Synthesis of (R)-(6-Methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methanol Tetraphenylborate Ion-Pair Complex: Characterization, Antimicrobial, and Computational Study
Abstract
:1. Introduction
2. Results and Discussion
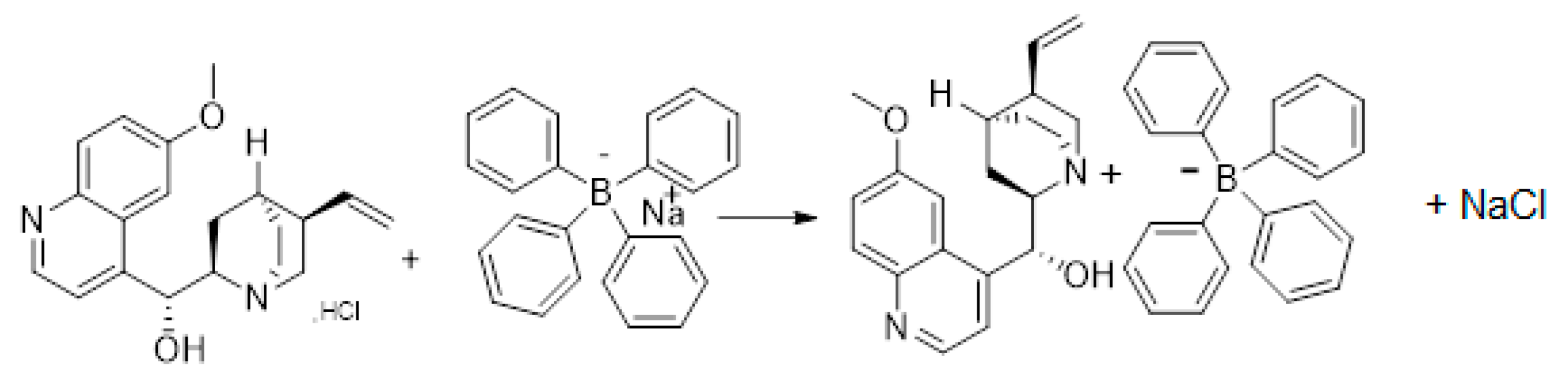
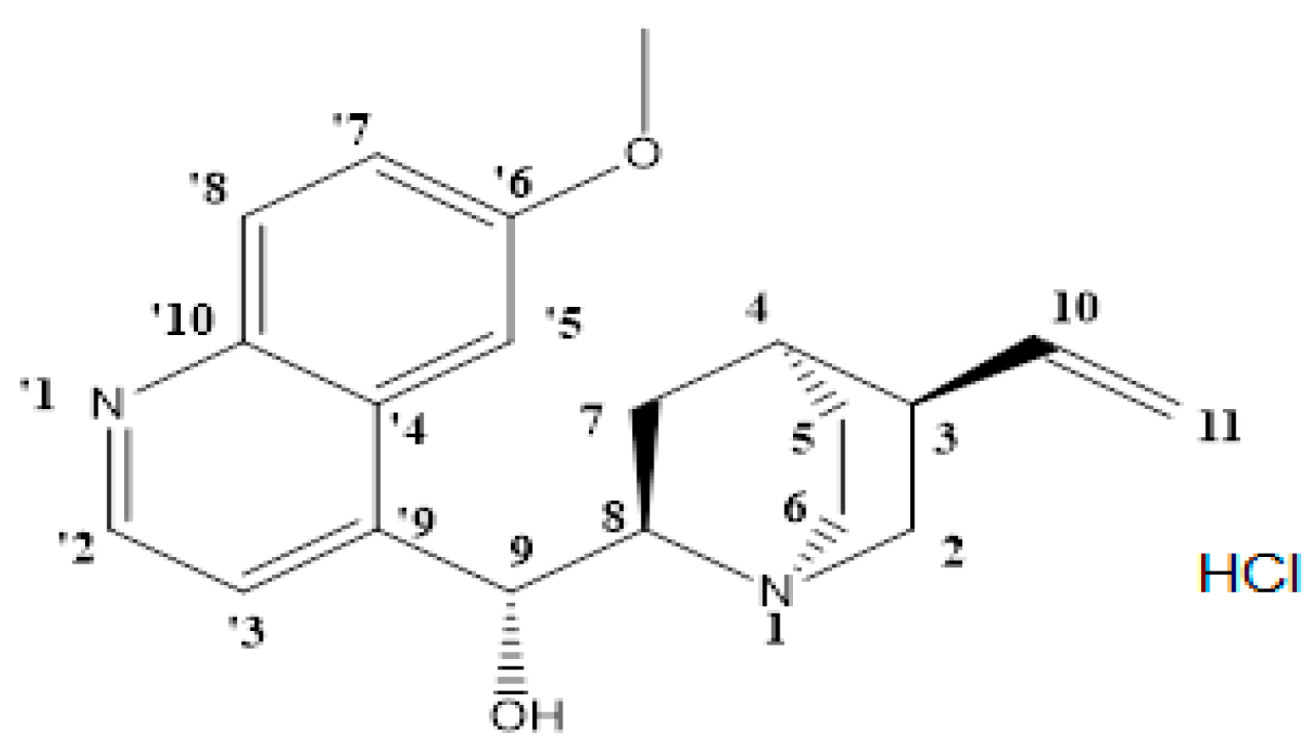
2.1. Chemistry
2.2. Antimicrobial Activities
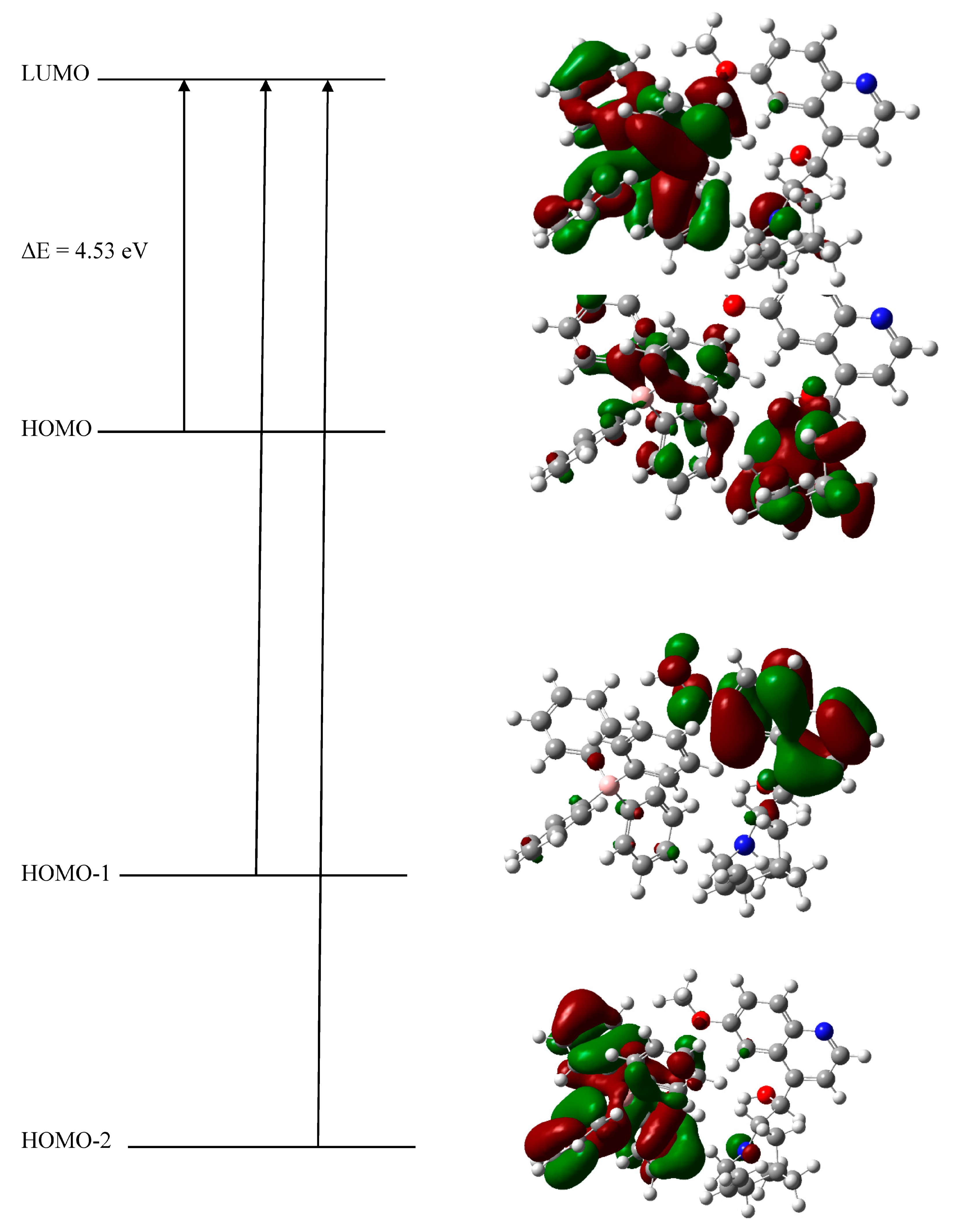
2.3. Computational Study
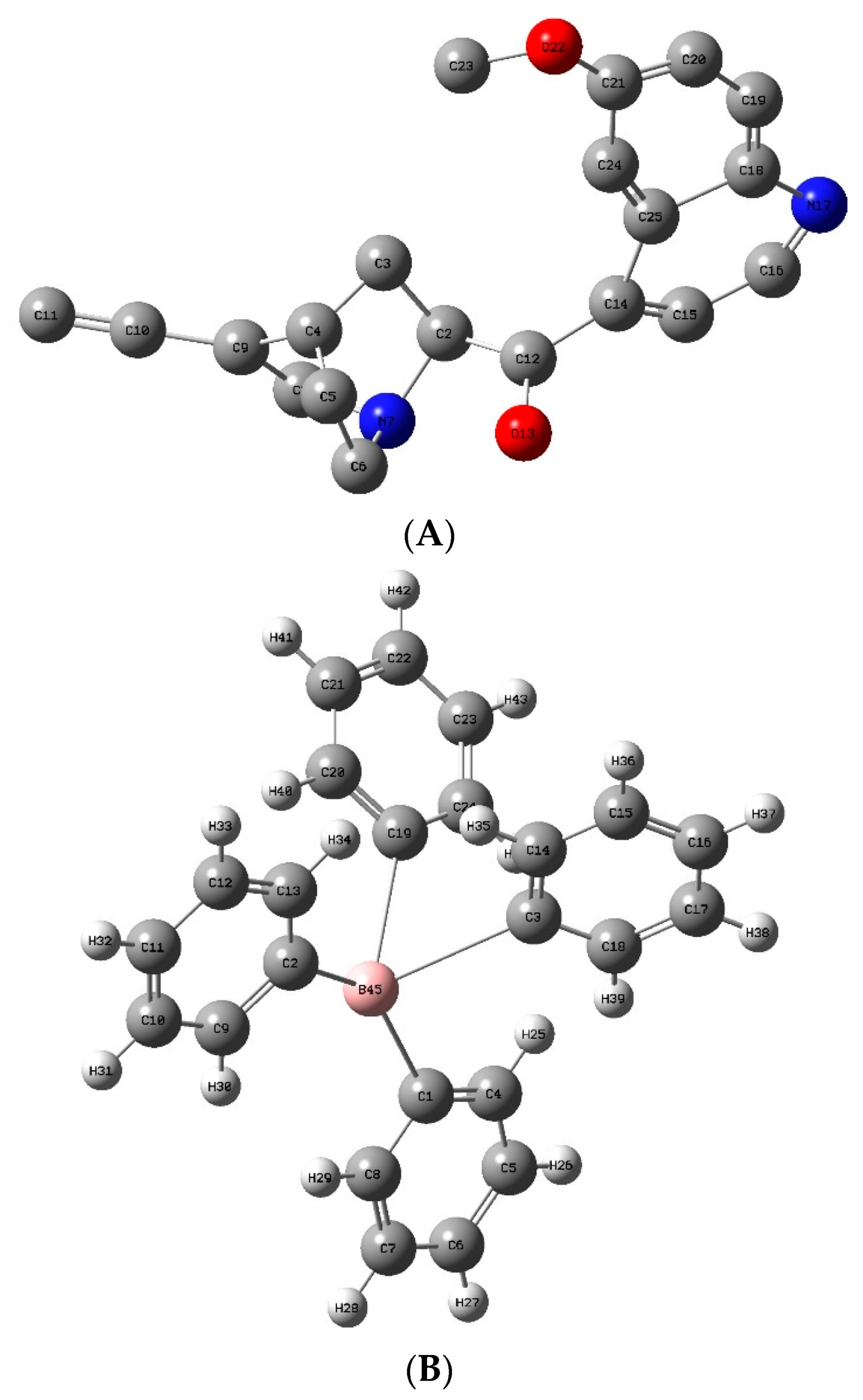
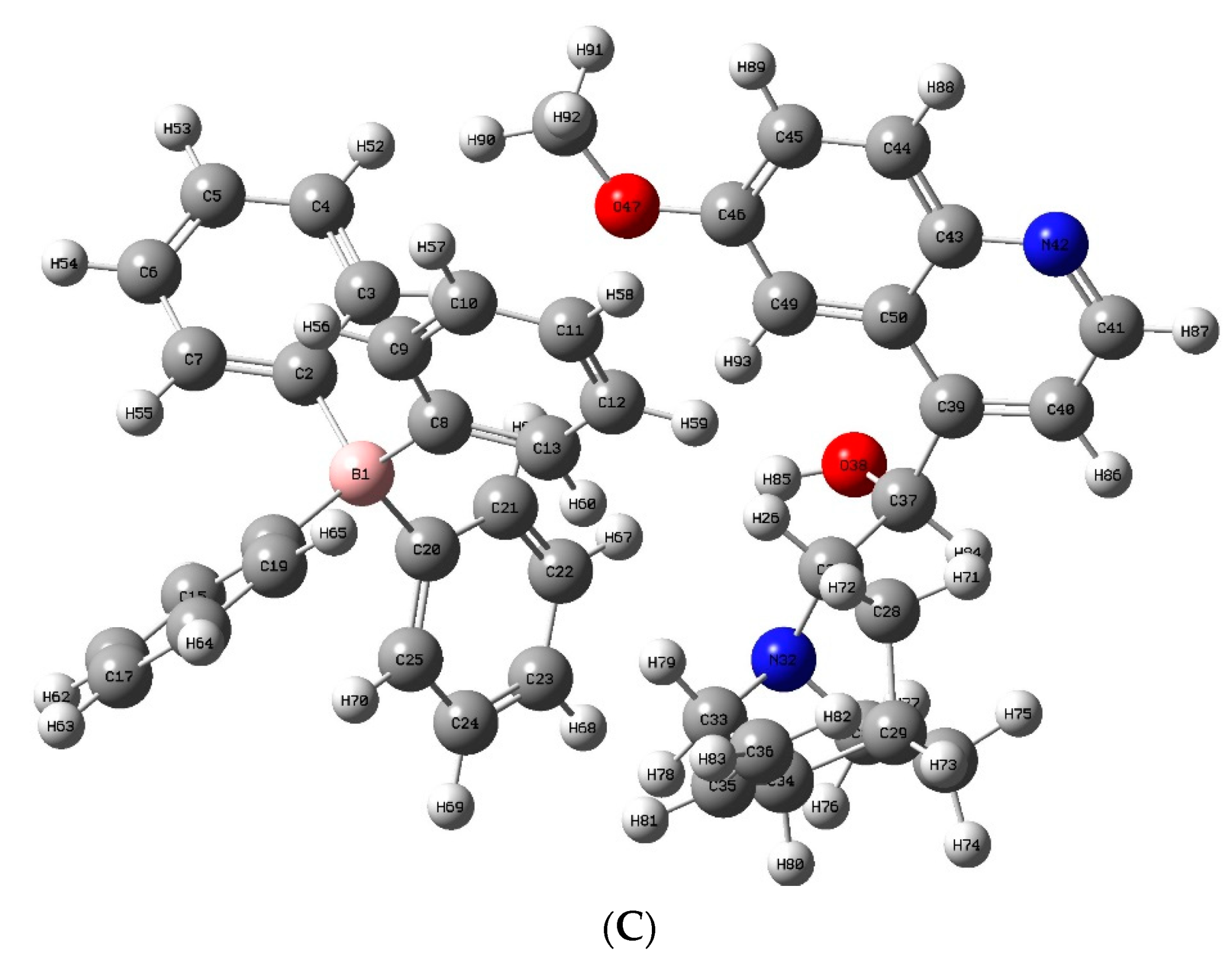
2.3.1. DFT Optimization
2.3.2. Interaction Energies (IEs)
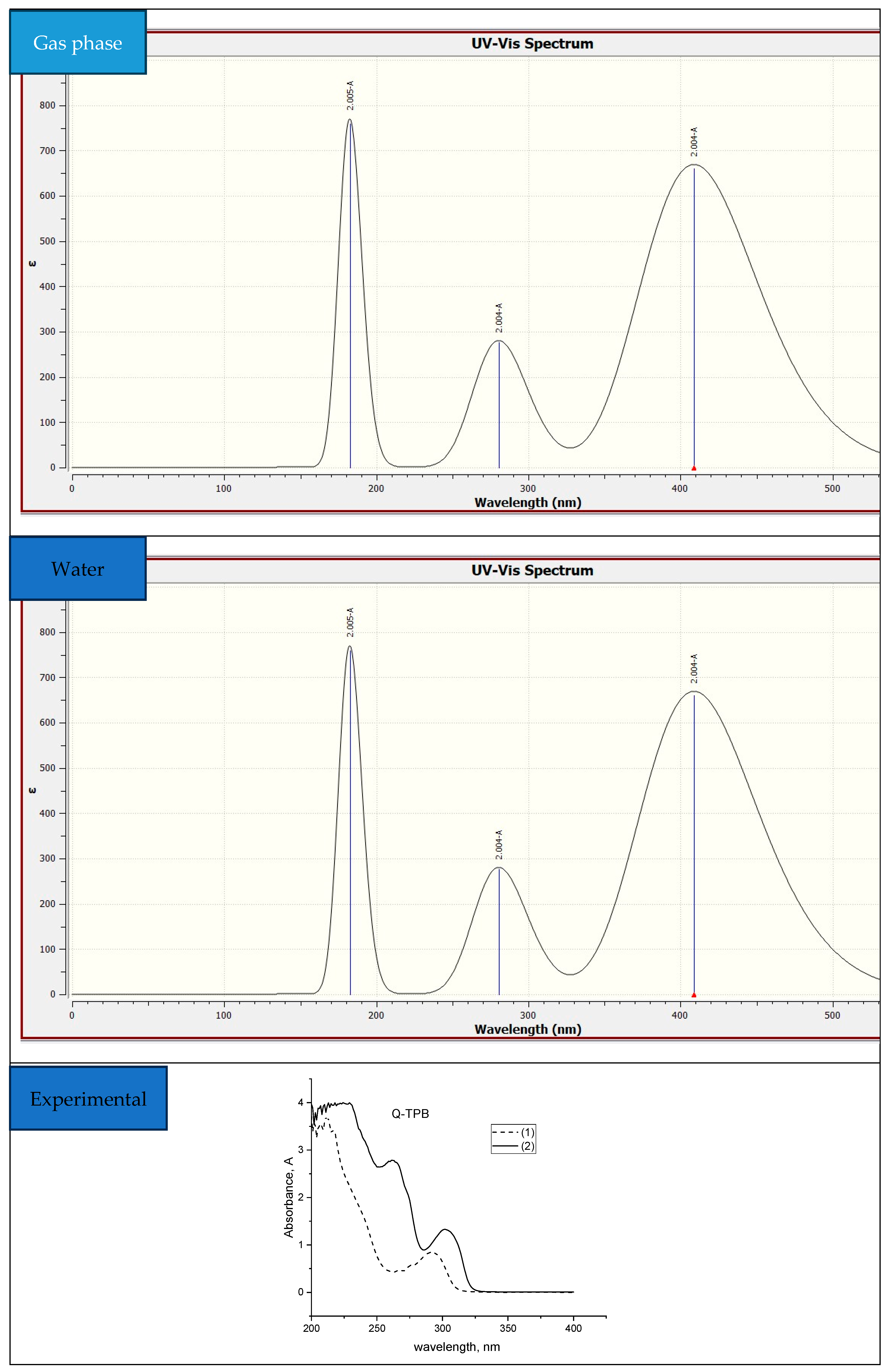
2.3.3. Electronic Transitions by UV/Visible
2.3.4. Vibrational Frequencies in IR-Spectrum
2.3.5. 1H NMR Chemical Shifts
2.3.6. Mulliken Charge
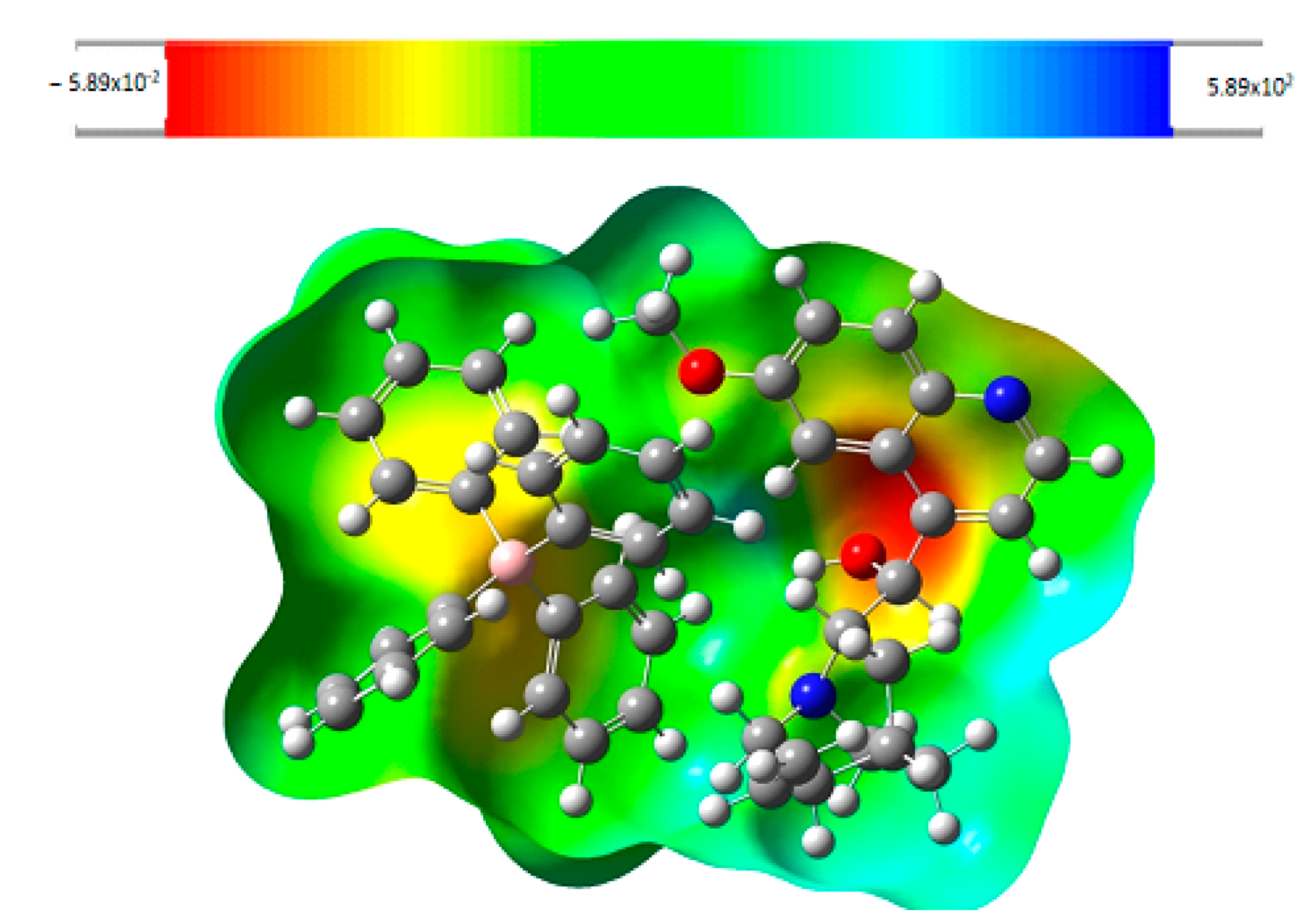
2.3.7. Molecular Electrostatic Potential (MEP)
3. Experimental
3.1. Materials and Instrument
3.2. Synthesis
3.3. Antimicrobial Susceptibility Testing
3.3.1. Disk Diffusion Method
3.3.2. Minimum Inhibitory Concentration (MIC)
3.4. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pósa, S.P.; Dargó, G.; Nagy, S.; Kisszékelyi, P.; Garádi, Z.; Hámori, L.; Szakács, G.; Kupai, J.; Tóth, S. Cytotoxicity of cinchona alkaloid organocatalysts against MES-SA and MES-SA/Dx5 multidrug-resistant uterine sarcoma cell lines. Bioorg. Med. Chem. 2022, 67, 116855. [Google Scholar]
- White, N.J. Antimalarial pharmacokinetics and treatment regimens. Br. J. Clin. Pharmacol. 1992, 34, 1. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, M. Drug development: Holding out for reinforcements. Nature 2012, 484, S16–S18. [Google Scholar] [CrossRef] [PubMed]
