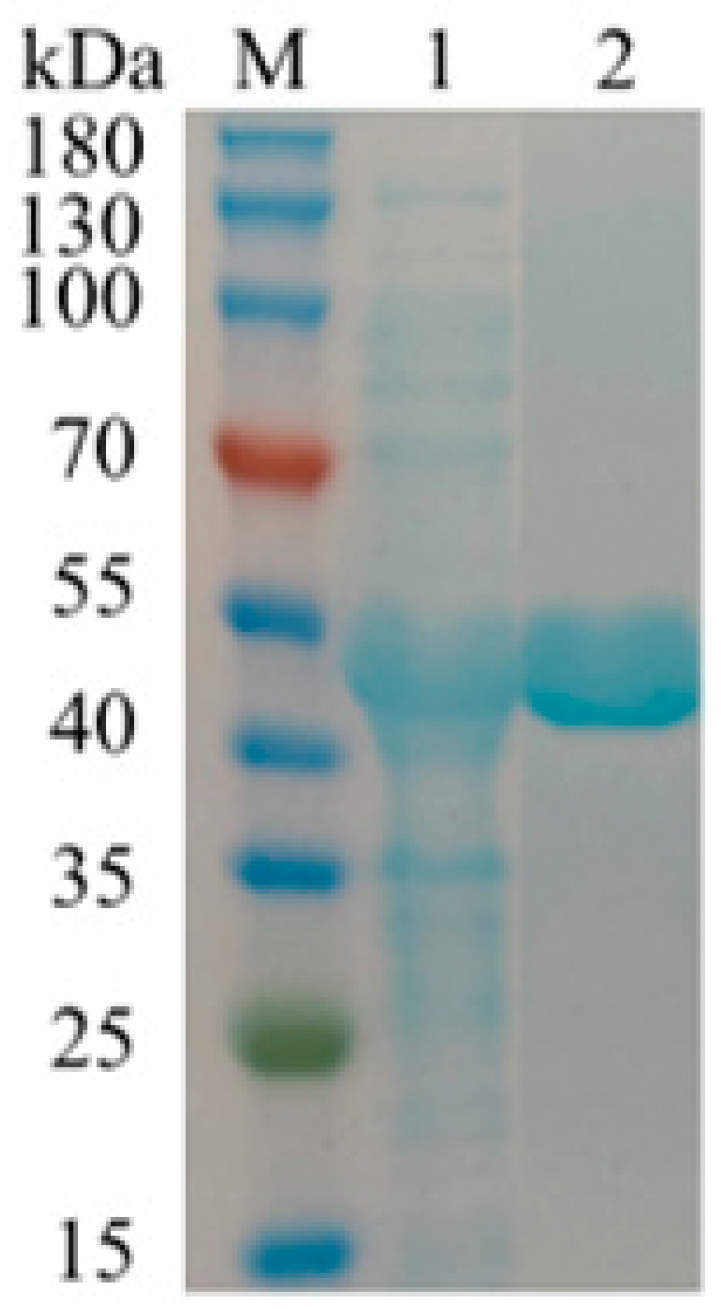
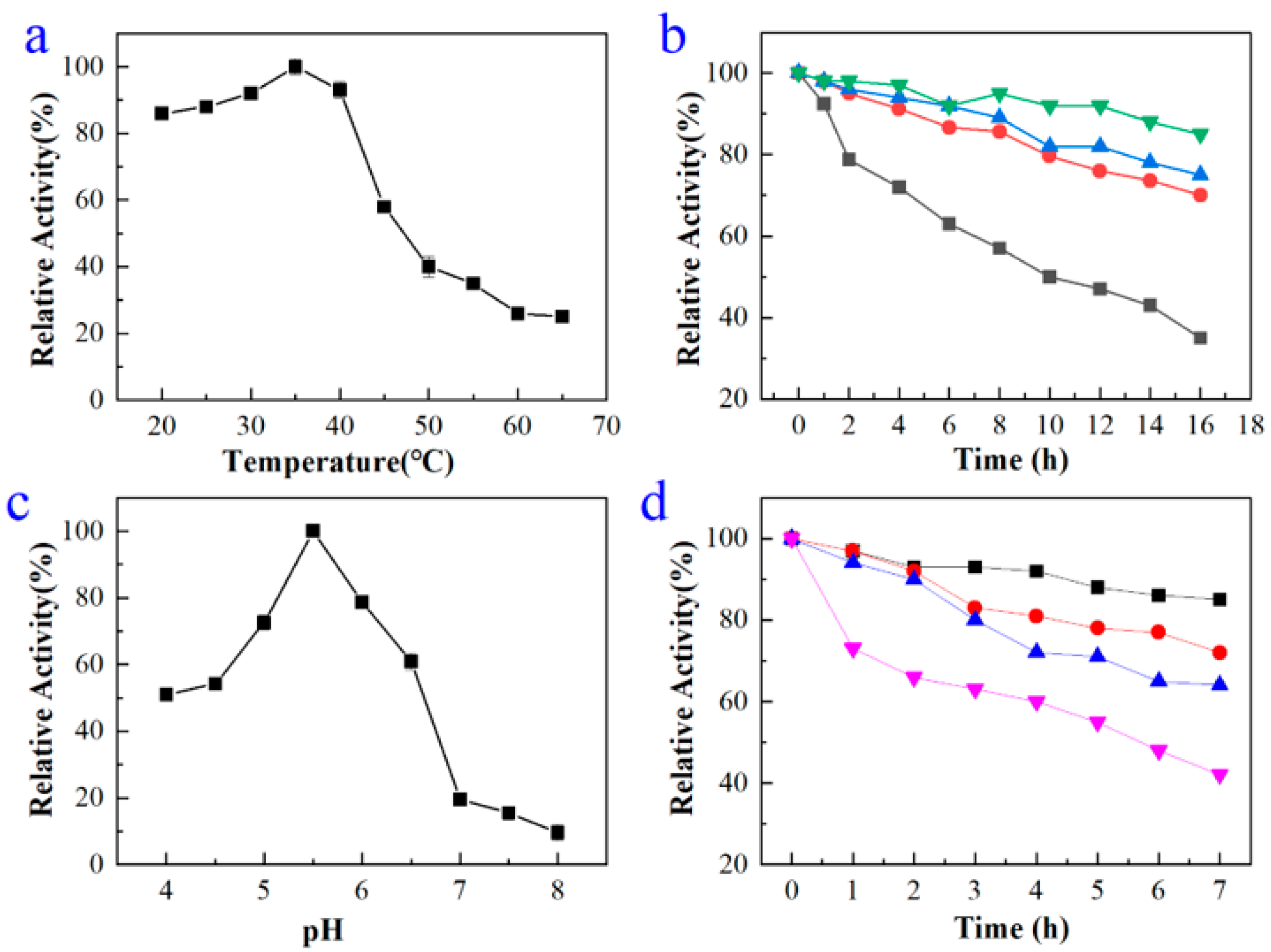
2.2. Expression, Purification, and Characterization of BdbglB from B. dentium
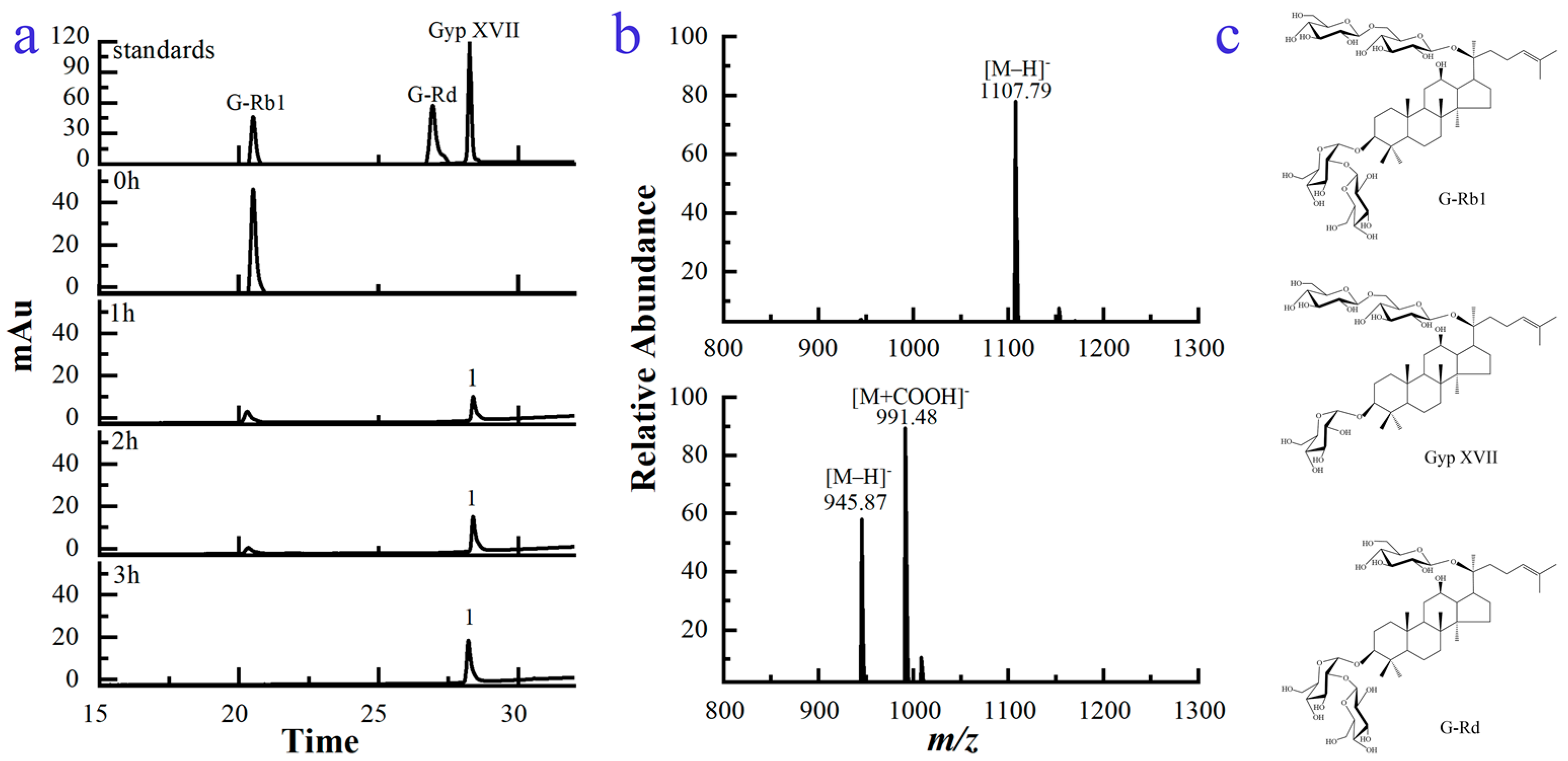
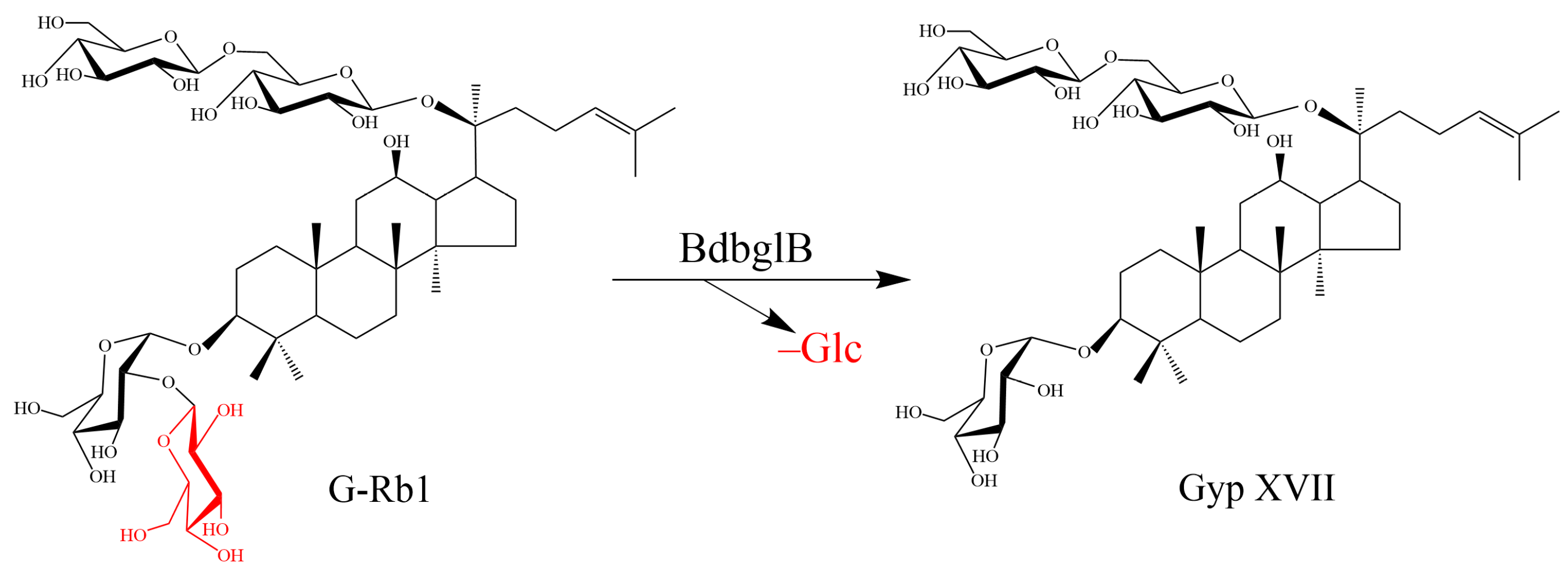
2.3. Structural Analysis of the Biotransformed Product of G-Rb1
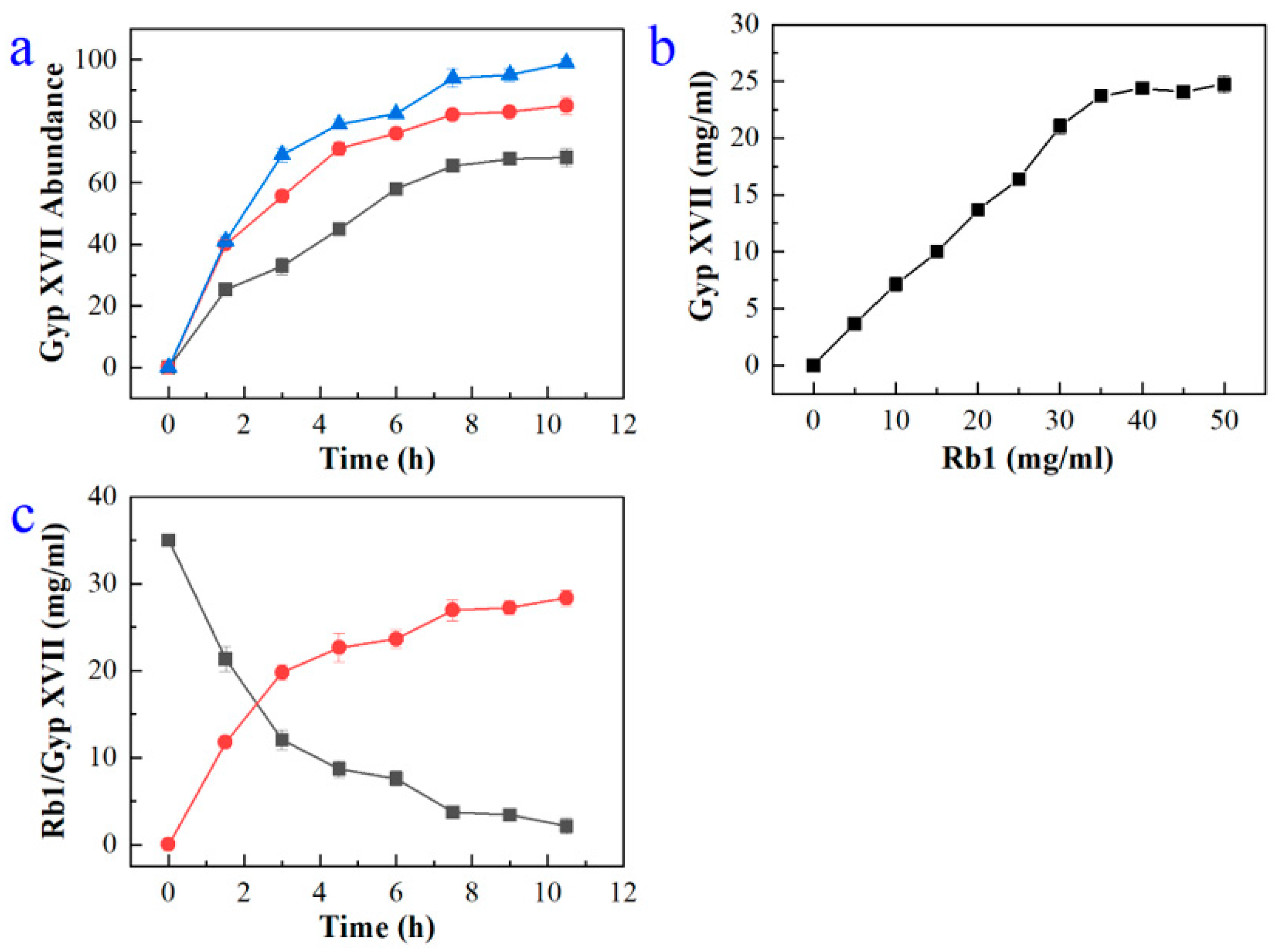
2.4. Large-Scale Production of Gyp XVII by Using the Recombinant Enzyme BdbglB
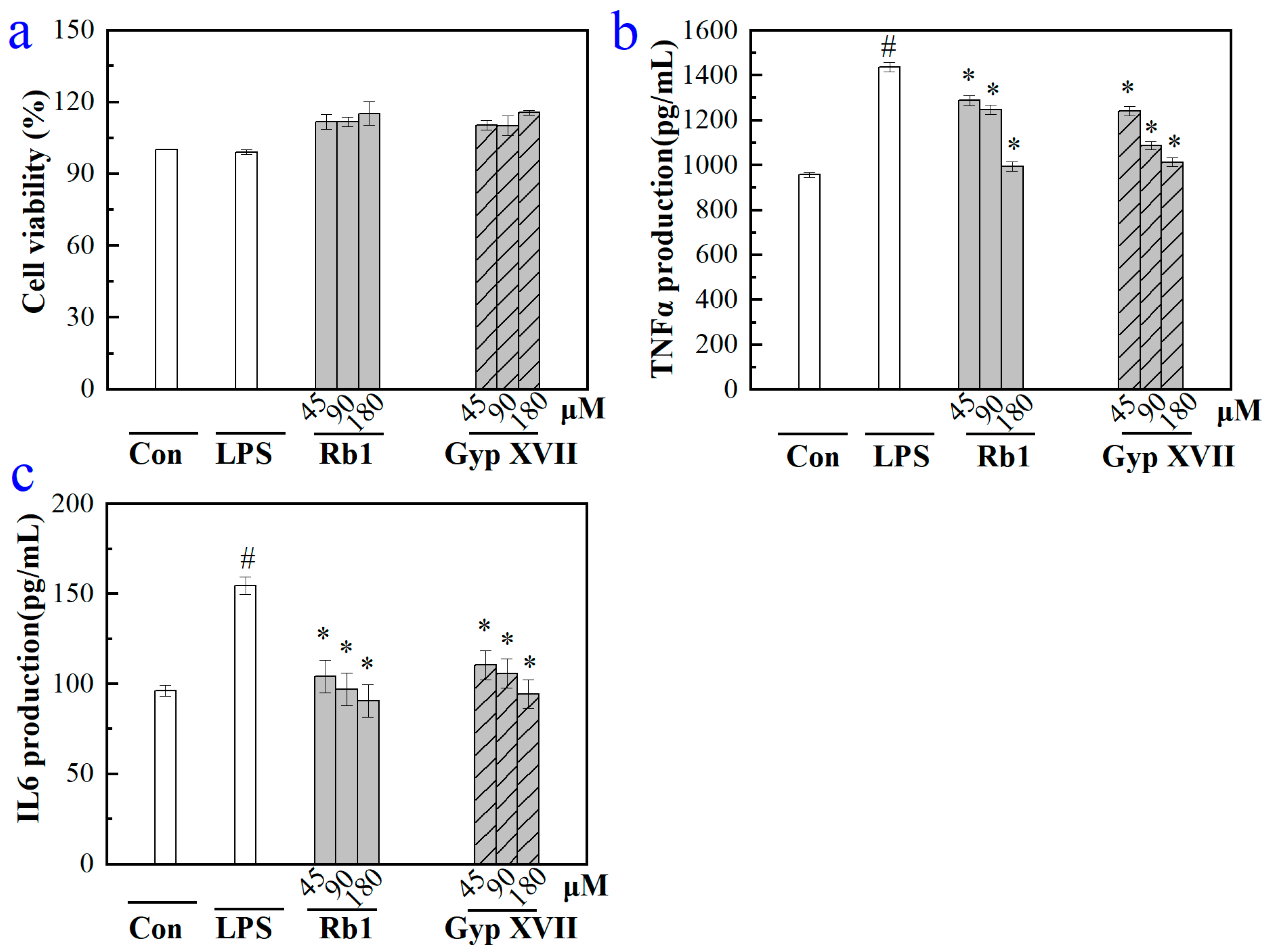
2.5. Effects of Gyp XVII and Its Precursor G-Rb1 on LPS-Stimulated RAW 264.7 Cell
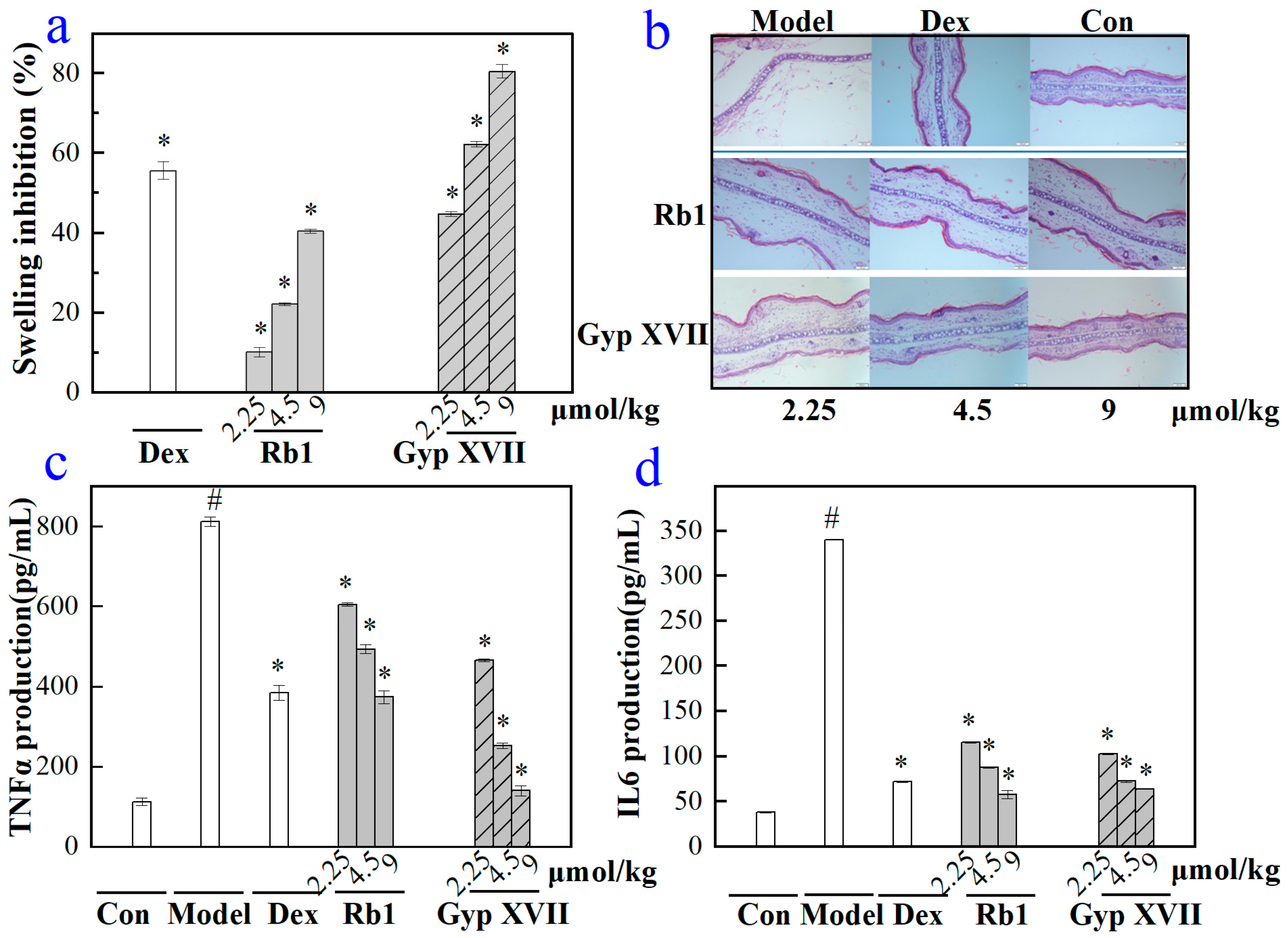
2.6. Effects of Gyp XVII and Its Precursor G-Rb1 on Mouse Ear Swelling Response
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sequence Analysis of BdbglB from B. dentium
4.3. Expression and Purification of BdbglB from B. dentium
4.4. Characterization of BdbglB from B. dentium
4.5. Structural Analysis of the Biotransformed Product of G-Rb1
4.5.1. HPLC Analysis
4.5.2. HPLC-MS Analysis
4.6. Large-Scale Production of Gyp XVII from G-Rb1 by BdbglB from B. dentium
4.7. Assessment of Anti-Inflammatory Activity In Vivo and In Vitro
4.7.1. Cell Culture and Treatment
4.7.2. Cell Viability
4.7.3. Analysis of Cytokines Using ELISA Assay
4.7.4. Xylene-Induced Ear Swelling and Histological Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [PubMed]
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin. Med. 2016, 11, 1–16. [Google Scholar]
- Wang, B.; Li, M.; Gao, H.; Sun, X.; Gao, B.; Zhang, Y.; Yu, L. Chemical composition of tetraploid Gynostemma pentaphyllum gypenosides and their suppression on inflammatory response by NF-κB/MAPKs/AP-1 signaling pathways. Food Sci. Nutr. 2020, 8, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, D.; Abula, S.; Hu, Y.; Zhao, X.; Huang, Y.; Liu, J.; Wu, Y.; Wang, D.; Tao, Y.; et al. The immunological adjuvant activity of gypenosides liposome against Newcastle disease vaccine. Int. J. Biol. Macromol. 2013, 60, 116–121. [Google Scholar] [CrossRef]
- Shen, C.Y.; Shi, M.M.; Yang, H.L.; Jiang, J.G.; Huang, C.L.; Zhu, W. Inhibitory effects of multi-components from Gynostemma pentaphyllum (Thunb.) Makino on macrophage foam cell formation exhibit multi-target characteristics. J. Funct. Foods 2019, 60, 103451. [Google Scholar] [CrossRef]
- Blythe, E.K.; Demirci, B.; Goger, F.; Tabanca, N. Characterization of Volatile and Polar Compounds of Jiaogulan Tea [Gynostemma pentaphyllum (Thunb.) Makino] by Hyphenated Analytical Techniques. Asian J. Chem. 2017, 29, 1285–1290. [Google Scholar] [CrossRef]
- Song, M.; Tan, D.; Li, B.; Wang, Y.; Shi, L. Gypenoside ameliorates insulin resistance and hyperglycemia via the AMPK-mediated signaling pathways in the liver of type 2 diabetes mellitus mice. Food Sci. Hum. Wellness 2022, 11, 1347–1354. [Google Scholar]
- Xing, S.F.; Lin, M.; Wang, Y.R.; Chang, T.; Cui, W.Y.; Piao, X.L. Novel dammarane-type saponins from Gynostemma pentaphyllum and their neuroprotective effect. Nat. Prod. Res. 2018, 34, 651–658. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Ha, T.K.Q.; Yang, J.L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar]
- Zheng, Y.; Zheng, Z.; Ming, Y.; Lin, M.; Chen, L.; Huang, W.; Xiao, J.; Lin, H. Gynosaponin TN-1 producing from the enzymatic conversion of gypenoside XLVI by naringinase and its cytotoxicity on hepatoma cell lines. Food Chem. Toxicol. 2018, 119, 161–168. [Google Scholar] [CrossRef]
- Cui, C.H.; Kim, D.J.; Jung, S.C.; Kim, S.C.; Im, W.T. Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming β-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property. Molecules 2017, 22, 844. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.L.; Dong, W.W.; Wu, S.; Jiang, J.; Yang, D.C.; Li, D.; Quan, L.H. Biotransformation of gypenoside XVII to compound K by a recombinantβ-glucosidase. Biotechnol. Lett. 2016, 38, 1187–1193. [Google Scholar]