- Wanli, M. Construction and performance of probe-type cells connected in a series assembly. Talanta 1992, 39, 1051–1055. [Google Scholar] [CrossRef]
- Anzai, J.; Isomura, C.; Osa, T. Fabrication of Quinine-Sensitive Membrane Electrodes and Their Properties. Chem. Pharm. Bull. 1985, 33, 236–241. [Google Scholar]
- Saad, B.; Bee-Leng, Y.; Saleh, M.I.; Rahman, I.A.; Mansor, S.M. Polyvinyl chloride-based membranes for flow injection analysis of quinine in beverages. J. AOAC Int. 2001, 84, 1151–1158. [Google Scholar]
- Jing, Y.; Watanabe, K.; Watanabe, T.; Kimura, S.; Toko, K. Development and Optimization of a Highly Sensitive Sensor to Quinine-Based Saltiness Enhancement Effect. Sensors 2023, 23, 3178. [Google Scholar] [CrossRef]
- Alrabiah, H.; Ali, E.A.; Alsalahi, R.A.; Attwa, M.W.; Mostafa, G.A.E. Fabrication and Applications of Potentiometric Membrane Sensors Based on γ-Cyclodextrin and Calixarene as Ionophores for the Determination of a Histamine H1-Receptor Antagonist: Fexofenadine. Polymers 2023, 15, 2808. [Google Scholar]
- Mostafa, G.A.E.; Ali, E.A.; Alsalahi, R.A.; Alrabiah, H. Fabrication and Applications of Potentiometric Membrane Sensors Based on Specific Recognition Sites for the Measurement of the Quinolone Antibacterial Drug Gemifloxacin. Molecules 2023, 28, 5144. [Google Scholar]
- AlRabiah, H.; Al-Majed, A.; Abounassif, M.; Mostafa, G.A.E. Ionophore-based potentiometric PVC membrane sensors for determination of phenobarbitone in pharmaceutical formulations. Acta Pharm. 2016, 66, 2016. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Kois, D.; Brinker, U.H. Metal Catalyzed Reductive CC Bond Formation; Springer: Berlin/Heidelberg, Germany, 1998; p. 4314. [Google Scholar]