- Zhao, H.X.; Jiao, W.B.; Xiu, Y.; Zhou, K.L.; Zhong, P.; Wang, N.; Yu, S.S. Enzymatic Biotransformation of Gypenoside XLIX into Gylongiposide I and Their Antiviral Roles against Enterovirus 71 In Vitro. Molecules 2022, 27, 4094. [Google Scholar] [PubMed]
- Shin, K.C.; Oh, D.K. Characterization of a novel recombinant β-glucosidase from Sphingopyxis alaskensis that specifically hydrolyzes the outer glucose at the C-3 position in protopanaxadiol-type ginsenosides. J. Biotechnol. 2014, 172, 30–37. [Google Scholar] [PubMed]
- Gao, H.; Kang, N.; Hu, C.; Zhang, Z.; Xu, Q.; Liu, Y.; Yang, S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine 2020, 69, 153197. [Google Scholar]
- Yu, T.; Rhee, M.H.; Lee, J.; Kim, S.H.; Yang, Y.; Kim, H.G.; Kim, Y.; Kim, C.; Kwak, Y.S.; Kim, J.H.; et al. Ginsenoside Rc from Korean Red Ginseng (Panax ginseng C.A. Meyer) Attenuates Inflammatory Symptoms of Gastritis, Hepatitis and Arthritis. Am. J. Chin. Med. 2016, 44, 595–615. [Google Scholar] [CrossRef]
- Aktan, F.; Henness, S.; Roufogalis, B.D.; Ammit, A.J. Gypenosides derived from Gynostemma pentaphyllum suppress NO synthesis in murine macrophages by inhibiting iNOS enzymatic activity and attenuating NF-κB-mediated iNOS protein expression. Nitric Oxide 2003, 8, 235–242. [Google Scholar]
- Wong, W.Y.; Lee, M.M.L.; Chan, B.D.; Ma, V.W.S.; Zhang, W.; Yip, T.T.C.; Wong, W.T.; Tai, W.C.S. Gynostemma pentaphyllum saponins attenuate inflammation in vitro and in vivo by inhibition of NF κB and STAT3 signaling. Oncotarget 2021, 8, 87401–87414. [Google Scholar]
- Wang, F.; Dang, Y.; Wang, J.; Zhou, T.; Zhu, Y. Gypenosides attenuate lipopolysaccharide-induced optic neuritis in rats. Acta Histochem. 2018, 120, 340–346. [Google Scholar]
- Wan, Z.H.; Zhao, Q. Gypenoside inhibits interleukin-1β-induced inflammatory response in human osteoarthritis chondrocytes. J. Biochem. Mol. Toxicol. 2017, 31, 21926. [Google Scholar]
- Liu, J.; Li, Y.; Yang, P.; Wan, J.; Chang, Q.; Wang, T.T.Y.; Lu, W.; Zhang, Y.; Wang, Q.; Yu, L.L. Gypenosides Reduced the Risk of Overweight and Insulin Resistance in C57BL/6J Mice through Modulating Adipose Thermogenesis and Gut Microbiota. J. Agric. Food Chem. 2017, 65, 9237–9246. [Google Scholar] [PubMed]
- Huang, T.H.W.; Li, Y.; Razmovski Naumovski, V.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Gypenoside XLIX isolated from Gynostemma pentaphyllum inhibits nuclear factor-kappaB activation via a PPAR-alpha-dependent pathway. J. Biomed. Sci. 2006, 13, 535–548. [Google Scholar] [PubMed]
- Lee, C.; Lee, J.W.; Jin, Q.; Jang, H.; Jang, H.J.; Rho, M.C.; Lee, M.K.; Lee, C.K.; Lee, M.K.; Hwang, B.Y. Isolation and Characterization of Dammarane-Type Saponins from Gynostemma pentaphyllum and Their Inhibitory Effects on IL-6-Induced STAT3 Activation. J. Nat. Prod. 2015, 78, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, H.; Zhang, X.; Yang, H.; Zhou, Q.; Yu, L. Two new saponins from tetraploid jiaogulan (Gynostemma pentaphyllum), and their anti-inflammatory and α-glucosidase inhibitory activities. Food Chem. 2013, 141, 3606–3613. [Google Scholar]
- Yang, F.; Shi, H.; Zhang, X.; Yu, L. Two Novel Anti-Inflammatory 21-Nordammarane Saponins from Tetraploid Jiaogulan (Gynostemma pentaphyllum). J. Agric. Food Chem. 2013, 61, 12646–12652. [Google Scholar] [CrossRef]
- Su, S.; Wang, J.; Wang, J.; Yu, R.; Sun, L.; Zhang, Y.; Song, L.; Pu, W.; Tang, Y.; Yu, Y.; et al. Cardioprotective effects of gypenoside XVII against ischemia/reperfusion injury: Role of endoplasmic reticulum stress, autophagy, and mitochondrial fusion fission balance. Phytother. Res. 2022, 36, 2982–2998. [Google Scholar] [CrossRef]
- Zhang, M.M.; Huo, G.M.; Cheng, J.; Zhang, Q.P.; Li, N.Z.; Guo, M.X.; Liu, Q.; Xu, G.H.; Zhu, J.X.; Li, C.F.; et al. Gypenoside XVII, an Active Ingredient from Gynostemma pentaphyllum, Inhibits C3aR-Associated Synaptic Pruning in Stressed Mice. Nutrients 2022, 14, 2418. [Google Scholar] [CrossRef]