- Chaffey, N. Molecular Biology of the Cell, 4th ed.; Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., Walter, P., Eds.; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Cebula, J.; Fink, K.; Boratyński, J.; Goszczyński, T.M. Supramolecular chemistry of anionic boron clusters and its applications in biology. Coord. Chem. Rev. 2021, 477, 214940. [Google Scholar]
- Casini, G.M.-A.A. Bioinorganic supramolecular coordination complexes and their biomedical applications. FEBS Lett. 2023, 597, 191. [Google Scholar]
- Shi, B.; Chai, Y.; Qin, P.; Zhao, X.-X.; Li, W.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q.; Yao, H.; Qu, W.-J. Detection of aliphatic aldehydes by a pillar[5]arene-based fluorescent supramolecular polymer with vaporchromic behavior. Chem. Asian. J. 2020, 17, e202101421. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, W.; Liu, M.; Zhao, J.; Fan, Y.; Bian, B.; Tao, Z.; Xiao, X. A supramolecular fluorescent probe based on cucurbit[10]uril for sensing the pesticide dodine. Chin. Chem. Lett. 2021, 32, 367. [Google Scholar] [CrossRef]
- Ailincai, D.; Morariu, S.; Rosca, I.; Sandu, A.I.; Marin, L. Drug delivery based on a supramolecular chemistry approach by using chitosan hydrogels. Int. J. Biol. Macromol. 2023, 248, 125800. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Kumar, N. (Eds.) Pharmaceutical Applications of Supramolecules; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Wang, H.; Tian, Q.; Quan, P.; Liu, C.; Fang, L. Probing the role of ion-pair strategy in controlling dexmedetomidine penetrate through drug-in-adhesive patch: Mechanistic insights based on release and percutaneous absorption process. AAPS PharmSciTech 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Wagay, S.A.; Khan, L.; Ali, R. Recent Advancements in Ion-Pair Receptors. Chem.–Asian J. 2023, 18, e202201080. [Google Scholar] [CrossRef]
- Kim, S.K.; Sessler, J.L. Ion pair receptors. Chem. Soc. Rev. 2010, 39, 3784. [Google Scholar] [CrossRef]
- Jagleniec, D.; Ziach, K.; Dąbrowa, K.; Romański, J. The effect of substitution pattern on binding ability in regioisomeric ion pair receptors based on an aminobenzoic platform. Molecules 2019, 24, 2990. [Google Scholar] [CrossRef]
- Xiong, S.; He, Q. Photoresponsive macrocycles for selective binding and release of sulfate. Chem. Commun. 2021, 57, 13514–13517. [Google Scholar] [CrossRef] [PubMed]
- Kokan, Z.; Chmielewski, M.J. A photoswitchable heteroditopic ion-pair receptor. J. Am. Chem. Soc. 2018, 140, 16010–16014. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Vervuurt, S.J.N.; Elmes, R.B.P.; Berry, S.N.; Proschogo, N.; Jolliffe, K.A. Extraction and transport of sulfate using macrocyclic squaramide receptors. Chem. Sci. 2020, 11, 201–207. [Google Scholar] [CrossRef]
- Zhang, C.; Mu, Y.; Zhao, S.; Zhang, W.; Wang, Y. Lithium extraction from synthetic brine with high Mg2+/Li+ ratio using the polymer inclusion membrane. Desalination 2020, 496, 114710. [Google Scholar] [CrossRef]