- Sun, T.; Duan, L.; Li, J.; Guo, H.; Xiong, M. Gypenoside XVII protects against spinal cord injury in mice by regulating the microRNA-21-mediated PTEN/AKT/mTOR pathway. Int. J. Mol. Med. 2021, 48, 146. [Google Scholar] [CrossRef]
- Espina, G.; Muñoz Ibacache, S.A.; Cáceres Moreno, P.; Amenabar, M.J.; Blamey, J.M. From the Discovery of Extremozymes to an Enzymatic Product: Roadmap Based on Their Applications. Front. Bioeng. Biotechnol. 2022, 9, 752281. [Google Scholar]
- Cui, C.H.; Kim, S.C.; Im, W.T. Characterization of the ginsenoside-transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl. Microbiol. Biotechnol. 2012, 97, 649–659. [Google Scholar]
- Wang, L.; Liu, Q.M.; Sung, B.H.; An, D.S.; Lee, H.G.; Kim, S.G.; Kim, S.C.; Lee, S.T.; Im, W.T. Bioconversion of ginsenosides Rb1, Rb2, Rc and Rd by novel β-glucosidase hydrolyzing outer 3-O glycoside from Sphingomonas sp. 2F2: Cloning, expression, and enzyme characterization. J. Biotechnol. 2011, 156, 125–133. [Google Scholar] [PubMed]
- An, D.S.; Cui, C.H.; Lee, H.G.; Wang, L.; Kim, S.C.; Lee, S.T.; Jin, F.; Yu, H.; Chin, Y.W.; Lee, H.K.; et al. Identification and Characterization of a Novel Terrabacter ginsenosidimutans sp. nov. β-Glucosidase That Transforms Ginsenoside Rb1 into the Rare Gypenosides XVII and LXXV. Appl. Environ. Microbiol. 2010, 76, 5827–5836. [Google Scholar] [PubMed]
- Kim, S.Y.; Lee, H.N.; Hong, S.J.; Kang, H.J.; Cho, J.Y.; Kim, D.; Ameer, K.; Kim, Y.M. Enhanced biotransformation of the minor ginsenosides in red ginseng extract by Penicillium decumbens β-glucosidase. Enzym. Microb. Technol. 2022, 153, 109941. [Google Scholar]
- Siddiqi, M.Z.; Shafi, S.M.; Im, W.T. Complete genome sequencing of Arachidicoccus ginsenosidimutans sp. nov., and its application for production of minor ginsenosides by finding a novel ginsenoside-transforming β-glucosidase. RSC Adv. 2017, 7, 46745–46759. [Google Scholar]
- Zheng, F.; Zhao, H.; Wang, N.; Zhong, P.; Zhou, K.; Yu, S. Cloning and characterization of thermophilic endoglucanase and its application in the transformation of ginsenosides. AMB Express 2022, 12, 1–13. [Google Scholar]
- Hu, Y.; Zhai, L.; Hong, H.; Shi, Z.; Zhao, J.; Liu, D. Study on the Biochemical Characterization and Selectivity of Three β-Glucosidases From Bifidobacterium adolescentis ATCC15703. Front. Microbiol. 2022, 13, 860014. [Google Scholar]
- Quan, L.H.; Piao, J.Y.; Min, J.W.; Kim, H.B.; Kim, S.R.; Yang, D.U.; Yang, D.C. Biotransformation of Ginsenoside Rb1to Prosapogenins, Gypenoside XVII, Ginsenoside Rd, Ginsenoside F2, and Compound K by Leuconostoc mesenteroides DC102. J. Ginseng Res. 2011, 35, 344–351. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, D.Y.; Kang, D.W.; Park, C.S. Hydrolysis of the outer β-(1,2)-d-glucose linkage at the C-3 postion of ginsenosides by a commercial β-galactosidase and its use in the production of minor ginsenosides. Biocatal. Biotransformation 2018, 37, 53–58. [Google Scholar] [CrossRef]
- Saraoui, T.; Parayre, S.; Guernec, G.; Loux, V.; Montfort, J.; Le Cam, A.; Boudry, G.; Jan, G.; Falentin, H. A unique in vivo experimental approach reveals metabolic adaptation of the probiotic Propionibacterium freudenreichii to the colon environment. BMC Genom. 2013, 14, 911. [Google Scholar]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar]
- Kenji, S.; Takashi, T.; Hidehiko, K.; Tatsurokuro, T. Isolation and char-acterization of two β-D-glucosidases from Bififidobacterium breve 203 (microbiology & fermentation industry). Agric. Biol. Chem. 1986, 50, 2287–2293. [Google Scholar]
- Matsumoto, T.; Shimada, S.; Hata, Y.; Tanaka, T.; Kondo, A. Multi-functional glycoside hydrolase: Blon_0625 from Bifidobacterium longum subsp. infantis ATCC 15697. Enzyme Microb. Technol. 2015, 68, 10–14. [Google Scholar] [PubMed]