- Paredes, C.; de San Miguel, E.R. Selective lithium extraction and concentration from diluted alkaline aqueous media by a polymer inclusion membrane and application to seawater. Desalination 2020, 487, 114500. [Google Scholar] [CrossRef]
- Mostafa, G.A.E.; Yousef, T.A.; ElGamal, A.A.; Homoda, A.M.A.; AlRabiah, H. Tamoxifen charge transfer complexes with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and 7,7,8,8-tetracyanoquinodimethan: Synthesis, spectroscopic characterization and theoretical study. Bioorg. Chem. 2022, 120, 105603. [Google Scholar] [CrossRef]
- Mostafa, G.A.E.; Yousef, T.A.; Gaballah, S.T.; Homoda, A.M.; Al-Salahi, R.; Aljohar, H.I.; AlRabiah, H. Quinine Charge Transfer Complexes with 2,3-Dichloro-5,6-Dicyano-Benzoquinone and 7,7,8,8-Tetracyanoquinodimethane: Spectroscopic Characterization and Theoretical Study. Appl. Sci. 2022, 12, 978. [Google Scholar] [CrossRef]
- Lewis, S.J. M100 Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, NJ, USA, 2022. [Google Scholar]
- Rex, J.H. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts: Approved Guideline. 2009, 2nd edition. Available online: https://clsi.org/media/1634/m44a2_sample.pdf (accessed on 5 October 2023).
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints—Bacteria (v 5.0); European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2020. [Google Scholar]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Eucast Definitive. Available online: https://clsi.org/media/1634/m44a2_sample.pdf(accessed on 5 October 2023).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009; Available online: https://www.gaussian.com (accessed on 5 October 2023).