- Florindo, R.N.; Souza, V.P.; Manzine, L.R.; Camilo, C.M.; Marana, S.R.; Polikarpov, I.; Nascimento, A.S. Structural and biochemical characterization of a GH3 β-glucosidase from the probiotic bacteria Bifidobacterium adolescentis. Biochimie 2018, 148, 107–115. [Google Scholar] [PubMed]
- Nunoura, N.; Ohdan, K.; Yamamoto, K.; Kumagai, H. Expression of the β-d-glucosidase I gene in Bifidobacterium breve 203 during acclimation to cellobiose. J. Ferment. Bioeng. 1997, 83, 309–314. [Google Scholar]
- Guadamuro, L.; Flórez, A.B.; Alegría, Á.; Vázquez, L.; Mayo, B. Characterization of four β-glucosidases acting on isoflavone-glycosides from Bifidobacterium pseudocatenulatum IPLA 36007. Food Res. Int. 2017, 100, 522–528. [Google Scholar]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D. The Proteomics Protocols Handbook; Humana Press: New York, NY, USA, 2005; pp. 571–607. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar]
| Additives | Relative Activity (%) | |
|---|---|---|
| Control | 100 | 100 |
| Metal ions | 5 mM | 10 mM |
| NH4Cl | 89.18 | 108.63 |
| NaCl | 109 | 119 |
| BaCl2 | 68.28 | 33.91 |
| KCl | 90.34 | 93.83 |
| MnCl2 | 135.66 | 93.81 |
| CaCl2 | 63 | 28 |
| ZnCl2 | 93.17 | 43.28 |
| CoCl2 | 80.42 | 50.11 |
| FeCl3 | 51.92 | 23.66 |
| Inhibitors | 5 mM | 10 mM |
| EDTA | 97.74 | 95.82 |
| Substrate | Relative Activity (%) |
|---|---|
| pNPβGlu | 100 ± 0.87 a |
| pNPαGlu | ND b |
| pNPαAraf | ND |
| pNPαArap | ND |
| pNPβLac | ND |
| pNPβGal | 7.5 ± 0.57 |
| pNPαMan | ND |
| pNPβMan | 6.4 ± 0.12 |
| pNPβXyl | 5.9 ± 0.85 |
| pNPβFuc | ND |
| pNPβCel | ND |
| pNPαRha | ND |
| Organism | Reaction Conditions | GH Family | Substrate | Product | Reaction Time | Yield | Reference |
|---|---|---|---|---|---|---|---|
| Sphingopyxis alaskensis | pH 5.5, 50 °C | — | G-Rb1 | Gyp XVII | 7 h | 87.5% | [14] |
| Actinosynnema mirum | pH 7.0, 37 °C | GH3 | G-Rb1 | Gyp XVII, Gyp LXXV | 18 h | — | [30] |