- Becke, A.D. Density-functional thermochemistry, I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Yousef, T.A.; Ezzeldin, E.; Abdel-Aziz, H.A.; Al-Agamy, M.H.; Mostafa, G.A.E. Charge transfer complex of neostigmine with 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone: Synthesis, spectroscopic characterization, antimicrobial activity, and theoretical study. Drug Des. Devel. Ther. 2020, 14, 4115. [Google Scholar] [CrossRef] [PubMed]
| Compound | S. aureus ATCC 25923 | B. subtilis ATCC 10400 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | C. albicans ATCC 90028 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inhibition Zone (mm) | MIC (μg/mL) | Inhibition Zone (mm) | MIC (μg/mL) | Inhibition Zone (mm) | MIC (μg/mL) | Inhibition Zone (mm) | MIC (μg/mL) | Inhibition Zone (mm) | MIC (μg/mL) | |
| Q-TPB | 25 | 16 | 20 | 32 | 9 | ND | 10 | ND | 20 | 32 |
| Ciprofloxacin | 34 | 0.25 | 30 | ≤0.25 | 32 | ≤0.25 | 29 | 0.25 | ND | ND |
| Fluconazole | ND | ND | ND | ND | ND | ND | ND | ND | 32 | 2 |
| Parameters | Q-TPB | |
|---|---|---|
| Solvent | Gas Phase | Water |
| ETotal | −45,833.05 | −46,087.18 |
| Dipole Moment | 2.45 | 6.95 |
| EHOMO | −6.12 | −6.32 |
| ELUMO | −1.99 | −1.79 |
| ΔE (ELUMO − EHOMO) | 4.13 | 4.53 |
| Structure | Solvent | λmax | ƒ | Electronic Transition | Major % Contribution |
|---|---|---|---|---|---|
| Q-TPB | Gas phase | 408.9 nm | 0.162 | H → L | 97% |
| Water | 0.165 | H → L | 99% | ||
| Gas phase | 280.6 nm | 0.077 | H-2 → L H → L | 84% 58% | |
| Water | 0.069 | H-2 → L H → L | 85% 55% | ||
| Gas phase | 182.5 nm | 0.186 | H-1 → L H → L | 97% 27% | |
| Water | 0.190 | H-1 → L H → L | 97% 29% |
| Band Assignments | Experimental | Calculated |
|---|---|---|
| νOH | 3487 cm−1 | 3552 cm−1 |
| νCH aliphatic | 3055, 3000, 2984 cm−1 | 3034, 3015, 2990 cm−1 |
| ν(CN)py | 1622 cm−1 | 1621 cm−1 |
| Phenyl | 1509 cm−1 | 1510 cm−1 |
| ν(OCH3) | 1267 cm−1 | 1257 cm−1 |
| Type | Label Numbers | Experimental Chemical Shift (ppm) | Calculated Chemical Shift (ppm) |
|---|---|---|---|
| ArH | 71 | 1.48 | 0.92 |
| ArH | 73 | 1.49 | 1.43 |
| ArH | 74 | 1.51 | 1.48 |
| ArH | 75 | 1.86 | 1.61 |
| ArH | 72, 79 | 2.02 | 1.85 |
| ArH | 80 | 2.07 | 2.17 |
| ArH, OH | 78, 85 | 2.51 | 2.43 |
| CH3 | 76, 92 | 2.72 | 2.98 |
| CH3 | 91 | 3.35 | 3.20 |
| CH2 | 77 | 3.60 | 3.38 |
| CH | 26 | 3.64 | 3.64 |
| CH3 | 90 | 3.99 | 3.74 |
| CH3 | 84 | 3.99 | 4.62 |
| ArH | 81 | 5.02 | 5.65 |
| ArH | 59 | 5.41 | 5.89 |
| ArH | 65 | 5.85 | 6.00 |
| ArH | 55 | 5.85 | 6.21 |
| ArH | 58, 64, 67 | 6.46 | 6.31 |
| ArH | 60 | 6.80 | 6.40 |
| ArH | 54, 52, 70, 57 | 6.80 | 6.51 |
| ArH | 53, 69 | 6.92 | 6.62 |
| ArH | 68 | 7.12 | 6.70 |
| ArH | 63, 89 | 7.12 | 6.78 |
| ArH | 86 | 7.19 | 6.87 |
| ArH | 93 | 7.19 | 6.94 |
| ArH | 62 | 7.38 | 7.04 |
| ArH | 56 | 7.38 | 7.11 |
| ArH | 66, 51 | 7.50 | 7.63 |
| ArH | 88 | 7.66 | 7.73 |
| ArH | 61 | 8.01 | 8.15 |
| ArH | 87 | 8.78 | 8.52 |
| Atoms | Quinine | TPB | Q-TPB Complex |
|---|---|---|---|
| B1 | −0.228 | 0.204 | |
| N32 | 0.228 | −0.059 | |