| Sphingomonas sp. 2F2 | pH 5.0, 37 °C | GH1 | G-Rb1 | Gyp XVII, F2 | 92 h | — | [31] |
| Terrabacter ginsenosidimutans sp. nov. | pH 7.0, 37 °C | GH3 | G-Rb1 | Gyp XVII, Gyp LXXV | 24 h | — | [32] |
| Penicillium decumbens | pH 4.0, 60 °C | — | G-Rb1 | Gyp XVII, F2, CK | 13 h | — | [33] |
| Arachidicoccus ginsenosidimutans sp. nov. | pH 7.5, 50 °C | GH1 | G-Rb1 | Gyp XVII, F2, CK | 4 h | — | [34] |
| Fervidobaterium pennivorans DSM9078 | pH 5.5, 90 °C | GH5 | G-Rb1 | Gyp XVII | 1 h | — | [35] |
| Bifidobacterium adolescentis ATCC15703 | pH 7.0, 30–50 °C | GH1 | G-Rb1 | Gyp XVII | 2 h | 72.16% | [36] |
| Leuconostoc mesenteroides DC102 | pH 6.0–8.0, 30 °C | — | G-Rb1 | Gyp XVII | 24 h | — | [37] |
| Aspergillus oryzae | pH 4.5, 50 °C | — | G-Rb1 | Gyp XVII | 1 h | 88.75% | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Zhang, Y.; Zhou, Y.; Xu, M.; Yu, S. Production of Gypenoside XVII from Ginsenoside Rb1 by Enzymatic Transformation and Their Anti-Inflammatory Activity In Vitro and In Vivo. Molecules 2023, 28, 7001. https://doi.org/10.3390/molecules28197001
Zhou K, Zhang Y, Zhou Y, Xu M, Yu S. Production of Gypenoside XVII from Ginsenoside Rb1 by Enzymatic Transformation and Their Anti-Inflammatory Activity In Vitro and In Vivo. Molecules. 2023; 28(19):7001. https://doi.org/10.3390/molecules28197001
Chicago/Turabian StyleZhou, Kailu, Yangyang Zhang, Yikai Zhou, Minghao Xu, and Shanshan Yu. 2023. "Production of Gypenoside XVII from Ginsenoside Rb1 by Enzymatic Transformation and Their Anti-Inflammatory Activity In Vitro and In Vivo" Molecules 28, no. 19: 7001. https://doi.org/10.3390/molecules28197001
APA StyleZhou, K., Zhang, Y., Zhou, Y., Xu, M., & Yu, S. (2023). Production of Gypenoside XVII from Ginsenoside Rb1 by Enzymatic Transformation and Their Anti-Inflammatory Activity In Vitro and In Vivo. Molecules, 28(19), 7001. https://doi.org/10.3390/molecules28197001