| O38 | −0.398 | −0.095 | |
| N42 | 0.194 | −0.032 | |
| O47 | −0.539 | −0.286 | |
| C48 | 0.087 | 0.154 | |
| C50 | 0.288 | 0.262 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, T.A.; Alrabiah, H.; Al-Agamy, M.H.; Al-Salahi, R.; Ali, E.A.; Mostafa, G.A.E. Synthesis of (R)-(6-Methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methanol Tetraphenylborate Ion-Pair Complex: Characterization, Antimicrobial, and Computational Study. Molecules 2023, 28, 6974. https://doi.org/10.3390/molecules28196974
Yousef TA, Alrabiah H, Al-Agamy MH, Al-Salahi R, Ali EA, Mostafa GAE. Synthesis of (R)-(6-Methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methanol Tetraphenylborate Ion-Pair Complex: Characterization, Antimicrobial, and Computational Study. Molecules. 2023; 28(19):6974. https://doi.org/10.3390/molecules28196974
Chicago/Turabian StyleYousef, Tarek A., Haitham Alrabiah, Mohamed H. Al-Agamy, Rashad Al-Salahi, Essam A. Ali, and Gamal A. E. Mostafa. 2023. "Synthesis of (R)-(6-Methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methanol Tetraphenylborate Ion-Pair Complex: Characterization, Antimicrobial, and Computational Study" Molecules 28, no. 19: 6974. https://doi.org/10.3390/molecules28196974
APA StyleYousef, T. A., Alrabiah, H., Al-Agamy, M. H., Al-Salahi, R., Ali, E. A., & Mostafa, G. A. E. (2023). Synthesis of (R)-(6-Methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methanol Tetraphenylborate Ion-Pair Complex: Characterization, Antimicrobial, and Computational Study. Molecules, 28(19), 6974. https://doi.org/10.3390/molecules28196974







